Abstract
A novel high-temperature proton exchange membrane (PEM) (PTFE/(PMo12O40)X-(PO-OH)Y composite membranes) was prepared by impregnating mixed acid into the porous PTFE membrane. The porous PTFE membrane serves as the support structure of the membrane, and (PMo12O40)X-(PO-OH)Y sol is used as the proton conductor. The solid poly acid (PMo12O40)X-(PO-OH)Y exhibits a proton conductivity of 0.025 S cm−1 at 180 °C, and the PTFE/(PMo12O40)X-(PO-OH)Y membrane presents a good proton conductivity (0.02 S cm−1) when the relative humidity is below 0.5% and temperature is 180 °C. Besides, the PTFE/(PMo12O40)X-(PO-OH)Y membrane performs moderate property as tested in hydrogen fuel cells. SEM and EDX reveal that the structure of (PMo12O40)X-(PO-OH)Y gel is uniformly distributed in the pores of PTFE, which enhanced the proton conductivity of the composite membranes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the intensification of energy and environmental issues, people’s demand for efficient and pollution-free energy is more urgent. Fuel cells have been recognized as one of the most promising energy conversion devices in the past few decades. Proton exchange membrane fuel cells (PEMFCs) attract people’s attention due to its high power density, pollution-free, and portable characteristics, and they are considered efficient power supplies for portable equipment, electric vehicles, and integrated cogeneration systems [1,2,3,4,5,6]. Compared with PEMFCs under the traditional working temperature (T < 80 °C), high-temperature (T > 100 °C) PEMFC (HT-PEMFC) is more attractive because of some advantages, such as higher catalyst activity, high tolerance of impurity (such as CO), simple hydrothermal management system, and high heat recovery [7,8,9].
Nowadays, PEMFC technology relies on perfluorosulfone (PFSA) polymer membranes (such as Nafion) as proton-conducting materials [10]. And the Nafion membranes are the leading and the most successful electrolytes used in PEMFCs, but the transfer of PEMFC protons in Nafion membranes must rely on liquid water. However, the Nafion membranes start dehydrating when the temperature is high, resulting in a sharp decrease in proton conductivity, so the Nafion membranes do not apply to HT-PEMFCs. Therefore, the key to the development of HT-PEMFC is to find a proton exchange membrane with high proton conductivity and high thermal stability. And some progress has been made, such as the water retention of Nafion can be improved by doping zirconium phosphate (ZrP), SiO2, and others [11,12,13,14,15], but Nafion’s cost makes commercialization extremely expensive. Hence, many nanopore polymers have aroused people’s interest and begun to be widely explored in HT-PEMFCs. [16] Among them, the polybenzimidazole (PBI) membrane was most commonly used. But the PBI membrane itself cannot transfer protons and electrons. It obtains proton conductivity by doping phosphoric acid, but excess acid reduces the mechanical strength and lifetime of the PBI membrane. [17,18,19,20,21,22,23,24] To solve this problem, the researchers increased the mechanical strength of the membranes by filling the nano-ion into the pores of the PBI, including SiO2 [25] and sulfophenylated TiO2 [26], but the synthesis process was very cumbersome. Besides, other membranes such as polyacrylamide (PAM) backbone [27] and polyvinyl alcohol (PVA)-based components [28] were also used. These membranes exhibited relatively low proton conductivity. In recent years, studies have found that porous PTFE can improve the mechanical strength of the membrane, and the polymer composite membrane prepared based on PTFE has high proton conductivity and thermal stability. For example, Park et al. [29] directly mixed PTFE and ZrP and then pressed them into composite membranes; zirconium phosphate increased the proton conductivity from PTFE 10−13 to 2.2 × 10−3 S cm−1. Based on this, Al-Othman et al. [30] added glycerol (GLY) to the synthesis of ZrP/PTFE to obtain a membrane with a conductivity of approximately 0.02–0.045 S cm−1; and then, Al-Othman et al. [31] prepared the modified Si–ZrP/PTFE/GLY membrane and S–ZrP/PTFE/GLY, and the addition of silicic acid increased proton conductivity. Recently, Al-Othman et al. [32] studied the proton conductivity of phosphotungstic acid (PWA) to ZrP/PTFE/GLY membrane and they found proton conductivity decreases when PWA was added to the ZrP/PTFE/GLY membrane. This may be that the addition of PWA has no positive effect on the modification of ZrP so that the PWA-ZrP/PTFE/GLY membrane has a proton conductivity of 0.003 S cm−1. In these studies, the ZrP is used as a proton conductor to settle in the pores of PTFE, and the addition of silicic acid/sulfuric acid/phosphoric acid is to change the diameter of ZrP. However, we want to explore the effect of solid polymer acid on proton conductivity, and now, reports on solid polymer acid as proton conductors are rare.
Herein, in our current work, we have synthesized a new membrane type of high-temperature PEMFC, which uses the porous PTFE membrane as a support structure. In the pores of the PTFE membranes, (PMo12O40)X-(PO-OH)Y sol is used as a proton conductor to prepare a proton exchange membrane with high temperature, thereby combining the advantages of PEMFC and PAFC. The composite membrane has good mechanical strength and high proton conductivity, and the performance of the membrane is evaluated in a hydrogen fuel cell test.
Experiment
Composite membrane preparation
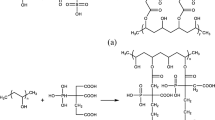
The PTFE/(PMo12O40)X-(PO-OH)Y membrane was prepared according to the procedure shown in Scheme 1. First, the porous polytetrafluoroethylene membrane was immersed in the mixed solution of H2SO4 (98 wt.% aqueous solution, purchased from Sigma-Aldrich) and H2O2 (30 wt.% aqueous solution, purchased from Sigma-Aldrich) with a mass ratio of 7:3 and soaked for 1 h at 80 °C for surface activation, and then rinsed with plenty of water. Then, it was immersed in a mixed solution having 1.0 mol/L NaOH (Sigma-Aldrich reagent grade), H2O2 (30 wt.% aqueous solution), and pure water with a mass ratio of 1:1:5, and soaked for 30 min at 70 °C to carry out further purification and rinsed with plenty of water. All chemicals are used directly without further treatment.
Next, the solid poly (mixed acid (PMo12O40)X-(PO-OH)Y) and (PMo12O40)X-(PO-OH)Y/PTFE membrane was prepared as follows. First, 1.0 g of H3PMo12O40 (Sigma-Aldrich reagent grade) inorganic powder was dispersed in CH3CH2OH (Sigma-Aldrich), and then 3 g of H3PO4 (85 wt.% solution, Sigma-Aldrich) was added, and after stirring for 4 h at 70 °C, a mixed solution was obtained. The mixed solution was heated at 250 °C for 7 h to obtain the solid polymer acid (PMo12O40)X-(PO-OH)Y. The treated PTFE membrane was immersed in the mixed acid solution obtained in the above step, and then dried at 250 °C for 7 h to get the (PMo12O40)X-(PO-OH)Y/PTFE membrane.
Characterization
The proton conductivity was measured by electrochemical impedance spectroscopy (EIS) four-probe method with the equipment (Parstat, 2273) and the frequency range from 1 to 20 kHz. Then, the proton conductivity (σ) is calculated by the formula σ = L/RA, where L and A are the thickness and effective area of the membrane, respectively, and R is the high frequency intercept of the impedance curve using the real axis on the complex impedance plane.
The membrane details were observed by SEM (JEOL JSM-5300lv) and analyzed by semi-quantitative EDX.
Membrane electrode assembly
At 150 °C and the pressure of 0.1 ton cm−2, the anode and cathode were hot pressed to the doping membranes for 10 min to prepare the membrane electrode assembly (MEA). The catalyst ink was prepared by the mixing the carbon-based catalyst (60% Pt on Vulcan XC-72, eTek Inc.) and PBI in DMAC (5%, w/w) and PVDF (1%, w/w) solutions for 12 h. And then, the ink was dropped to a carbon paper carrier covered with a carbon gas diffusion layer (eTek) to prepare electrodes.
Fuel cell test
The MEA (of the active cross section area of 1 cm2) was sandwiched between two high-density graphite blocks impregnated with phenolic resin, and then parallel gas flow channels were processed thereon. Using H2 and O2 for cell testing, the gas flow rate used was about 40 times higher than the stoichiometric requirement for the maximum current obtained from the cell test. Tube electric heaters were installed in the graphite block to achieve the required temperature.
Results and discussion
SEM observation and morphological study of composite membrane
Figure 1 exhibits the SEM of the surface and the cross section of the PTFE/(PMo12O40)X-(PO-OH)Y composite membrane. Figure 1a shows the result of electron microscopy after × 5000 magnification of the PTFE surface. It presents the surface of PTFE is coarse, loose, and porous. Figure 1b and c present that the plate-like material is formed and the PTFE skeleton was covered. Figure 1c is the result of further magnifying the surface of the PTFE/(PMo12O40)X-(PO-OH)Y by 5000, exhibiting that the pores are densely packed and significantly smaller compared with those of the PTFE in Fig. 1a. This is because during the experiment, small particles of poly acid (PMo12O40)X-(PO-OH)Y enter the pores of PTFE and form sol clusters in the pores. All sol clusters are joined together and cohered to PTFE polymer. The distribution of the total elements of the cross section of the (PMo12O40)X-(PO-OH)Y composite membrane in Fig. 2a also confirms the tight connection between the poly acid clusters and PTFE pores, and it delivers the dispersion density of P and Mo is much higher than that of F and C, and the elements of P, Mo, F, and C are homogeneously distributed, indicating a good dispersion of the (PMo12O40)X-(PO-OH)Y cluster in the pores of PTFE. The pores of PTFE are densely packed by sol, which can prevent the gas cross between the anode and the cathode of the fuel cell. On the other hand, part of the pores of PTFE/(PMo12O40)X-(PO-OH)Y provides space for the flow of reactants and product after the catalyst is added [31].
Proton conductivity of PTFE/(PMo12O40)X-(PO-OH)Y membrane
Figure 3a shows the influence of temperature on proton conductivity of (PMo12O40)X-(PO-OH)Y solid acid. As can be seen from Fig. 3a, (PMo12O40)X-(PO-OH)Y produces a high proton conductivity (0.025 S cm−1) with the temperature from 80 °C increased to 180 °C. The effect of RH on proton conductivity of (PMo12O40)X-(PO-OH)Y is shown in Fig. 3b. It exhibits that the proton conductivity increases greatly with the relative humidity, when the RH% is in the range of 0.5 to 8.5. However, when the RH% exceeds 8.5, the proton conductivity tends to be flat and between 0.04 and approximately 0.045 S cm−1. This is because the phosphomolybdic acid (PMo) itself is hydrophilic, and as the relative humidity increases, the water content increases. Besides, the phosphoric acid used in the experiment also contains water, and Ganapathy et al. [33] have reported that heteropoly acids have a high proton mobility in the hydrated state. All in all, the solid acid (PMo12O40)X-(PO-OH)Y prepared has great potential as proton conductors.
Figure 3c presents the proton conductivity of PTFE/(PMo12O40)X-(PO-OH)Y membrane. The proton conductivity is about 25–50% higher than that of (PMo12O40)X-(PO-OH)Y solid acids shown in Fig. 3a, when the temperature is 80~160 °C. And at 180 °C, the proton conductivity of PTFE/(PMo12O40)X-(PO-OH)Y membrane is 0.02 S cm−1, and it presents great potential in the application of high-temperature proton exchange fuel cell. The enhancement of proton conductivity of PTFE/(PMo12O40)X-(PO-OH)Y composite membrane at high temperature can be attributed to the following reasons: during the preparation of composite membrane, the addition of heteropoly acid increases the number of OH− groups and provides more jumping paths for protons; phosphomolybdic acid connects with phosphate clusters, which may create new proton transport pathways; phosphomolybdic acid is a hydrophilic inorganic material, and the connection of (PMo12O40)X-(PO-OH)Y to PTFE may improve water absorption and increase the amount of water molecules in the composite membranes and increase their conduction path; and then, as seen in SEM, plate-like solid acids (PMo12O40)X-(PO-OH)Y are filling the PTFE holes, leading to an increase in the total surface area and therefore the proton conductivity.
Fuel cell performance
Figure 4 indicates the performance of the fuel cell with the PTFE/(PMo12O40)X-(PO-OH)Y composite membrane of 70 μm thickness. The cell voltage shows a notable loss of polarization voltage; for example, when the battery voltage is 0.4 V, the current density is only 200 mA cm−2. The reason for the relatively poor performance of the fuel cell is that the structure of the catalyst layer is poor, and the catalyst layer is a simple PVDF-bonded carbon having no proton conductor. By optimizing the catalyst layer structure, including the proton-conducting composite, the performance of the current composite membrane fuel cells can be improved, and this is also the direction we need to work next.
Conclusion
A high-temperature proton-conductive membrane was made using porous PTFE membrane as a supporting structure and (PMo12O40)X-(PO-OH)Y as a proton conductor. The PTFE/(PMo12O40)X-(PO-OH)Y membrane has good proton conductivity of 0.02 S cm−1 when the RH is under 0.5% and the temperature is 180 °C. The membrane exhibited modest voltage performance when tested in hydrogen fuel cells, which was partly due to the preparation method of the catalyst layer, and this is what the next work needs to improve. However, the PTFE/(PMo12O40)X-(PO-OH)Y composite membrane still has great potential in the application of high-temperature PEMFCs.
References
Barbir F, Yazici S (2008) Status and development of PEM fuel cell technology. Int J Energy Res 32:369–378
Schlapbach L (2009) Technology: hydrogen-fuelled vehicles. Nature 460:809–811
Hou S et al (2015) Enhanced low-humidity performance in a proton exchange membrane fuel cell by the insertion of microcrystalline cellulose between the gas diffusion layer and the anode catalyst layer. Int J Hydrogen Energy 40:15613–15621
Debe MK (2012) Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486:43–51
Barbir F (2005) PEM fuel cells theory and practice. Elsevier Academic Press, New York
Nalbant Y, Colpan CO, Devrim Y (2019) Energy and exergy performance assessments of a high temperature-proton exchange membrane fuel cell based integrated cogeneration system. Int J Hydrogen Energy online
Zhang L, Xie Z, Zhang J, Wilkinson, Liu Z, Holdcroft S (2006) High temperature PEM fuel cells. J Power Sources 160:872–891
Li Q, He R, Jensen JO (2003) Approaches and recent development of polymer electrolyte membranes for fuel cells operating above 100 °C. Chem Mater 15:4896–4915
Ahmed DH, Sung HJ, Bae J (2008) Reactants flow behaviour and water management for different current densities in PEMFC. Int J Heat Mass Transf 51:2006–2019
Velayutham G, Subramaniam CK, Rajalakshmi N, Dhathathreyan KS (2006) Effect of solvents on the characteristics of Nafion®/PTFE composite membranes for fuel cell applications. J Power Sources 160:10–17
Yang C, Srinivasan S, Aricò A (2001) Composite Nafion/zirconium phosphate membranes for direct methanol fuel cell operation at high temperature. Electrochem Solid-State Lett 4:31–34
Si Y, Kunz HR, Fenton JM (2004) Nafion-Teflon-Zr(HPO4)2 composite membranes for high-temperature PEMFCs. J Electrochem Soc 151(4):A623–A631
Jung U, Park K, Park E (2006) Improvement of low-humidity performance of PEMFC by addition of hydrophilic SiO2 particles to catalyst layer. J Power Sources 159:529–532
Pereira F, Vallé K, Belleville P (2008) Advanced mesostructured hybrid silica−Nafion membranes for high-performance PEM fuel cell. Chem Mater 205:1710–1718
Adjemian KT, Srinvasan S, Benzigerb J (2002) Investigation of PEMFC operation above 100 °C employing perfluorosulfonic acid silicon oxide composite membranes. J Power Sources 109:343–356
Li Q, Jensen JO, Savinell RF, Bjerrum NJ (2009) High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog Polym Sci 34:449–477
Haque MA, Sulong AB, Loh KS (2017) Acid doped polybenzimidazoles based membrane electrode assembly for high temperature proton exchange membrane fuel cell: a review. Int J Hydrog Energy 42:9156–9179
Heo P, Kajiyama N, Kobayashi K (2008) Proton conductor in Sn0.95Al0.05P2O7-PBI-PTFE composite membrane. Electrochem Solid State Lett 11:91–95
Krishnan NN, Joseph D, Duong NH (2017) Phosphoric acid doped crosslinked polybenzimidazole (PBI-OO) blend membranes for high temperature polymer electrolyte fuel cells. J Membr Sci 544:416–424
Wang K, Yang L, Wei W (2018) Phosphoric acid-doped poly(ether sulfone benzotriazole) for high-temperature proton exchange membrane fuel cell applications. J Membr Sci 549:23–27
Ma YL, Wainright JS, Litt MH, Savinell RF (2004) Conductivity of PBI membranes for high-temperature polymer electrolyte fuel cells. J Electrochem Soc 151:8–16
Asensio JA, Borrós SR, Romero PG (2004) Proton-conducting membranes based on poly(2,5-benzimidazole) (ABPBI) and phosphoric acid prepared by direct acid casting. J Membr Sci 241:89–93
Thomas S, Araya SS, Frensch SH (2019) Hydrogen mass transport resistance changes in a high temperature polymer membrane fuel cell as a function of current density and acid doping. Electrochim Acta 317:521–527
Li M, Shao Z, Scott K (2008) A high conductivity Cs2.5H0.5PMo12O40/ polybenzimidazole (PBI)/H3PO4 composite membrane for proton-exchange membrane fuel cells operating at high temperature. J Power Sources 183:69–75
Devrim Y, Devrim H, Eroglu I (2016) Polybenzimidazole/SiO2 hybrid membranes for high temperature proton exchange membrane fuel cells. Int J Hydrog Energy 41:10044–10052
Krishnan NN, Lee S, Ghorpade RV (2018) Polybenzimidazole (PBI-OO) based composite membranes using sulfophenylated TiO2 as both filler and crosslinker, and their use in the HT-PEM fuel cell. J Membr Sci 560:11–20
Qin Q, Tang Q, Li Q (2014) Incorporation of H3PO4 into three-dimensional polyacrylamide-graft-starch hydrogel frameworks for robust high-temperature proton exchange membrane fuel cells. Int J Hydrog Energy 39:4447–4458
Guisasola CG, Greus AR (2018) Dielectric relaxations and conductivity of cross-linked PVA/SSA/GO composite membranes for fuel cells. Polym Test 67:55–67
Park Y, Jae-Dong K, Nagai M (2000) High proton conductivity in ZrP-PTFE composites. J Mater Sci Lett 19:1735
Al-Othmanab A, Tremblaya AY, Pell W (2012) The effect of glycerol on the conductivity of Nafion-free ZrP/PTFE composite membrane electrolytes for direct hydrocarbon fuel cells. J Power Sources 199:14–21
Al-Othmanab A, Tremblaya AY, Pell W (2013) A modified silicic acid (Si) and sulphuric acid (S)–ZrP/PTFE/glycerol composite membrane for high temperature direct hydrocarbon fuel cells. J Power Sources 224:158–167
Al-Othman A, Zhu Y, Tawalbeh M (2017) Proton conductivity and morphology of new composite membranes based on zirconium phosphates, phosphotungstic acid, and silicic acid for direct hydrocarbon fuel cells applications. J Porous Mater 24:721–729
Ganapathy S, Fournier M, Paul JF (2002) Location of protons in anhydrous Keggin heteropolyacids H3PMo12O40 and H3PW12O40 by 1H{31P}/31P{1H} REDOR NMR and DFT quantum chemical calculations. J Am Chem Soc 124:7821–7828
Acknowledgements
This work was finished in Keith Scott Group of Newcastle University, UK.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, X., Li, M. A composite membrane based on PTFE and solid poly (mixed acid (PMo12O40)X-(PO-OH)Y) for high-temperature fuel cells. Ionics 26, 1003–1009 (2020). https://doi.org/10.1007/s11581-019-03263-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03263-3