Abstract
The exploration of high-performance supercapacitors (SCs) has attracted wide attention worldwide. Herein, iron cobalt oxide (FeCo2O4) nanocomposites were prepared by using soft-template and hydrothermal method. The prepared FeCo2O4 electrode material presented good dispersivity and had different flower-like structures and flocculent structures, which exhibited high capacitance and cyclic stability. Specially, the FeCo2O4 electrode with the usage of 0.5 g polyvinylpyrrolidone (PVP), sodium dodecyl sulfate (SDS), cetyltrimethylammonium bromide (CTAB), and none template agents showed high capacitance of 1231.1, 956.2, 1027.8, and 677.3 F g−1 at 1 A g−1. Furthermore, we used an optimized FeCo2O4 electrode material as the positive electrode, activated carbon (AC) as the negative electrode, and 6 M KOH solution as the electrolyte to assemble an asymmetric supercapacitor (ASC). The resulting ASC device exhibits a favorable energy density of 51.2 Wh kg−1 and a high power density of 775.4 W kg−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the demand for sustainable and environmentally friendly energy storage equipment is increasing, supercapacitors with more efficient energy harvest and energy delivery have received widespread attention [1,2,3,4,5,6]. The cobaltite’s spinal oxides can be used in a variety of applications due to their low cost and simple preparation methods [7]. According to different energy storage mechanisms, supercapacitors can be divided into two types, namely, electrochemical double-layer capacitors and capacitor with fast faradaic oxidation-reduction reaction [8,9,10]. The difference between these two types of SCs is that there is a fast and reversible faradic redox reaction between the electrode and electrolyte in the charge-discharge processes of the pseudocapacitors [11,12,13,14,15]. The capacitive charge-storage aptitude offers advantages over the traditional battery system, which has long-term cycling stability, the ability to charge within seconds, and the capability to release energy ~ 10 times faster than the present battery systems [16,17,18]. These advantages can be applied to a variety of applications, including hybrid vehicles and equipment used in the biomedical, aerospace, and military fields. However, the low energy-storage capacity (5–30 Wh kg−1) of capacitive energy-storage devices (supercapacitors) greatly limits their auxiliary applications [19,20,21]. The energy density of SCs was increased through efforts to explore new high-performance electrode materials and their manufacturing methods [22, 23]. Transition metal oxides (TMOs), a typical pseudocapacitor material, attract worldwide attention because of the more complex valence states and the faster reversible redox action. RuO2 is a typical single-metal TMOs, which exhibits good electrochemical performances but has been restricted in the further applications for its expensive costs and high toxicity [24, 25]. Spinel-structured FeCo2O4 is one of the candidates for traditional TMOs, Fe replaces a Co in Co3O4, and Co can be replaced with any other transition metals Ni, Mn, Zn, or Cu to enhance conductivity, reduce toxicity, and improve the electrochemical performance of the electrode [26]. This transition metal oxide (MCo2O4) exhibits excellent electrochemical performance and structural stability. MCo2O4, which benefits from the synergy between M and cobalt ions, shows improved redox reactions and recognized structural variations [27]. There are many methods for preparing MCo2O4, such as sol-gel method [28], oxalate decomposition method [29], solid-phase reaction method [30], and coprecipitation method [31]. Compared with the above methods, the hydrothermal method does not require complicated equipment and can reduce the agglomeration of materials. Yuan group has reported the ultrathin mesoporous NiCo2O4 nanosheets, which deliver ultrahigh specific capacitance of 2010 and 1450 F g−1 at current densities of 2 and 20 A g−1, respectively [32]. Mohamed et al. [33] synthesized nanoflakes-like FeCo2O4 electrode by hydrothermal method on nickel foam substrate with specific capacitance of 135 F g−1. The nanoflakes-like FeCo2O4 electrode fabricated by Xu et al. [34] has specific capacitance of 969 F g−1.
Template method is an effective method for preparing different morphological structures and specific surface areas. In this paper, PVP, SDS, and CTAB were used as templates by hydrothermal method and then annealed to improve their electrochemical performance. PVP acts as a template, and metal ions are coordinated through -N and C=O functional groups, which can affect the morphology of the nanoparticles [35,36,37,38]. Using SDS as a template can promote the connection of nanostructures during the dissolution and recrystallization process, and long molecular chains can reduce the surface tension of the particles and provide more active space to enhance the capacitance performance [39]. CTAB can ionize CTAB+ ions, and these cations will adsorb on the particle surface to form a dense double-layer protective layer.
Herein, the FeCo2O4 was obtained by simple hydrothermal method and subsequent annealing treatment. The microstructure of FeCo2O4 was controlled by adding different template agents. The prepared electrode material has excellent electrochemical characteristics and remarkable cycle stability. In addition, we use the prepared electrode material as the positive electrode and activated carbon as the negative electrode to manufacture asymmetric supercapacitors for practical applications, which delivered an ultrahigh energy density of 51.2 Wh kg−1 at a power density of 775.4 W kg−1 and outstanding reliability.
Experimental details
Material preparation
In this experiment, all chemical reagents are of analytical purity and can be used without further purification. In order to remove the oxide layer on the Ni foam (10 × 10 × 1.5 mm), we used 6 M hydrochloric acid, absolute ethanol, and deionized water to ultrasonically treat Ni foam and dried at room temperature to obtain a clean foamed nickel.
In a typical synthesis, 1 mmol Fe(NO3)2·9H2O [98%, Tianjin Baodi Chemical Co. Ltd.], 2 mmol Co(No3)2·6H2O [99%, Tianjin Fengchuan Chemical Reagent Co. Ltd.], 6 mmol NH4F [96%, Tianjin Damao Chemical Reagent Factory], and 12 mmol CO(NH2)2 [99%, Tianjin Guangfu Co. Ltd.] were dissolved in 40 mL deionized water and magnetically stirred for about 30 min at room temperature. Subsequently, a certain amount of the template agent dissolved in 40 ml of deionized water was mixed with the above solution, and the magnetic stirring was continued for 30 min. The solution mixture was transferred into 100-ml Teflon liner autoclave, and the treated foamed nickel was immersed therein, and the mixture was held at 140 °C for 10 h. When the hydrothermal reaction was complete, the Ni foam substrate mantled with precursor and the corresponding powder products were washed several times with deionized water and absolute ethanol and then dried overnight at 60 °C under vacuum. Finally, the precursor was calcined in a muffle furnace at 250 °C for 2 h in air, and final samples were collected for further characterization. The synthetic schematic of the electrode material is shown in Fig. 1. The samples with none template agent and the samples adding with 0.5 g template agents of PVP [Tianjin Fuchen Chemical Reagent Factory], SDS [Tianjin Fuchen Chemical Reagent Factory], and CTAB [99%, Tianjin Fuchen Chemical Reagent Factory] were labeled as FCO, P-FCO, S-NCO, and C-FCO, respectively.
Material characterizations and electrochemical measurements
The prepared sample was characterized by powder X-ray diffraction (XRD, D8-Fouse, Germany) with a Cu-Kα (λ = 0.15406 nm) radiation at a scanning speed of 12°/min and scanning range of 10~80° to determine the crystallographic structure of the material. The surface morphology of the products was characterized by the field emission electron microscope (FE-SEM, Nova NanoSEM 450 FEI). The electrochemical properties of the prepared materials were measured in a three-electrode system. The FeCo2O4 grown on nickel foam was used as the working electrode, the Hg/HgO was used as the reference electrode, and platinum electrode was used as the counter electrode, respectively. Using 6 M KOH [85%, Tianjin Fengchuan Chemical Reagent Co. Ltd.] as electrolyte, all electrochemical properties were measured using an electrochemistry workstation (CHI660C, Shanghai). Cyclic voltammetry (CV) tests were performed between 0 and 0.6 V. Galvanostatic charge-discharge (GCD) was performed in the range of 0–0.45 V. The electrochemical impedance spectroscopy (EIS) measurement was performed in the frequency range of 100 kHz to 0.01 Hz.
The specific capacity is calculated from the discharge process of GCD curves according to the following formula [40]: C = I·Δt / (m·ΔV), in which I is the discharge current, Δt is the discharge time, m is the mass of the active material in the electrode, and ΔV is the voltage window for discharge.
Assembly of asymmetric supercapacitors
Manufacturing and electrochemical measurement of asymmetric supercapacitors: The electrode material supported on nickel foam was used as the positive electrode, and activated carbon was used as the negative electrode. The fabrication of the negative electrode is as follows: Activated carbon, acetylene black, and polyvinylidene fluoride (PVDF) with a weight ratio of 8:1:1 were dissolved in N-methyl-2-pyrrolidinone (NMP) solvent to form a slurry. The homogeneous slurries were coated on foamed nickel and dried at 60 °C for 12 h. The loading mass of materials on positive and negative electrode was controlled precisely based on the charge balance mechanism (m+ / m− = C−·V− / C+·V+). Energy density (E, Wh kg−1) and power density (P, W kg−1) of the ASC were calculated respectively by the formulas: E = 0.5·Cs·V2, P = E /Δt, where V represents the voltage except resistance drop (V) and Δt is the discharge time (s).
Results and discussion
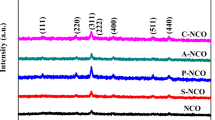
In this work, FeCo2O4 material was successfully grown on nickel foam by a simple hydrothermal process followed with annealing treatment. Figure 2 shows a typical XRD pattern of the prepared FeCo2O4. It can be seen from the XRD measurement results that most of the main diffraction peaks of the FeCo2O4 spinel oxide can be easily identified. The diffraction peaks at the 2θvalues of 19.0°, 31.3°, 36.7°, 59.5°, and 65.4°can be assigned to the (111), (220), (311), (511), and (440) planes of the FeCo2O4 spinel phase (JCPDS 42-1467), respectively. The peak at the 2θ values of 44.5° could be indexed to (111) crystalline planes of foamed nickel (JCPDS no. 87-0712). This is consistent with previous reports, and no other impurity peaks were observed, indicating that the crystallinity and purity of the sample are better after calcination [41]. The elemental composition of P-FCO by energy-dispersed X-ray spectroscopy (EDS) spectrum (Fig. 3) shows the characteristic peaks of elements. The results show that the prepared sample is composed of Fe, Co, O, and Ni. The results demonstrate the accuracy of the prepared sample.
Scanning electron microscope (SEM) was used to characterize the morphology and microstructure of the prepared samples. Figure 4 shows the morphology of FeCo2O4 synthesized on nickel foam with different templating agents. We can observe that the three-dimensional network structure of the foamed nickel is not damaged, and the sample particles can be well attached to the foamed nickel. Figure 4 a–c show the SEM images of FeCo2O4 material prepared without the template at different magnifications, which presents that it is a flower-like structure composed of conical nanowires. Figure 4 d–f and j–l show the morphology of FeCo2O4 materials prepared with PVP and CTAB as template, which reveals that the material is also a flower-like structure composed of conical nanowires uniformly dispersed on the foamed nickel with web-like interconnect structure. As their structure is more uniformly dispersed, which greatly improves the spatial structure of FeCo2O4, thereby promoting charge transport and ion diffusion. The flocculent microsphere-type FeCo2O4 structure was obtained by using SDS as a template (Fig. 4 g–i), and the image shows that it provides a larger surface area for redox reaction, which increases the material’s energy storage capacity. The resulted templating agent can effectively boost the surface morphology and microstructure of the material.
The electrochemical performance of FeCo2O4 electrodes was evaluated in a three-electrode system by cyclic voltammetry (CV) and galvanostatic charge-discharge (GCD). Current collector skeleton used foamed nickel substrate as electrode material. Figure 5a exhibits a typical CV curve without template and after adding template of FeCo2O4. The scan rate is 5 mV s−1, and the potential window is between 0 and 0.45 V. Obviously, the CV curve has a couple of obvious redox peaks, which are generated by the related redox reactions of Fe2+ and Fe3+ and Co2+ and Co3+ pairs in an alkaline electrolyte [42]. This result is due to the reversible Faraday of M-O/M-O-OH reaction (M represents Co or Fe) which indicates the conventional faradaic behavior of battery-type electrodes [43]. The reaction mechanisms of FeCo2O4 in alkaline electrolyte are represented as follows [38]:
a CV curves of FCO, P-FCO, S-FCO, and C-FCO samples at a scan rate of 5 mV s−1. b CV curves of P-FCO sample at different scan rates. c Charge-discharge curves of FCO, P-FCO, S-FCO, and C-FCO samples at the current density of 1 A g−1. d Charge-discharge curves of P-FCO sample at different current densities. e Capacity of FCO, P-FCO, S-FCO, and C-FCO samples at different current densities. f Cycling stability of FCO, P-FCO, S-FCO, and C-FCO samples at current density of 10 A g−1
As we all know, the closed area of the CV curve can reflect the electrochemical performance of the material [44]. Compared with FCO, the CV curve of P-FCO, S-FCO, and C-FCO has larger closed area and higher peak current. In addition, the current response for P-FCO was higher than others, indicating that it has the best charge storage capabilities. Figure 4b presents the CV curves of P-FCO at different scan rates (5, 10, 15, 20, 30, and 50 mV s−1). It can be seen from Fig. 5b that the CV curve is symmetrical when the scan rate is small, and the shape of the CV curve can be well retained even at high scan rates. The result indicated that the electrode material has good redox reversibility at the same time. In addition, as the scanning rate increases, the closed area of the CV curve also increases, and the oxidation and reduction peaks move to positive and negative potentials, respectively. This is usually considered to be related to the charge diffusion polarization in the electrode material, that is, the reversibility of the redox reaction is sacrificed [45, 46]. In order to further investigate the electrochemical properties of materials, the GCD curves of FCO, P-FCO, S-FCO, and C-FCO at a constant current density of 1 A g−1 are displayed in Fig. 5c. From the figure, we can see that the charge-discharge curve is not an ideal straight line, and the obvious charge-discharge plateaus could demonstrate the Faradaic behavior of the electrode [47]. The above results are consistent with the CV results. The specific capacitances of the four electrode materials are 677.3, 1231.1, 956.2, and 1027.8 F g−1, respectively. As expected, the material with the templating agent has a much higher specific capacitance than the material without the templating agent. Among them, P-FCO has the largest specific capacitance at 1 A g−1, which conforms to the results of CV curves. Meanwhile, the voltage plateau shown in the GCD curve indicates the presence of a typical pseudocapacitive behavior, which is also consistent with the CV results. Comparison of specific capacitance values of four oxide electrode materials is exhibited in Fig. 5e. Generally, due to the voltage drop (IR), the capacitance value decreases as the current density increases. The results show that when the current density increases from 1 to 10 A g−1, the capacitance retention rates of FCO, P-FCPO, S-FCO, and C-FCO are 54.1%, 71.5%, 60.7%, and 78.7%, respectively. Table 1 describes the capacitance and corresponding retentions of four samples under the current densities of 10 A g−1, exhibiting considerable capacitance and a high retention rate while the current density increased from 1 to 10 A g−1, which demonstrated high capacitance and rate capability. By adding a templating agent, the capacity retention rate of FCO was effectively improved, which indicates that the electrode materials with templating agent have better magnification performance, and the result is consistent with the symmetry of the CV curve. Figure 5d shows the GCD curves of P-FCO materials at different current densities. The charge-discharge curve is usually symmetrical, which indicates that the prepared material has good reversibility, and the slightly curved linear characteristic is the typical characteristic of chirped capacitor characteristics. The lower capacitance at higher current densities may be due to the penetration of a smaller number of electrolyte ions in the electrode surface [48]. The specific capacitance of P-FCO is 1231.1, 1137.3, 1058, 1001.1, 945.8, and 880 F g−1 when the current density is 1, 2, 3, 5, 7, and 10 A g−1, respectively. Even at high current density (10 A g−1), P-FCO can still achieve relatively high capacitance.
The cyclic stability of the electrode material is also a vital factor for the practical application of supercapacitors. Figure 5f shows the results of 2000 cycles of electrode material at a current density of 10 A g−1. It can be seen from the figure that the specific capacity has initially increased significantly, which may be the result of activation. The retention rates of FCO, P-FCO, S-FCO, and C-FCO are 61.4%, 79.6%, 68.9%, and 82.7%, respectively. By adding templating agent, the cycling stability of the material is stably improved, and the material exhibits excellent kinetic reversibility.
Further electrochemical impedance spectroscopy (EIS) measurements and Nyquist plots were used to evaluate electrochemical performance closely related to ion diffusion and charge transfer processes. Figure 6 a and b show the impedance and Nyquist plots of the material. For each EIS spectra, it consists of a semicircle along the solid axis in the high frequency region and a straight line in the low frequency region, which can be simulated by equivalent circuits. In the high frequency region, the intersection of the curve on the real axis represents equivalent series resistance (Rs) of electrochemical system, including the ionic resistance of the electrolyte, the intrinsic resistance of the electrode active material, and the contact resistance between the material and the substrate. The Rs of FCO, P-FCO, S-FCO, and C-FCO are 0.8381 Ω, 1.167 Ω, 0.7772 Ω, and 0.8505 Ω, respectively. Obviously, the semicircle of the high frequency part indicates the charge-discharge transfer resistance (Rct) of the active material during the redox reaction. The values are 0.4494 Ω, 0.4114 Ω, 1.1 Ω, and 0.04329 Ω. Cdl means an electric double-layer capacitor located at the interface between the electrode and the electrolyte. The straight part represents Warburg impedance. The P-FCO has a steeper straight line, indicating less resistance to ion diffusion, which is one of the characteristics of capacitive behavior. The above setting is connected in series with the pseudocapacitance (CF). The specific parameters are shown in Table 2. Above results indicate that P-FCO electrode materials have a lower charge transfer resistance and diffusion resistance.
Electrochemical properties of FeCo2O4-based hybrid supercapacitor
To further evaluate the practical potential of FCO, an asymmetric supercapacitor device (P-FCO // AC) has been assembled; although the cycle rate performance of P-FCO material is not the best, it has a high specific capacitance of 1231.1 F g−1 at a current density of 1 A g−1, and the remaining 880 F g−1 capacitors remain at a current density of 10 A g−1, so we choose P-FCO as the positive electrode, activated carbon as the negative electrode, and 6 M KOH solution as the electrolyte. As shown in Fig. 7a, the activated carbon electrode and the FCO electrode have stable voltage windows of − 1–0 V and 0–0.6 V, respectively. Therefore, the assembled supercapacitor can operate at a potential window of 0–1.6 V. Figure 7b displays the CV curves of the assembled device at different scan rates. It can be seen that the CV curve of the device has the capacitance characteristics of Faradaic capacitor and an electric double-layer capacitor. The CV curve is not a standard rectangle, but an electric double-layer capacitor and pseudocapacitor are generated in low potential region and high potential region, respectively. As the scan rate increases, the shape of the CV curve hardly changes, which confirms good rate capability and reversibility of device. The GCD test was performed at different current densities to further explore the electrochemical performance of the fabricated ASC devices, as shown in Fig. 7c. When the current density is 1, 2, 3, 5, 7, and 10 A g−1, according to the discharge cross section on the GCD curve, we calculate the specific capacitances of 153.4, 131.6, 121.5, 105.8, 98, and 87.7 F g−1, respectively. In addition, we can see that the GCD curve is nearly symmetrical, which indicates that the ASC device has a better Coulomb efficiency and electrochemical reversibility. And when the current density is from 1 to 10 A g−1, the capacity retention rate reaches 57.2%, as shown in Fig. 7d.
a CV curves of the P-FCO and AC samples at a scan rate of 5 mV s−1. b CV curves of the ASC device at different scan rates. c Galvanostatic charge-discharge curves of the ASC device at different current densities. d Specific capacity diagram of the ASC device at different current densities. e The energy and power density of the ASC device at various charge-discharge rates. f Cycling performance of the ASC device at current densities of 10 A g−1
Calculations of the energy density (E) and power density (P) were based on the GCD curve and the following formula: E = 0.5·Cs·V2, P = E /Δt. The energy density of ASC equipment is calculated to be 51.2, 43.9, 40.6, 35.3, 32.7, and 29.1 Wh kg−1, and the power density is 775.4, 1549.4, 2327.4, 3874.4, 5424.9, and 7760 W kg−1. A figure of the device describing the relationship between capability density and power density is shown in Fig. 7e. It is worth noting that energy density decreases with increasing power density, which is a common phenomenon in supercapacitor applications. Stable and long-lasting cycle life is also a key factor in the practical application of supercapacitor. Figure 7f shows 2000 cycles of the assembled ASC device at a current density of 10 A g−1. In the first two hundred cycles, the capacitance value increased due to activation and then decreased slowly. After the end of 2000 cycles, the capacitance retention rate was 76.8%. The result suggests that the sample has good cycle stability. We speculate that nanowire flower-like structures can provide fast, convenient, and long-term Faraday reactions. In addition, due to the stable 3D Internet-like structure of the foamed nickel substrate, the mechanical adhesion of the active material can be ensured.
Conclusion
In summary, we successfully prepared the FeCo2O4 electrode material by simple hydrothermal method and annealing treatment and adjusted its structure and morphology by adding template agents. The results show that the capacities of FCO, P-FCO, S-FCO, and C-FCO at the current density of 1 A g−1 were 677.3, 1231.1, 956.2, and 1027.8 F g−1, respectively. Especially, P-FCO material has excellent rate performance and superior cycling stability (79.6% capacitance retention after 2000 cycles). In addition, we used P-FCO material as the positive electrode and activated carbon as the negative electrode to assemble the ASC device. The resulting device had an energy density of 51.2 Wh kg−1 at a power density of 775.4 W kg−1. To conclude, this work demonstrates an exciting pathway for design of a novel composite electrode for next generation high-performance flexible supercapacitors.
References
Xiao J, Wan L, Yang S, Xiao F, Wang S (2014) Design hierarchical electrodes with highly conductive NiCo2S4 nanotube arrays grown on carbon fiber paper for high-performance pseudocapacitors. Nano Lett 14:831–838. https://doi.org/10.1021/nl404199v
Yang J, Yu C, Fan X, Liang S, Li S, Huang H, Ling Z, Hao C, Qiu J (2016) Electroactive edge site-enriched nickel–cobalt sulfide into graphene frameworks for high-performance asymmetric supercapacitors, energy environ. Sci 9:1299–1307. https://doi.org/10.1039/C5EE03633J
Wang Y, Song Y, Xia Y (2016) Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem Soc Rev 45:5925–5950. https://doi.org/10.1039/c5cs00580a
Zhang D, Miao M, Niu H, Wei Z (2014) Core-spun carbon nanotube yarn supercapacitors for wearable electronic textiles. ACS Nano 8:4571–4579. https://doi.org/10.1021/nn5001386
Yu X, Lu B, Xu Z (2014) Super long-life supercapacitors based on the construction of nanohoneycomb-like strongly coupled CoMoO4-3D graphene hybrid electrodes. Adv Mater 26:1044–1051. https://doi.org/10.1002/adma.201304148
Xu GB, Li W, Yang LW, Wei XL, Ding JW, Zhong JX et al (2015) Highly-crystalline ultrathin Li4Ti5O12 nanosheets decorated with silver nanocrystals as a high-performance anode material for lithium ion batteries. J Power Sources 276:247–254. https://doi.org/10.1016/j.jpowsour.2014.11.108
Yadav AA, Lokhande AC, Kim JH, Lokhande CD (2016) Supercapacitive activities of porous La2O3 symmetric flexible solid-state device by hydrothermal method. Int J Hydrog Energy 41:18311–18319. https://doi.org/10.1016/j.ijhydene.2016.08.028
Cao X, Shi Y, Shi W, Lu G, Huang X, Yan Q, Zhang Q, Zhang H (2011) Preparation of novel 3D graphene networks for supercapacitor applications. Small 7:3163–3168. https://doi.org/10.1002/smll.201100990
Cao X, Yin Z, Zhang H (2014) Three-dimensional graphene materials: preparation, structures and application in supercapacitors. Energy Environ Sci 7:1850–1865. https://doi.org/10.1039/c4ee00050a
Ma X, Zhang L, Xu G, Zhang C, Song H, He Y et al (2017) Facile synthesis of NiS hierarchical hollow cubes via Ni formate frameworks for high performance supercapacitors. Chem Eng J 320:22–28. https://doi.org/10.1016/j.cej.2017.03.033
Xiao Z, Xu B, Zhang S, Yang Z, Mei Y, Fan W et al (2018) Balancing crystallinity and specific surface area of metal-organic framework derived nickel hydroxide for high-performance supercapacitor. Electrochim Acta 284:202–210. https://doi.org/10.1016/j.electacta.2018.07.173
Yu G, Xie X, Pan L, Bao Z, Cui Y (2013) Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2:213–234. https://doi.org/10.1016/j.nanoen.2012.10.006
Hall PJ, Mirzaeian M, Fletcher SI, Sillars FB, Rennie AJR, Shitta-Bey GO et al (2010) Energy storage in electrochemical capacitors: designing functional materials to improve performance. Energy Environ Sci 3:1238. https://doi.org/10.1039/c0ee00004c
Eskusson J, Jänes A, Kikas A, Matisen L, Lust E (2011) Physical and electrochemical characteristics of supercapacitors based on carbide derived carbon electrodes in aqueous electrolytes. J Power Sources 196:4109–4116. https://doi.org/10.1016/j.jpowsour.2010.10.100
Wang X, Tian W, Zhai T, Zhi C, Bando Y, Golberg D (2012) Cobalt (II, III) oxide hollow structures: fabrication, properties and applications. J Mater Chem 22:23310. https://doi.org/10.1039/c2jm33940d
Liu S, Jun SC (2017) Hierarchical manganese cobalt sulfide core–shell nanostructures for high-performance asymmetric supercapacitors. J Power Sources 342:629–637. https://doi.org/10.1016/j.jpowsour.2016.12.057
Ghosh S, Jeong SM, Polaki SR (2018) A review on metal nitrides/oxynitrides as an emerging supercapacitor electrode beyond oxide. Korean J Chem Eng 35:1389–1408. https://doi.org/10.1007/s11814-018-0089-6
Lee W-J, Jeong S, Lee H, Kim B-J, An K-H, Park Y-K, Jung SC (2017) Facile synthesis of iron-ruthenium bimetallic oxide nanoparticles on carbon nanotube composites by liquid phase plasma method for supercapacitor. Korean J Chem Eng 34:2993–2998. https://doi.org/10.1007/s11814-017-0205-z
Hu Q, Gu Z, Zheng X, Zhang X (2016) Three-dimensional Co3O4@NiO hierarchical nanowire arrays for solid-state symmetric supercapacitor with enhanced electrochemical performances. Chem Eng J 304:223–231. https://doi.org/10.1016/j.cej.2016.06.097
Chodankar NR, Dubal DP, Kwon Y, Kim D-H (2017) Direct growth of FeCo2O4 nanowire arrays on flexible stainless steel mesh for high-performance asymmetric supercapacitor. NPG Asia Materials 9:e419-e. https://doi.org/10.1038/am.2017.145
Chodankar NR, Dubal DP, Ji S-H, Kim D-H (2018) Superfast electrodeposition of newly developed RuCo2O4 nanobelts over low-cost stainless steel mesh for high-performance aqueous supercapacitor. Adv Mater Interfaces 5:1800283. https://doi.org/10.1002/admi.201800283
Li T, Li GH, Li LH, Liu L, Xu Y, Ding HY, Zhang T (2016) Large-scale self-assembly of 3D flower-like hierarchical Ni/Co-LDHs microspheres for high-performance flexible asymmetric supercapacitors. ACS Appl Mater Interfaces 8:2562–2572. https://doi.org/10.1021/acsami.5b10158
Chang Z-H, Feng D-Y, Huang Z-H, Liu X-X (2018) Electrochemical deposition of highly loaded polypyrrole on individual carbon nanotubes in carbon nanotube film for supercapacitor. Chem Eng J 337:552–559. https://doi.org/10.1016/j.cej.2017.12.095
Zhu B, Tang S, Vongehr S, Xie H, Meng X (2016) Hierarchically MnO2-nanosheet covered submicrometer-FeCo2O4-tube forest as binder-free electrodes for high energy density all-solid-state supercapacitors. ACS Appl Mater Interfaces 8:4762–4770. https://doi.org/10.1021/acsami.5b11367
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41:797–828. https://doi.org/10.1039/c1cs15060j
Yadav AA, Hunge YM, Mathe VL, Kulkarni SB (2018) Photocatalytic degradation of salicylic acid using BaTiO3 photocatalyst under ultraviolet light illumination. J Mater Sci Mater Electron 29:15069–15073. https://doi.org/10.1007/s10854-018-9646-3
Liu B, Kong D, Huang ZX, Mo R, Wang Y, Han Z, Cheng C, Yang HY (2016) Three-dimensional hierarchical NiCo2O4 nanowire@Ni3S2 nanosheet core/shell arrays for flexible asymmetric supercapacitors. Nanoscale 8:10686–10694. https://doi.org/10.1039/c6nr02600a
Lobo LS, Kalainathan S, Kumar AR (2015) Investigation of electrical studies of spinel FeCo2O4 synthesized by sol-gel method. Superlattice Microst 88:116–126. https://doi.org/10.1016/j.spmi.2015.09.010
Darbar D, Anilkumar MR, Rajagopalan V, Bhattacharya I, Elim HI, Ramakrishnappa T, Ezema FI, Jose R, Reddy MV (2018) Studies on spinel cobaltites, MCo2O4 (M=Mn, Zn, Fe, Ni and Co) and their functional properties. Ceram Int 44:4630–4639. https://doi.org/10.1016/j.ceramint.2017.12.010
Zhu Y, Pu X, Song W, Wu Z, Zhou Z, He X et al (2014) High capacity NiCo2O4 nanorods as electrode materials for supercapacitor. J Alloys Compd 617:988–993. https://doi.org/10.1016/j.jallcom.2014.08.064
Wang C, Zhang X, Zhang D, Yao C, Ma Y (2012) Facile and low-cost fabrication of nanostructured NiCo2O4 spinel with high specific capacitance and excellent cycle stability. Electrochim Acta 63:220–227. https://doi.org/10.1016/j.electacta.2011.12.090
Yuan C, Li J, Hou L, Zhang X, Shen L, Lou XWD (2012) Ultrathin mesoporous NiCo2O4 nanosheets supported on Ni foam as advanced electrodes for supercapacitors. Adv Funct Mater 22:4592–4597. https://doi.org/10.1002/adfm.201200994
Mohamed SG, Chen CJ, Chen CK, Hu SF, Liu RS (2014) High-performance lithium-ion battery and symmetric supercapacitors based on FeCo2O4 nanoflakes electrodes. ACS Appl Mater Interfaces 6:22701–22708. https://doi.org/10.1021/am5068244
Xu G, Zhang Z, Qi X, Ren X, Liu S, Chen Q et al (2018) Hydrothermally synthesized FeCo2O4 nanostructures: structural manipulation for high-performance all solid-state supercapacitors. Ceram Int 44:120–127. https://doi.org/10.1016/j.ceramint.2017.09.146
Zhu Y, Wang J, Wu Z, Jing M, Hou H, Jia X et al (2015) An electrochemical exploration of hollow NiCo2O4 submicrospheres and its capacitive performances. J Power Sources 287:307–315. https://doi.org/10.1016/j.jpowsour.2015.04.053
Khalid S, Cao C, Wang L, Zhu Y (2016) Microwave assisted synthesis of porous NiCo2O4 microspheres: application as high performance asymmetric and symmetric supercapacitors with large areal capacitance. Sci Rep 6:22699. https://doi.org/10.1038/srep22699
An C, Wang Y, Huang Y, Xu Y, Xu C, Jiao L et al (2014) Novel three-dimensional NiCo2O4 hierarchitectures: solvothermal synthesis and electrochemical properties. CrystEngComm 16:385–392. https://doi.org/10.1039/c3ce41768a
Zhang Y, Ma M, Yang J, Su H, Huang W, Dong X (2014) Selective synthesis of hierarchical mesoporous spinel NiCo2O4 for high-performance supercapacitors. Nanoscale 6:4303–4308. https://doi.org/10.1039/c3nr06564b
Zhang H, Gu J, Jiang Y, Zhao J, Zhang X (2014) Wang C effects of sodium dodecyl sulfate on the electrochemical behavior of supercapacitor electrode MnO2. J Solid State Electrochem 18(1):235–247. https://doi.org/10.1007/s10008-013-2266-1
Dong XC, Xu H, Wang XW, Huang YX, Chan-Park MB, Zhang H, Wang LH, Huang W (2012) Chen P 3D graphene-cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 6(4):3206–3213. https://doi.org/10.1021/nn300097q
Zhang J, Cheng JP, Li M, Liu L, Liu F, Zhang XB (2015) Flower-like nickel–cobalt binary hydroxides with high specific capacitance: tuning the composition and asymmetric capacitor application. J Electroanal Chem 743:38–45. https://doi.org/10.1016/j.jelechem.2015.02.021
Ma F-X, Yu L, Xu C-Y, Lou XW (2016) Self-supported formation of hierarchical NiCo2O4 tetragonal microtubes with enhanced electrochemical properties. Energy Environ Sci 9:862–866. https://doi.org/10.1039/c5ee03772g
Zhang Z, Huang Z, Ren L, Shen Y, Qi X, Zhong J (2014) One-pot synthesis of hierarchically nanostructured Ni3S2 dendrites as active materials for supercapacitors. Electrochim Acta 149:316–323. https://doi.org/10.1016/j.electacta.2014.10.097
Liu J, Wang J, Ku Z, Wang H, Chen S, Zhang L, Lin J, Shen ZX (2016) Aqueous rechargeable alkaline CoxNi2-xS2/TiO2 battery. ACS Nano 10:1007–1016. https://doi.org/10.1021/acsnano.5b06275
Ji J, Zhang LL, Ji H, Li Y, Zhao X, Bai X, Fan X, Zhang F, Ruoff RS (2013) Nanoporous Ni(OH) thin film on 3D ultrathin-graphite foam for asymmetric supercapacitor. ACS Nano 7:6237–6243. https://doi.org/10.1021/nn4021955
Wang B, Williams GR, Chang Z, Jiang M, Liu J, Lei X et al (2014) Hierarchical NiAl layered double hydroxide/multiwalled carbon nanotube/nickel foam electrodes with excellent pseudocapacitive properties. ACS Appl Mater Interfaces 6:16304–16311. https://doi.org/10.1021/am504530e
Sahoo S, Nguyen TT, Shim J-J (2018) Mesoporous Fe–Ni–Co ternary oxide nanoflake arrays on Ni foam for high-performance supercapacitor applications. J Ind Eng Chem 63:181–190. https://doi.org/10.1016/j.jiec.2018.02.014
Shen L, Uchaker E, Zhang X, Cao G (2012) Hydrogenated Li4Ti5O12 nanowire arrays for high rate lithium ion batteries. Adv Mater 24:6502–6506. https://doi.org/10.1002/adma.201203151
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Du, C., Han, E., Gao, L. et al. Soft-template and simple hydrothermal method to synthesize Fe-Co oxide on nickel foam and apply it to supercapacitors. Ionics 26, 4009–4018 (2020). https://doi.org/10.1007/s11581-020-03535-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03535-3