Abstract
In this study, a series of cobalt-free Sn-doped cathode materials of Li1.15Ni0.27Mn0.58-xSnxO2 are prepared by the solvothermal method for the first time. The structural characterization of the material reveals that all the samples consist of hexagonal layered structural LiMO2 (M = Mn/Ni), monoclinic-layered structural Li2MnO3, and cubic spinel structural LiMn2O4. Moreover, the interplanar spacing of the layered structure increases in the material, and the electrochemical impedance of the material declines by Sn doping. As a result, all Sn-doped samples exhibit better electrochemical performance than pristine Li1.15Ni0.27Mn0.58O2 materials. Among them, Li1.15Ni0.27Mn0.56Sn0.02O2 delivers comprehensively improved electrochemical performance. The initial coulombic efficiency of the Li1.15Ni0.27Mn0.56Sn0.02O2 sample is 84.4%, which is nearly 10% higher than the pristine material, and Li1.15Ni0.27Mn0.56Sn0.02O2 exhibits an initial discharge specific capacity of 260.8 mAh/g at a current density of 0.1 C, and the capacity retention after first 100 cycles at 1 C reached 94.67%. Rate capability of Li1.15Ni0.27Mn0.58-xSnxO2 is significantly improved by Sn doping. The specific discharge capacity of the Li1.15Ni0.27Mn0.56Sn0.02O2 sample at 5 C is two times higher than that of Li1.15Ni0.27Mn0.58O2. Moreover, the Li1.15Ni0.27Mn0.56Sn0.02O2 material can still maintain a discharge capacity of 220 mAh/g when the current density returns to 0.1 C after a large current density cycling process. These results show that the proper amount of Sn doping can effectively improve the electrochemical performance of Li1.15Ni0.27Mn0.58O2, due to the fact that Sn ions have larger ionic radii than the transition metal (Mn/Ni) ions and Sn can partially replace the transition metal element ions in the layered structure, thereby expanding the lithium ion diffusion channel and inhibiting instability of the material structure during the cycle. However, an excessive amount of Sn (x > 0.03) generates a Li2SnO3 impurity in the material, resulting in deterioration of material properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lithium-rich manganese-based cathode material xLi2MnO3·(1-x)LiMO2 (0< x <1, M = Mn, Ni, Co) has a specific capacity of 250 mAh/g, an operating voltage exceeding 4.5 V, and a high energy density [1]. Currently, Co-free lithium-rich manganese-based cathode materials are generating widespread interest, due to their low cost and environmental friendliness [2, 3]. The lithium-rich manganese-based material can be considered as a matrix of layered Li2MnO3 and layered LiMO2 (M = Mn, Ni, Co) forming a solid solution. Li2MnO3 and LiMO2 are layered rock salt structures, similar to the structure of α-NaFeO2, and Li2MnO3 can also be described in the form of Li[Li1/3Mn2/3]O2. Therefore, the lithium-rich manganese-based cathode material xLi2MnO3·(1-x)LiMO2 can be regarded as derived from layered Li2MnO3, in which a part of the transition metal M replaces Li+ in Li[Li1/3Mn2/3]O2. However, the Li2MnO3 component in the material undergoes an irreversible structural change during charge and discharge, which results in poor performance during charge–discharge cycles. The main property deficiencies in lithium-rich manganese-based materials are as follows: the large capacity loss during the first charge and discharge process, poor rate capability, and unsatisfactory cycle performance. Elemental doping is considered as an effective way to improve the electrochemical properties of lithium-rich manganese-based materials [4].
Usually, an element with ionic radius close to the ions of the substituted elements is selected to dope in lithium-rich cathode materials. Element doping can improve the conductivity of the material, increase the lattice parameters, form stronger M–O bonds, which promotes Li+ migration, and thus can significantly improve the structural stability and rate performance of the material. Doping methods include anionic doping, cationic doping, and anion–cation co-doping.
Anion doping generally selects F− and S2− to partially replace oxygen. Partial substitution of oxygen in the Li[Li1/6Ni1/4Mn7/12]O2-xFx material by fluorine can facilitate the growth of primary particles [5]. With increasing fluorine content, the cycle performance significantly improves. This is because fluorine substitution reduces irreversible oxygen loss in the lattice during the first cycle and promotes the formation of a layered-spinel symbiotic structure in the material. Li1.2Mn0.6Ni0.2O1.97S0.03 obtained by substituting oxygen with a small amount of sulfur shows extremely high coulombic efficiency in the first cycle [6], due to reversible intercalation and deintercalation of Li+ in S-doped materials.
Cation doping elements are generally used to replace the position of Mn in the material, and Li is substituted by Na+ and K+. Na+ and Al3+ double doping can inhibit the side reaction between the NaxLi1.2-xMn0.6-xAlxNi0.2O2 lithium-rich electrode and the electrolyte during the cycle [7], which greatly improves the high-temperature cycle stability of the material. Different from the nickel doping of the general lithium-rich manganese-based cathode material, only doping a small amount of nickel into Li[Li1/3-2x/3Mn2/3-x/3Nix]O2 material can promote the progressive activation of Li2MnO3 [8], thereby obtaining a larger specific capacity and better cycle performance. In contrast, excess Ni overactivates Li2MnO3 and causes a large capacity loss in the first cycle. Cr doping in Li1.1Ni0.25Mn0.75O2.3 not only can effectively reduce the voltage attenuation [9] but also reduces the Rct (charge transfer resistance) of the material, thus improving the cycle performance. In addition, Cr doping can reduce the content of Mn3+ in Li1.2Ni0.2Mn0.6O2 during the cycling process [10], thereby inhibiting the formation of the spinel-like structure and facilitating the increase in intermediate voltage. Moreover, Cr doping increases the lithium ion diffusion coefficient and electron conductivity of the Li1.2Ni0.2Mn0.6O2 cathode material, thereby improving the rate performance. Adding a small amount of B3+ ions to the tetrahedral site in Li-rich material can prevent the transition metal ions in the material from migrating to the tetrahedral position [11], and can stabilize the material structure. Mg substitution in lithium-rich materials can increase the interlayer spacing and the lithium ion diffusion coefficient of the material [12], which significantly improves the insertion and deintercalation of lithium ions, rate capability, and cycle performance. In addition, Nb [13] or Y [14] substitution in lithium-rich materials has a similar effect to Mg. Zn substitution in lithium-rich materials reduces charge transfer resistance and increases exchange current density [15], improving the cycling performance, because of excellent structural stability and conductivity of the active electrode. Zr doping in lithium-rich materials can increase the lattice parameters and the structural stability of materials and suppress electrode polarization [16]. Moreover, Zr-doped lithium-rich materials have better cycle stability at large current densities, so the enhanced electrochemical performance is ascribed to the improved structural stability and lithium ion diffusion of bulk electrode materials. In summary, elements with a larger ionic radius can be used to replace transition metal elements in lithium-rich manganese-based materials, and they can generally increase the interplanar spacing without drastically changing the material structure.
In this work, we partially substituted Mn in the lithium-rich manganese-based cathode material Li1.15Ni0.27Mn0.58O2 with Sn. Sn has a larger ionic radius than Mn and Ni, which may improve the material structure and thus the material electrochemical performance. Although many researchers have tried to modify the positive electrode material with Sn, its mechanism of action is still unclear and worthy of further investigation.
Experimental methods
Synthesis of Li1.15Ni0.27Mn0.58-xSnxO2 (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) materials
Li1.15Ni0.27Mn0.58-xSnxO2 (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) cathode materials were synthesized by the solvothermal method. Three solutions were prepared. First, manganese acetate and nickel acetate were dissolved in ethanol together with tin acetate according to the molar ratio in the target material Li1.15Ni0.27Mn0.58-xSnxO2 and mechanically stirred until completely dissolved. Then, an appropriate amount of oxalic acid was dissolved in ethanol by ultrasonication. Finally, 5 wt% excess of lithium acetate was dissolved in ethanol by ultrasonication according to the molar ratio in the Li1.15Ni0.27Mn0.58-xSnxO2 material, where excess lithium was used to compensate for the loss of lithium during calcination.
The prepared oxalic acid and lithium acetate solution was added dropwise to the metal cation ethanol solution at room temperature, followed by continuous stirring for 8 h. Then, the mixture was subjected to a solvothermal reaction in a reaction vessel at 200 °C for 20 h.
After naturally cooling to room temperature, the reacted solution was stirred and evaporated in a water bath at 80 °C to obtain a precursor. After the precursor was treated with 450 °C pre-calcination and 650 °C high-temperature calcination in a furnace, the target Li1.15Ni0.27Mn0.58-xSnxO2 material was obtained.
Structure and morphology analysis
X-ray powder diffraction (XRD, Rigaku D/max-2500PC) with Cu Kα radiation (40 kV, 200 mA) between 10 and 80° with a scan rate of 5° min−1 was employed to analyze the phase composition and crystal structure of the as-prepared material. The morphology and particle size of Li1.15Ni0.27Mn0.58-xSnxO2 (x = 0.01, 0.02, 0.03) materials were analyzed by scanning electron microscopy (SEM, Hitachi S4700). X-ray photoelectron spectroscopy (XPS, PHI 5000 VersaProbe II, Al-Ka) was used to detect the valence states of transition metal elements (Mn and Ni) and Sn in Li1.15Ni0.27Mn0.58-xSnxO2 samples. In addition, the lattice fringes and microstructure of Li1.15Ni0.27Mn0.58-xSnxO2 materials were observed by transmission electron microscopy (TEM, Tecnai G2 Spirit, 120 kV).
Electrochemical measurements
The prepared cathode material Li1.15Ni0.27Mn0.58-xSnxO2 was sufficiently dissolved in N-Methyl pyrrolidone (NMP) solvent with polyvinylidene fluoride (PVDF) binder and carbon black at a weight ratio of 8:1:1 to prepare a slurry, which was coated on aluminum foil, dried, and then cut into an electrode. Subsequently, the electrode was assembled into a CR2032 coin cell with a lithium anode and a Celgard 2320 separator in a glove box for electrochemical performance testing. A 1 M LiPF6 solution (solvent is a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) with volume ratio 1:1) was used as the electrolyte.
Results and discussion
Morphology and structure analysis
SEM images in Fig. 1 show that all the secondary particles of Sn-doped Li1.15Ni0.27Mn0.58-xSnxO2 samples exhibit loose aggregate morphology with many pores in their structure, and their sizes are similar with radii of approximately 1–2 μm. The primary particle of all samples is approximately 40 nm and is uniform in diameter. This fine-particle structure with loose pores facilitates the reaction of the electrode material with the electrolyte and the bulk transport of lithium ions.
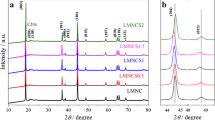
The XRD patterns of different contents of Sn-doped samples of Li1.15Ni0.27Mn0.58-xSnxO2 (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) are shown in Fig. 2. Compared with the Li1.15Ni0.27Mn0.58O2 sample, there is no obvious change in the diffraction peaks of Sn-doped samples, and no new diffraction peaks appeared in the diffraction pattern of the samples with x = 0.01 and x = 0.02; however, two new weak diffraction peaks emerged in the material when x ≥ 0.03. These new diffraction peaks belong to Li2SnO3, because excess Sn forms a Li2SnO3 heterophase in Li1.15Ni0.27Mn0.58-xSnxO2 cathode material. The diffraction peaks (400) and (511) of the cubic spinel phase appear in the spectrum of all Li1.15Ni0.27Mn0.58-xSnxO2 materials, indicating the presence of the spinel phase LiMn2O4 in the material. Additionally, the two pairs of (006)/(102) and (108)/(110) diffraction peaks are clearly split, which proves that Li1.15Ni0.27Mn0.58-xSnxO2 materials still maintain the LiMO2 (M = Mn, Ni) layered structure [17]. In addition, the weak diffraction peaks of monoclinic Li2MnO3 phase appeared in all Li1.15Ni0.27Mn0.58-xSnxO2 samples between 20 and 25° [18]. Furthermore, with increasing Sn content, the positions of (003) and (104) diffraction peaks shift to a small angle, which signals an increase in the lattice constant of Sn-doped samples [19].
TEM characterization results of Li1.15Ni0.27Mn0.56Sn0.02O2 (x = 0.02) are shown in Fig. 3. Figure 3a exhibits the HRTEM image of the i region with a crystal plane spacing of 0.492 nm. The fast Fourier transform (FFT) pattern of Li1.15Ni0.27Mn0.56Sn0.02O2 in Fig. 3d characterizes a C2/m monoclinic-layered Li2MnO3 structure [20]. Figure 3 a and d demonstrates that the i region describes the (001) plane in the monoclinic structure. The interplanar spacing of the (001) plane in Fig. 3a increases compared with the pristine Li1.15Ni0.27Mn0.58O2 material (Fig. S1, 0.470 nm). The interplanar spacing of the ii region in Fig. 3b is 0.476 nm, and the FFT pattern of the ii region (Fig. 3e coincides with the features of the Fd\( \overline{3} \)m space group [21], showing that the ii region belongs to the (111) crystal plane of the spinel LiMn2O4 structure with Fd\( \overline{3} \)m space group. The interplanar spacing of the iii region in Fig. 3c is 0.480 nm, which is slightly increased compared with the pristine Li1.15Ni0.27Mn0.58O2 material, and its FFT pattern conforms to the characteristics of the R\( \overline{3} \)m hexagonal layered LiMO2 structure [22], demonstrating that the iii region belongs to the (003) crystal plane in the layered structure. TEM characterization of Li1.15Ni0.27Mn0.56Sn0.02O2 material indicates that the (001) plane interplanar spacing of the monoclinic-layered structure and the (003) plane of the hexagonal layered structure in the material increases, which is consistent with the XRD characterization results, proving that Sn enters the transition metal layer, while Sn ions with larger radii than the transition metal element ions Ni2+ and Mn4+ increase the interplanar spacing.
XPS analysis is performed on Li1.15Ni0.27Mn0.58-xSnxO2 cathode materials with different Sn contents to investigate the state of Sn in the material. The XPS spectra of Li1.15Ni0.27Mn0.58-xSnxO2 samples with x = 0 and x = 0.05 are shown in Fig. 4. Figure 4a shows the full XPS spectrum for x = 0 and x = 0.05 samples. Figure 4 b, c, and d illustrate XPS spectra of Mn2p, Ni2p, and Sn3d. Compared with the pristine Li1.15Ni0.27Mn0.58O2 sample, Sn-doped samples show two new peaks at the binding energies of 494.5 eV and 486.5 eV (as shown in Fig. 4d), corresponding to Sn3d, demonstrating that Sn exists in the form of Sn4+ [23]. In addition, the positions of other peaks in the spectra of x = 0 and x = 0.05 are similar; a pair of split 2p3/2 and 2p1/2 peaks appear in the spectrum of Mn and Ni [24]. The 2p3/2 peak at 642.1 eV corresponds to Mn4+ [25], and the 2p3/2 peak at the binding energy of 854.5 eV corresponds to Ni2+. Furthermore, a satellite peak commonly found in Ni-containing oxides such as LiNiO2 also appears near the binding energy of Ni 2p3/2 [26,27,28]. Quantitative analysis of the XPS spectra of Ni2p and Mn2p shows that the ratio of Mn3+ and Mn4+ content in Li1.15Ni0.27Mn0.58O2 material is 1.079, and that in the Li1.15Ni0.27Mn0.53Sn0.05O2 sample with Sn-doping amount x = 0.05 is 1.103. Similarly, by quantitative analysis of the XPS spectra of Ni2p, the ratios of Ni2+ and Ni3+ content in the samples with x = 0 and x = 0.05 are 1.021 and 1.025, respectively. Quantitative analysis results show that Sn doping has little effect on the valence state of transition metal elements in the material.
Electrochemical performance
Comparing the electrochemical impedance spectroscopy (EIS) results of all samples with different Sn contents in Fig. 5 (In the EIS test, when preparing electrode pads of different samples, we kept the thickness/weight of the electrode pads of each sample be consistent to exclude other factors from interfering with the test results), it is found that the electrochemical impedances of all Sn-doped Li1.15Ni0.27Mn0.58-xSnxO2 samples are lower than that of the pristine Li1.15Ni0.27Mn0.58O2 sample, and the Li1.15Ni0.27Mn0.56Sn0.02O2 sample with x = 0.02 has the lowest impedance. It is worth noting that even in the optimal sample, the electrochemical impedance is higher than that of other reported materials; this can be attributed to the increase in Li/Ni cation mixing in the material. In a layered structure, the ratio of the intensity of the X-ray diffraction peaks corresponding to the (003) crystal plane and the (104) crystal plane can be used to characterize the degree of cation mixing in the structure [29]. As shown in Fig. 2, with the increase of the doping amount of Sn, the I(003)/I(104) ratio of Li1.15Ni0.27Mn0.58-xSnxO2 decreases continuously, indicating the degree of cation misalignment in Sn-doped samples increases, which leads to an increase in electrochemical impedance in the material.
The first charge–discharge curves of Li1.15Ni0.27Mn0.58-xSnxO2 materials in the range of 2–4.8 V at C/10 (1 C = 250 mAh/g) rate are shown in Fig. 6. The first charge curve of all samples consists of two parts: a slope between 3.8–4.5 V and a plateau with a voltage above 4.5 V. The slope on the curve represents the oxidation process of the transition metal element in the layered structure of the material, such as the oxidation of Ni and Mn to higher valence states. The plateau above 4.5 V corresponds to the process of Li+ deintercalation accompanied by O loss in Li2MnO3, which provides most of the charging capacity. After this reaction process, the Li2MnO3 phase is converted to electrochemically active MnO2, which could accommodate one Li in the subsequent discharge process and then regenerate the layered LiMnO2 phase. On the discharge curve of all the samples, two adjacent short voltage platforms appear between 3.5 and 2.5 V, corresponding to the reduction reaction of the transition metal ions in the layered structure and the spinel structure.
As indicated in Fig. 6b, all Sn-doped samples exhibit higher capacity and initial coulombic efficiency than the undoped Li1.15Ni0.27Mn0.58O2 sample. The Li1.15Ni0.27Mn0.56Sn0.02O2 material with x = 0.02 shows the best performance with a discharge specific capacity of 260.8 mAh/g and initial coulombic efficiency of 84.4%, which are much higher than those of the Li1.15Ni0.27Mn0.58O2 material (210.3 mAh/g and 76.8%). However, it is worth noting that as the amount of Sn doping increases, the capacity and initial coulombic efficiency of the Li1.15Ni0.27Mn0.58-xSnxO2 material increase first and then decrease, and the performance of the sample with x = 0.05 is only slightly higher than the pristine Li1.15Ni0.27Mn0.58O2 sample. The preferred Sn-doping level is x = 0.02, which increases the capacity of Li1.15Ni0.27Mn0.58-xSnxO2 while improving the initial coulombic efficiency. Compared with ternary cathode materials containing cobalt, this discharge capacity value is slightly lower. This is because there is a serious Li/Ni cation mixing in the material. When Ni2+ occupies the position of Li+ in the lithium layer, the transmission of Li+ is hindered and part of Li+ are unable to smoothly return to the crystal lattice, resulting in large capacity loss [30], while cobalt in the ternary material can play a role in stabilizing the structure and can reduce cation mixing in the material to provide a higher discharge capacity [31].
The data in Table 1 also proves that Sn doping can effectively reduce the irreversible capacity loss of Li1.15Ni0.27Mn0.58-xSnxO2 material during the first charge–discharge process. According to the structural analysis of all the samples, Sn can be used as a structural support to stabilize the layered structure. Therefore, more Li+ can be embedded back into the MnO2 phase in the first cycle, eliminating part of the irreversible capacity loss.
The capacity differential (dQ/dV) curves of Li1.15Ni0.27Mn0.58-xSnxO2 cathode materials with different Sn-doping amounts are shown in Fig. 7. As can be seen from Fig. 7a, during the first charging process, the first oxidation peak appears between 2.9–3.1 V in Li1.15Ni0.27Mn0.58O2, indicating that Mn in the spinel structure phase LiMn2O4 is oxidized; when the charging voltage reaches region between 4.1 and 4.4 V, the second oxidation peak emerges. In this process, Li+ is removed from the layered structure and oxidation of the transition metal occurs at the same time (Ni2+ to Ni4+); when charging increases to approximately 4.6 V, a strong oxidation peak appears, which corresponds to the voltage plateau on the charging curve during the first charge–discharge cycle. In this process, Li+ is deintercalated from Li2MnO3 accompanied by the loss of O; then, MnO2 generates during this reaction, and Li2MnO3 phase is activated. During the discharge process, a reduction peak arises around 3.2 V, the transition metal element ions Ni4+ and Mn4+ in the material are reduced in this process, while the reaction of Li+ intercalation into MnO2 occurs; the reduction peak at 2.5 V represents the reduction of Mn in the spinel phase LiMn2O4.
The dQ/dV curves of all Sn-doped samples are similar to the Li1.15Ni0.27Mn0.58O2 sample. It is worth noting that, compared with the undoped Li1.15Ni0.27Mn0.58O2 sample, the oxidation peak of transition metal ions on the dQ/dV curve of Sn-doped samples shifts to a lower voltage during the charging process, while the reduction peaks of transition metal element ions shift to higher voltages in the discharge process. This is because the doping by Sn reduces the electrochemical impedance of the Li1.15Ni0.27Mn0.58-xSnxO2 material [32] and enhances the electrochemical activity. However, the positions of Li and O deintercalation peaks do not change significantly, indicating that Sn doping does not affect the activity of the Li2MnO3 phase.
The discharge capacity of Li1.15Ni0.27Mn0.58-xSnxO2 material with different content of Sn in the first 100 cycles at 1 C rate is shown in Fig. 8. During the first ten cycles of Sn-doped Li1.15Ni0.27Mn0.58-xSnxO2 samples, the discharge capacity gradually increases, which is related to the activation process of the Li2MnO3 phase [8]. The Li1.15Ni0.27Mn0.56Sn0.02O2 sample shows the best cycling performance without significant attenuation during 100 cycles. The discharge capacity of Li1.15Ni0.27Mn0.56Sn0.02O2 after 100 cycles at 1 C is as high as 182.6 mAh/g accompanied with a capacity retention of 94.67%, while the discharge capacity of pristine Li1.15Ni0.27Mn0.58O2 is only 119.1 mAh/g after 100 cycles at 1 C accompanied with a capacity retention of 90.32%. Li1.15Ni0.27Mn0.55Sn0.03O2 maintains a high specific capacity of 170.5 mAh/g after 100 cycles at the 1 C rate, and the capacity retention rate is extremely high at 98.10% among all Li1.15Ni0.27Mn0.58-xSnxO2 samples. Li1.15Ni0.27Mn0.54Sn0.04O2 shows a discharge capacity comparable with that of Li1.15Ni0.27Mn0.58O2 sample after 100 cycles; however, its capacity retention rate is inferior to that of the undoped sample. Li1.15Ni0.27Mn0.53Sn0.05O2 delivers a discharge specific capacity of only 63.5 mAh/g after 100 cycles at 1 C, and its capacity retention rate is the lowest among all samples, only 61.18%. According to above analysis, an appropriate Sn content can improve structural stability of Li1.15Ni0.27Mn0.58-xSnxO2 materials, which increases their cycle performances, although excessive Sn doping can degrade the cycle performance of Li1.15Ni0.27Mn0.58-xSnxO2 material. These results are in good agreement with the conclusion of the previous material structure characterization.
Figure 9 shows the rate performance of all Li1.15Ni0.27Mn0.58-xSnxO2 samples. It is illustrated that the rate capability of Li1.15Ni0.27Mn0.58-xSnxO2 material has been significantly improved due to Sn doping, and the Li1.15Ni0.27Mn0.56Sn0.02O2 sample exhibits the best rate performance among all samples. Li1.15Ni0.27Mn0.56Sn0.02O2 sample shows a discharge capacity of approximately 65 mAh/g at 5 C, which is three times that of the undoped Li1.15Ni0.27Mn0.58O2. Additionally, when the current density returns to 0.1 C, the Li1.15Ni0.27Mn0.56Sn0.02O2 sample acquires a specific discharge capacity of 220 mAh/g, which is nearly twice that of Li1.15Ni0.27Mn0.58O2. By investigating the rate capability of various Sn-doped samples at different current densities, the proper content of Sn doping (x = 0.02) can effectively promote the rate capability of Li1.15Ni0.27Mn0.58-xSnxO2 materials, but excessive Sn doping is detrimental to the rate capability of the material. The specific discharge capacity of Li1.15Ni0.27Mn0.53Sn0.05O2 at 5 C is only half that of Li1.15Ni0.27Mn0.57Sn0.01O2, and the discharge capacity is only approximately 170 mAh/g when the current density returns to 0.1 C. It is concluded that Sn doping can significantly improve the rate capability of Li1.15Ni0.27Mn0.58-xSnxO2, which is related to the change in the material structure. The structural characterization of Li1.15Ni0.27Mn0.58-xSnxO2 materials has proven that Sn doping increases the interplanar spacing of the monoclinic and hexagonal layered structures in Li1.15Ni0.27Mn0.58-xSnxO2 materials, which are more conducive to the transport of lithium ions. In addition, Sn ions can be used as a support to stabilize the material structure to maintain the structural stability during charge–discharge processes.
Conclusions
Li1.15Ni0.27Mn0.58-xSnxO2 samples with different Sn content were prepared by the solvothermal method. Structural analysis shows that a small amount of Sn doping (x ≤ 0.02) does not introduce impurities into the material, but when the content of Sn exceeds 0.03, Li2SnO3 impurity phases begin to appear in the material. With increasing Sn content, the main diffraction peaks of (003) and (104) in the XRD pattern shift to a small angle, indicating that the crystal plane spacing of the monoclinic-layered structure and the hexagonal layered structure in Li1.15Ni0.27Mn0.58-xSnxO2 increases, which is also confirmed by TEM characterization results. The particles of Li1.15Ni0.27Mn0.58-xSnxO2 are microspheres composed of primary particles around 40 nm. There are many pores on the spherical particles, which are beneficial to the penetration of the electrolyte and diffusion of lithium ions.
All Sn-doped samples show higher discharge capacities and initial coulombic efficiencies than those of pristine Li1.15Ni0.27Mn0.58O2 cathode material. Li1.15Ni0.27Mn0.56Sn0.02O2 reached the highest discharge capacity of 260.8 mAh/g in the first cycle at 0.1 C, and its first coulombic efficiency was as high as 84.4%. During the 100 cycles at the 1 C rate, it is demonstrated that the proper Sn-doping content (x ≤ 0.02) can simultaneously improve the discharge capacity and cycle retention of Li1.15Ni0.27Mn0.58-xSnxO2 cathode materials; however, excess Sn doping (x >0.03) deteriorates cycle performance. Li1.15Ni0.27Mn0.56Sn0.02O2 maintains the highest discharge specific capacity of 182.6 mAh/g after 100 cycles at the 1 C rate, and the capacity retention rate is also high (94.67%); Li1.15Ni0.27Mn0.55Sn0.03O2 shows the highest capacity retention among all samples after 100 cycles (98.1%), while holding a high discharge specific capacity of 170.5 mAh/g. The improvement in the rate capability of Li1.15Ni0.27Mn0.58-xSnxO2 material due to Sn doping is particularly significant. The discharge specific capacity of Li1.15Ni0.27Mn0.56Sn0.02O2 at 5 C is three times that of the undoped Li1.15Ni0.27Mn0.58O2 material. In addition, when the current density returns to 0.1 C, the Li1.15Ni0.27Mn0.56Sn0.02O2 sample still shows a high discharge capacity of 220 mAh/g.
The conclusions in this study demonstrate that Sn doping can increase the interplanar spacing of the layered structure in Li1.15Ni0.27Mn0.58-xSnxO2 cathode material and reduce the electrochemical impedance; thus, the electrochemical performance of the cathode material is comprehensively improved.
References
Xu CS, Jiang WF, Yu HT, Guo CF, Xie Y, Ren N, Yi TF (2019) Hollow and hierarchical Li1.2Mn0.54Ni0.13Co0.13O2 micro-cubes as promising cathode materials for lithium ion battery. J Alloys Compd 807:151686
Li X, Qian K, He YB, Liu C, An D, Li Y, Kang F (2017) A dual-functional gel-polymer electrolyte for lithium ion batteries with superior rate and safety performances. J Mater Chem A 5:18888–18895
Yi TF, Zhu YR, Tao W, Luo S, Xie Y, Li XF (2018) Recent advances in the research of MLi2Ti6O14 (M= 2Na, Sr, Ba, Pb) anode materials for Li-ion batteries. J Power Sources 399:26–41
Yi TF, Wei TT, Li Y, He YB, Wang ZB (2020) Efforts on enhancing the Li-ion diffusion coefficient and electronic conductivity of titanate-based anode materials for advanced Li-ion batteries. Energy Storage Materials
Liu C, Qian K, Lei D, Li B, Kang F, He YB (2017) Deterioration mechanism of LiNi0.8Co0.15Al0.05O2/graphite-SiOx power batteries under high temperature and discharge cycling conditions. Journal of Materials Chemistry A:6
An J, Shi L, Chen G, Li M, Liu H, Yuan S, Zhang D (2017) Insights into the stable layered structure of a Li-rich cathode material for lithium-ion batteries. J Mater Chem A 5(37):19738–19744
Xie D, Li G, Li Q, Fu C, Fan J, Li L (2016) Improved cycling stability of cobalt-free Li-rich oxides with a stable interface by dual doping. Electrochim Acta 196:505–516
Ye D, Sun C, Chen Y, Ozawa K, Hulicova-Jurcakova D, Zou J, Wang L (2015) Ni-induced stepwise capacity increase in Ni-poor Li-rich cathode materials for high performance lithium ion batteries. Nano Res 8(3):808–820
Liu Y, Gao Y, Wang Q, Dou (2014) A influence of coated MnO2 content on the electrochemical performance of Li1.2Ni0.2Mn0.6O2 cathodes. Ionics 20.6: 825–831
Liu Y, Zhang Z, Gao Y, Yang G, Li C, Zheng J, Su M (2016) Mitigating the voltage decay and improving electrochemical properties of layered-spinel Li1.1Ni0.25Mn0.75O2.3 cathode material by Cr doping. J Alloys Compd 657:37–43
Pan L, Xia Y, Qiu B, Zhao H, Guo H, Jia K, Liu Z (2016) Structure and electrochemistry of B doped Li(Li0.2Ni0.13Co0.13Mn0.54)1-xBxO2 as cathode materials for lithium-ion batteries. J Power Sources 327:273–280
Jin X, Xu Q, Liu H, Yuan X, Xia Y (2014) Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery. Electrochim Acta 136:19–26
Li X, Xin H, Liu Y, Li D, Yuan X, Qin X (2015) Effect of niobium doping on the microstructure and electrochemical properties of lithium-rich layered Li[Li0.2Ni0.2Mn0.6]O2 as cathode materials for lithium ion batteries. RSC Adv 5(56):45351–45358
Li N, An R, Su Y, Wu F, Bao L, Chen L, Chen S (2013) The role of yttrium content in improving electrochemical performance of layered lithium-rich cathode materials for Li-ion batteries. J Mater Chem A 1(34):9760–9767
Zhao J, Wang Z, Guo H, Li X, He Z, Li T (2015) Synthesis and electrochemical characterization of Zn-doped Li-rich layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material. Ceram Int 41(9):11396–11401
Chen H, Hu Q, Huang Z, He Z, Wang Z, Guo H, Li X (2016) Synthesis and electrochemical study of Zr-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 as cathode material for Li-ion battery. Ceram Int 42(1):263–269
Fu F, Xu GL, Wang Q, Deng YP, Li X, Li JT, Sun SG (2013) Synthesis of single crystalline hexagonal nanobricks of LiNi1/3Co1/3Mn1/3O2 with high percentage of exposed {010} active facets as high rate performance cathode material for lithium-ion battery. J Mater Chem A 1(12):3860–3864
Wang C C, Jarvis K A, Ferreira P J, Manthiram (2013) A effect of synthesis conditions on the first charge and reversible capacities of lithium-rich layered oxide cathodes. Chem Mater 25.15: 3267–3275
Ho CS, Kang YC (2014) Uniform decoration of vanadium oxide nanocrystals on reduced graphene-oxide balls by an aerosol process for lithium-ion battery cathode material. Chem Eur J 20(21):6294–6299
Mohanty D, Sefat AS, Kalnaus S, Li J, Meisner RA, Payzant EA, Daniel C (2013) Investigating phase transformation in the Li1.2Co0.1Mn0.55Ni0.15O2 lithium-ion battery cathode during high-voltage hold (4.5 V) via magnetic, X-ray diffraction and electron microscopy studies. J Mater Chem A 1(20):6249–6261
Mohanty D, Sefat AS, Li J, Meisner RA, Rondinone AJ, Payzant EA, Daniel C (2013) Correlating cation ordering and voltage fade in a lithium-manganese-rich lithium-ion battery cathode oxide: a joint magnetic susceptibility and TEM study. Phys Chem Chem Phys 15(44):19496–19509
Jung SK, Gwon H, Hong J, Park KY, Seo DH, Kim H, Kang K (2014) Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Advanced Energy Materials 4.1:1300787
Li J, He X, Zhao R, Wan C, Jiang C, Xia D, Zhang S (2006) Stannum doping of layered LiNi3/8Co2/8Mn3/8O2 cathode materials with high rate capability for Li-ion batteries. J Power Sources 158(1):524–528
Zheng Z, Hua WB, Yu C, Zhong YJ, Xu BB, Wang JZ, Zhang ZY (2015) Heterogeneous intergrowth xLi1.5Ni0.25Mn0.75O2.5·(1-x)Li0.5Ni0.25Mn0.75O2 (0≤ x≤ 1) composites: synergistic effect on electrochemical performance. Dalton Trans 44(32):14255–14264
Nakayama M, Watanabe K, Ikuta H, Uchimoto Y, Wakihara M (2003) Grain size control of LiMn2O4 cathode material using microwave synthesis method. J Solid State Ionics 164(1–2):35–42
Shaju KM, Rao GVS, Chowdari BVR (2003) X-ray photoelectron spectroscopy and electrochemical behaviour of 4 V cathode, Li(Ni1/2Mn1/2)O2. Electrochim Acta 48(11):1505–1514
Carley AF, Jackson SD, O'shea JN, Roberts MW (1999) The formation and characterisation of Ni3+-an X-ray photoelectron spectroscopic investigation of potassium-doped Ni (110)-O. Surf Sci 440(3):L868–L874
Amine K, Tukamoto H, Yasuda H, Fujita Y (1996) A new three-volt spinel Li1+xMn1.5Ni0.5O4 for secondary lithium batteries. J Electrochem Soc 143(5):1607–1613
Wang CC, Jarvis KA, Ferreira PJ, Manthiram A (2013) Effect of synthesis conditions on the first charge and reversible capacities of lithium-rich layered oxide cathodes. Chem Mater 25(15):3267–3275
Xia H, Liu C, Shen L, Yu J, Li B, Kang F, He YB (2020) Structure and thermal stability of LiNi0.8Co0.15Al0.05O2 after long cycling at high temperature. J Power Sources 450:227695
He YS, Pei L, Liao XZ, Ma ZF (2008) Preparation and performance of LiNi0.8Co0.2O2 cathode material based on co-substituted α-Ni(OH)2 precursor. Chin Sci Bull 09:46–50
Chen H, Hu Q, Peng W, Guo H, Yan G, Wu X (2017) New insight into the modification of Li-rich cathode material by stannum treatment. Ceram Int 43(14):10919–10926
Acknowledgments
This work is supported by the Shenzhen Basic Research Free Exploration Project (No. JCYJ20180306171650007), the National Natural Science Foundation of China (21905069), and the Shenzhen Science and Technology Innovation Committee (JCYJ20180507183907224, KQTD20170809110344233)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1521 kb)
Rights and permissions
About this article
Cite this article
Li, H., Zhu, Z., Zhang, J. et al. Electrochemical performance of Sn-doped cobalt-free Li1.15Ni0.27Mn0.58-xSnxO2 cathode material for Li-ion batteries. Ionics 26, 3785–3794 (2020). https://doi.org/10.1007/s11581-020-03543-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03543-3