Abstract
Herein, we present the use of lithium tetrafluoroborate (LiBF4) as an electrolyte salt for wide-temperature electrolytes in lithium-ion batteries. The research focused on the application of blend salts to exhibit their synergistic effect especially in a wide temperature range. In the study, LiCoO2 was employed as the cathode material; LiBF4 and lithium difluoro(oxalate)borate (LiODFB) were added to an electrolyte consisting of ethylene carbonate (EC), propylene carbonate (PC), and ethyl methyl carbonate (EMC). The electrochemical performance of the resulting electrolyte was evaluated through various analytical techniques. Analysis of the electrical conductivity showed the relationship among solution conductivity, the electrolyte composition, and temperature. Cyclic voltammetry (CV), charge-discharge cycling, and AC impedance measurements were used to investigate the capacity and cycling stability of the LiCoO2 cathode in different electrolyte systems and at different temperatures. Scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS) were applied to analyze the surface properties of the LiCoO2 cathode after cycling. The results indicated that the addition of a small amount of LiODFB into the LiBF4-based electrolyte system (LiBF4/LiODFB of 8:2) may enhance the electrochemical performance of the LiCoO2 cell over a relatively wide temperature range and improve the cyclability of the LiCoO2 cell at 60 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries (LIBs) have advantages, such as a high discharging platform, large power and energy density, a long cycling life, no memory effect, and being environmentally friendly [1, 2]. LIBs are widely used in small appliances, such as portable mobile devices and digital terminals, large power storage stations, space technologies, and in the area of traffic and transportation [3]. However, their utilization in electric automobiles has been hindered by the relatively narrow application temperature range [2]. Thus, in order for LIBs to be successfully applied in other fields of technology, the application temperature range of the LIBs must be broadened. A major contributor to this limitation is the electrolyte, which is one of the three essential elements of LIBs. LIB electrolytes currently available on the market have application temperature range of − 20 and 60 °C and show a significantly lower capacity outside these temperatures [4, 5]. Ongoing attempts to broaden their working temperature range mostly focus on improvements of the solvent system [6, 7] and the use of lithium salts [7,8,9,10].
Currently, the most commonly used commercial electrolyte is a carbonate solvent system mixed with lithium hexafluorophosphate (LiPF6) salt. While the LiPF6-based electrolyte system has a relatively high conductivity and can form a stable solid electrolyte interface (SEI) [11], it is also moisture sensitive and thermally unstable.
When exposed to high temperature, LiPF6 decomposes into lithium fluoride (LiF) and phosphorus pentafluoride (PF5) and PF5 readily hydrolyzes to form HF reacting with trace amount of water. The two products, PF5 and HF, can then react with the cathode and anode of the LIBs, leading to reduced cell performances [12, 13]. Thus, in order to circumvent these disadvantages, it is crucial that a new and optimized electrolyte system is invented.
Even though the electrolyte system based on LiBF4 shows great potential, it is not widely applied because of its susceptibility to hydrolysis, relatively low conductivity, and hindered SEI formation [14]. However, compared to the currently used LiPF6 system, LiBF4 has several advantages. Zhang et al. reported that although LiBF4 has lower conductivity than LiPF6 at low temperature, it displays better performance because it has a lower electric charge transfer (Rct) than LiPF6. In addition, LiBF4 is less moisture sensitive and has comparable cycling properties to LiPF6 at room temperature [11, 15]. All of these properties make LiBF4 a more suitable system for low-temperature applications. In contrast, the application of LiBF4 in high-temperature environments represents a challenge. Even though LiBF4 has better thermal stability than LiPF6 [16], which allows it to be applied in high-temperature environments, it exhibits poor film-forming performance. The increase in temperature of the LiBF4-based electrolyte system causes a rapid decrease in the LIB capacity decreases, which leads to a large reduction in the coulombic efficiency of the battery [11]. Thus, in order for LiBF4 to be applied to wide-temperature electrolytes, there are a few important changes that need to be made. First, the film-forming performance of LiBF4 needs to be improved, and second, the conductivity of the electrolyte should be increased so that its performance at high and low temperatures can be enhanced.
Lithium difluoro(oxalate)borate (LiODFB) is a novel lithium salt, which has properties that are similar to both lithium bis(oxalato)borate (LiBOB) and LiBF4. It exhibits very good film-forming performance, which may lead to the inhibition of PC co-intercalation in graphite and the formation of a stable SEI at high temperature [17, 18]. Additionally, LiODFB electrolyte maintains high conductivity within a relatively wide temperature range. The novel lithium salt is compatible with nearly all common cathode materials, including LiMn2O4, LiFePO4, and LiNil/3Mnl/3Co1/3O2, and assembled cells can realize stable cycling without significant fade at 60 °C [8, 18,19,20,21].
Currently, LiODFB is used as a lithium salt additive that improves the performance of cells. A small addition of LiODFB into LiPF6 not only ensures the formation of an effective SEI but also greatly increases the capacity retention and life of the cell. Moreover, it leads to the significant decrease of the capacity fade of LIBs at high temperatures [8]. Monikowska et al. [15] pointed out that LiODFB/LiBF4-based electrolytes display high conductivity in a solution of EC/PC/DMC (1:1:3, v/v/v) at room temperature, which enhances the cycling performance of the cells. Based on these results, we propose the use of blend salts to employ their synergistic effects on the LIBs in an effort to broaden the application temperature range of the electrolyte. In this work, we attempted to extend the lower limit of the application temperature of the electrolyte. PC was the solvent of choice for this study, due to its low melting point (− 48.8 °C). Additionally, fluoroethylene carbonate (FEC) [22, 23], a film-forming additive, was applied in combination with LiODFB in order to explore its synergistic effect in forming a stable interfacial protection film at the surface of the electrode.
Experimental
Preparation of the electrolyte
The battery grade LiBF4, LiODFB, LiPF6, PC, EC, EMC, and FEC were purchased from Guangzhou Tinci Materials Technology Co., Ltd., China. All electrolytes were prepared in an argon-filled glove box (LG1200/750TS, Vigor Gas Purification Technology (Suzhou Co., Ltd., China)) with water and oxygen content lower than 1 ppm. First, PC and EMC with a volume ratio of 1:3 were mixed. Since EC is a solid at room temperature, it was heated to dissolve. Part of the liquefied EC was added to PC/EMC solution, and the resulting solution had a volume ratio of 1:1:3 (PC/EC/EMC). The lithium salts, LiBF4 and LiODFB, were then added to the formed mixture. Add FEC, which accounts for 5% (weight ratio) of the above electrolyte to improve the cycling stability of the cell. The electrolyte consisted of 1 M (1-x) LiBF4-xLiODFB (x is the molar ratio of LiODFB in the LiBF4-LiODFB mixture). An electric conductivity meter (Smart tester CPC-505, Germany) was used to measure the electric conductivity of the prepared electrolyte system in the temperature range of − 40 to 60 °C. The high- and low-temperature environments were controlled using a high-low temperature test box made by Shanghai Linpin Instrument Stock Co., Ltd., China.
Cell assembly
The cathode material LiCoO2, Super P, and the adhesive (polyvinylidene fluoride, PVDF) were mixed in a weight ratio of 8:1:1. A solution of N-methylpyrrolidone (NMP) was added, and the mixture was ball milled. The slurry was spread out on aluminum foil and dried at 120 °C under vacuum for 12 h. After being pressed at 10 MPa, the aluminum foil coated with the slurry was cut into cathode plates with a 12 mm diameter. The LiCoO2 cathodes were assembled in CR2025-type coin cells with lithium metal as a counter electrode separated by a Celgard 2400 separator. The prepared electrolytes were injected in cells accordingly.
Electrochemical performance
CV and electrochemical impedance spectroscopy (EIS) measurements were performed in order to evaluate the electrochemical stability of the electrode. CV and EIS were carried out in cells with three electrodes: LiCoO2 electrode served as the working electrode and a lithium metal disk was applied as a counter and reference electrode. CV test was performed with a scan rate of 0.1 mV s−1 and scanning voltage ranging from 2.5 to 5.0 V, and EIS was performed at a frequency range from 0.1 to 105 Hz and amplitude of 5 mV using an electrochemical workstation (Princeton, PARSTAT MC). The LAND CT2001A battery test system (Wuhan, China) was applied to test the charge-discharge performance of the LiCoO2/Li half cells at 25, − 20, and 60 °C, between 3 and 4.2 V (vs. Li/Li+). The button cells were cycled twice at the rate of 0.1 C, and then, cycling tests were carried out at the rate of 1 C.
Characterization of the electrode/electrolyte Interface
The morphology and composition of the film on the cathode surface were investigated by cycling it in different electrolytes. The cycled cells were disassembled in the glove box, and the residual electrolyte on the cathode surface was washed off repeatedly with dimethyl carbonate (DMC). The cathodes were dried at 50 °C under vacuum for 2 h, and their morphology was examined with a field emission scanning electron microscopy (FESEM, Hitachi SU8010, Japan). X-ray photoelectron spectroscopy (XPS, Axis Ultar DLD, Kratos Analytical Ltd.) was used to analyze the composition of the film on the cathode surface.
Results and discussions
Ionic conductivity
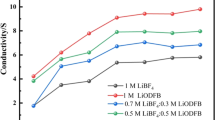
Conductivity is an important parameter to measure the performance of an electrolyte, and it determines the internal resistance and rate of an electrode. Figure 1 shows the ionic conductivity of the electrolytes at different concentrations of blended salts in a temperature range of − 40 to 60 °C.
The results showed temperature- and concentration-dependent conductivity of different electrolyte systems. It is evident that the conductivity of the electrolyte increased significantly proportional to the rising temperature. This was due to the decreased viscosity of the electrolyte and increased transfer rate of Li+ [24]. Meanwhile, the conductivity of the electrolyte increased proportionally to the rising LiODFB concentration causing the pure LiODFB-based electrolyte to have the highest conductivity. This phenomenon may be ascribed to the fact that LiODFB has a greater anionic radius and less ionic association than LiBF4 in the PC/EC/EMC (1:1:3, v/v) + FEC (5%) solution system. However, this trend is not apparent at low temperatures. This is because the viscosity of the electrolyte is directly proportional to the size of the anion. The larger radius of ODFB− in comparison to BF4− causes LiODFB to have a higher viscosity than BF4−, which causes the reduced conductivity of the electrolyte [25]. In other words, LiBF4-containing electrolyte systems exhibit a preferable conductivity property at low temperature. This result is consistent with that reported by Zhang [26]. In conclusion, LiODFB can enhance both the conductivity and the viscosity of LiBF4-based electrolytes. We should optimize ratio of the two lithium salts, so that the cells have the best electrochemical performance.
The CV of LiCoO2/Li half cell
Figure 2a–c presents CV curves of LiCoO2/Li half cells, assembled using the electrolytes prepared in this paper, at 25, − 20, and 60 °C, respectively. All CV curves displayed an apparent pair of redox peaks. At 25 °C (Fig. 2a), the pure LiBF4-based electrolyte had the smallest. Considering the results at this temperature, the rise in concentration of LiODFB led to an increase of the potential difference. Moreover, the results indicate that as the concentration of LiODFB increases, the reversible performance of the cell declines. The reason behind this phenomenon can be related to the decomposition of LiODFB into the film on the electrode surface. Due to the fact that LiODFB has good film-forming properties, it can form a dense and effective interfacial protection film on the surface of the electrode with a small amount of decomposition. This film could enhance the cycling stability of the cell, but it could also induce an increase in the interfacial resistance of the cell [27].
When the temperature was reduced to − 20 °C, as illustrated in Fig. 2b, the oxidation and reduction peaks of the cells exhibited a significant shift in potential. More specifically, the oxidation peak shifted to a higher voltage and the reduction peak to a lower voltage. Also, the difference in the voltage levels increased significantly. The fact that this change was more pronounced in the pure LiODFB electrolyte indicates that the reversible performance of the cell may be affected to a greater extent by the decomposition products of LiODFB at lower temperature. Furthermore, the lower temperature caused a decrease in the peak intensities and increase in peak widths. This indicated that the reversible intercalation and deintercalation of the Li+ into or out of the LiCoO2 cathode are hindered due to weakening dynamic factors [28].
At 60 °C (Fig. 2c), the potential difference in the oxidation and reduction peaks in each electrolyte system was relatively small. This indicated that the LiCoO2/Li cells exhibit good dynamic properties at high temperature. The pure LiBF4 electrolyte system at this temperature showed relatively small peak intensity and rather wide peak width. The addition of a small amount of LiODFB to the system led to a further decrease in the potential difference in the oxidation and reduction peaks. This can be contributed to the fact that at the higher temperature, LiODFB enhances the conductivity of the electrolyte, which improves the cycling performance of the cell.
The results from the CV measurements in different temperatures indicated that LiBF4/LiODFB lithium salt mixture combines the good low-temperature performance of LiBF4 and good high-temperature performance of LiODFB. This demonstrates the good synergistic effect created from the combination of different lithium salts.
The cycling performance of the LiCoO2/Li cell
The loss in capacity of LIBs in the cycling process is due to the loss of Li+, caused by the formation and growth of the SEI film. Additionally, capacity loss was caused by the gradual increase in interfacial resistance between the electrode and electrolyte during the charge-discharge process of the cells [29]. Figure 3 shows the cycling performances of the LiCoO2/Li half cells in different electrolytes at different rates (1, 0.1, and 1 C) and temperatures (25, − 20, and 60 °C).
The LiCoO2/Li cells in pure LiBF4-based electrolyte exhibited a significant fade and relatively low capacity after cycling at 25 and 60 °C (Fig. 3a, c). However, as the concentration of LiODFB increased, the capacity and cycling performance of the cell significantly improved. Following this trend, the best performance was measured in the pure LiODFB-based electrolyte. The capacity retention efficiencies of the cells with pure LiBF4-based and LiBF4/LiODFB (8:2)-based electrolyte were 77.86 and 87.5%, respectively, after 500 cycles at 25 °C and 89.13 and 93.50%, respectively, after 100 cycles at 60 °C. The increase in efficiency could be contributed to the fact that LiODFB significantly increased the conductivity of the electrolyte and enhanced the stability of the interface film. This, in turn, improved the discharge capacity and cycling stability of the electrolyte.
The cycling performances of LiCoO2/Li half cells in different electrolytes at − 20 °C are displayed in Fig. 3b. The results indicate that the pure LiBF4 and mixed-lithium salts system exhibit better cycling performance than the LiODFB-based electrolyte. After 300 cycles, the LiBF4-based and LiBF4/LiODFB (8:2)-based electrolytes showed an excellent cycling stability and capacity efficiencies of 94.64 and 98.67%, respectively. Conversely, the capacity of the LiODFB-based electrolyte decreased severely and the efficiency was merely 89.47%. This change was due to the thick electrode interfacial film that was formed by LiODFB, which caused a severe electrode polarization and destroyed the cycling stability of the cell. This effect is consistent with the phenomenon observed in the CV measurements at the low temperature. The results from the cycling performance showed that the LiBF4/LiODFB(8:2)-based electrolyte displays a good cycling performance at wide temperature range. This implies that a small amount of LiODFB increases the conductivity of the electrolyte, which makes it easy to form a dense and stable interface film.
The EIS of the LiCoO2/Li cell
The internal resistance of a cell is a key parameter of performance of LIBs. The internal resistance affects the power and discharge rate of a cell. Moreover, it evaluates the internal structure of a cell and the characteristics of the interface between an electrode and electrolyte. By testing the fully charged LiCoO2/Li half cells assembled with different electrolytes, via the AC impedance method, the influence of the working temperature on the electrochemical process of the cell can be further analyzed, and the results are shown in Fig. 4.
The semi-cycles in the high-frequency zone represent the resistance of the surface films on the cathode and anode (Rf), which reflects the migration of Li+ on the film surface. The middle-frequency zone was assigned to Rct, which reflects the migration of Li+ on the surface of the active material. The inclined line in the low-frequency represents the Warburg resistance (Zw), which represents the diffusion of Li+ inside the active material, and its slope is the diffusion rate of Li+ in the electrode material [30]. The cell resistance of pure LiBF4-based electrolyte was the lowest (Fig. 4). However, as the concentration of LiODFB increased, the resistance of the cells became higher. Following this trend, the cell with pure LiODFB-based electrolyte exhibited the highest resistance. This effect was due to the fact that LiODFB was preferentially oxidized on the electrode surface and formed a thick interfacial film, which increased the cell resistance.
At 25 °C (Fig. 4a), the rise in LiODFB concentration did not cause a significant increase in the resistance. This is mainly due to fact that the cell resistance does not have a large impact on the discharge capacity and cycling stability at room temperature. These results were consistent with the ones obtained in the cycling performance tests. Therefore, it is evident that pure LiODFB-based electrolyte has good performances. In other words, a high conductivity electrolyte shows relatively good discharge capacity and cycling stability at room temperature. At lower temperature (− 20 °C), in contrast, the resistance increased significantly as the amount of LiODFB increased (Fig. 4b). In this case, the electrochemical performance of the cell was mainly determined by the resistance. This result was consistent with the results from the CV and cycling performance measurements. Consequently, the increase in the concentration of LiODFB improved the conductivity of the electrolyte. However, it also increased the electrochemical resistance, which affected the discharge capacity of the cell at low temperatures. At higher temperature (60 °C), the resistance of the cell decreased significantly, which indicated that the resistance of Li+ migration is relatively low (Fig. 4c). In this case, the electrochemical performance may be affected by both the conductivity of the electrolyte and the interfacial resistance.
Morphology and composition analysis of the LiCoO2 cathode surface
The complete passivation of the electrode surface and the formation an interfacial film with good Li+ transfer properties are key factors that influence wide temperature performance of LIBs [10]. The oxidation effect of solvent molecules on the surface of the cathode at room temperature is often very weak. Thus, the thickness of the film on the cathode surface is much thinner than the SEI film on the anode surface. However, the oxidation reaction rate may drastically increase during the process of storage or in the presence of high temperature, which would lead to the more oxidation products. In this case, the thickness of the cathode interfacial film would be similar to that of the SEI film. This, in turn, has a crucial effect on the cycling stability of the cathode [10]. Therefore, the impact of different electrolyte systems on the LiCoO2 cathode at high temperature was investigated. SEM was used to obtain scanning measurements of the surface and cross-sectional of the cathode after 100 cycles at 60 °C (Fig. 5). SEM images of the surface and cross-section of the uncycled cathode showed smooth and clean surfaces and a well-grown crystal structure (Fig. 5a, b). Additionally, measurements of the morphology of the surface and cross-section of the cathode after 100 cycles using the pure LiBF4-based electrolyte were obtained (Fig. 5c, d). The SEM images showed that a layer of loose and uneven polymer covers the cathode surface, which led to a relatively poor thermal stability of the interfacial film. Additionally, a large amount of cracks were observed on the surface of the particles, which indicates that the cathode was eroded. This causes the electrolyte to decompose continuously and accounts for the poor high-temperature performance of cells using the LiBF4-based electrolyte. Next, the morphology of the cathode in a cell using pure LiODFB-based electrolyte was investigated (Fig. 5g, h). The surface of the cathode was relatively smooth and did not show any signs of erosion. This was due to the fact that LiODFB decomposed at a relatively high temperature and formed a dense and stable passivation film on surface of the cathode. This film prevented the electrolyte from reacting further with the cathode material. This phenomenon explains the good capacity retention of the cell using pure LiODFB-based electrolyte at high temperature. Finally, the morphology of the cathode cycled in a LiBF4/LiODFB(8:2)-based electrolyte was measured (Fig. 5e, f). The SEM images revealed no cracks on the surface of the electrode and showed a relatively dense film on the cathode surface. A comparison of these results with the ones obtained from the pure LiBF4-based electrolyte revealed that the addition of LiODFB significantly improve the thermal stability of the interface. This effect is mainly caused by the stable and dense interface film formed by LiODFB on the surface of the electrode. Thus, it can be concluded that the electrolyte system with the mixed lithium salts of LiBF4/LiODFB (8:2) has relatively good wide temperature performance.
SEM images of LiCoO2 electrode from LiCoO2/Li cells a, b uncycled at 60 °C; c, d LiBF4-based electrolyte system, after 100 cycles at 60 °C; e, f LiBF4/LiODFB blend salt with a molar ratio of 8:2 electrolyte system, after 100 cycles at 60 °C; g, h LiODFB-based electrolyte system, after 100 cycles at 60 °C
In order to examine the effect of the electrolytes on the interfacial film of the cathode further, the composition of the film on the cathode surface after 100 cycles at 60 °C was measured by XPS (Fig. 6). Survey scans of four samples were carried out (Fig. 6a): LiCoO2 cathode uncycled (sample 1); LiCoO2 cathode after 100 cycles in the LiBF4-based electrolyte (sample 2); LiCoO2 cathode after 100 cycles in the LiBF4/LiODFB (8:2)-based electrolyte(sample 3); and LiCoO2 cathode after 100 cycles in the LiODFB-based electrolyte (sample 4). The peaks that were observed from these scans were identified as follows: carbon auger electron peak (~ 1220 eV), oxygen auger electron peak (~ 980 eV), fluoride electron peak (878, 860, and 833 eV), and photoelectron peaks: Co2p (~ 780 eV), F1s (~ 689 and 687 eV), O1s (~ 532 eV), C1s (~ 285 eV), B1s (~ 194 eV), and Li1s (~ 57 eV).
XPS patterns of the LiCoO2 cathode uncycled (sample 1), after 100 cycles in the 1 mol L−1 LiBF4 electrolyte system (sample 2), after 100 cycles in the 1 mol L−1 LiBF4-LiODFB (8:2) electrolyte system (sample 3) and after 100 cycles in the 1 mol L−1 LiODFB electrolyte system (sample 4), respectively. a Survey, b F 1s, c C 1s, d O 1s, e B 1s, and f Li 1s
Comparison of the scans in the areas of the fluorine, carbon, oxygen, boron and lithium photoelectron peaks revealed significant differences. In the F1s spectrum (Fig. 6b), all four LiCoO2 samples showed a peak at ~ 688.5 eV (–CF2), which is believed to result from the binder PVDF. In contrast, the uncycled LiCoO2 cathode (sample 1) showed a single peak of –CF2 at ~ 688.5 eV, another peak at ~ 686.5 eV present in the photoelectron spectra of samples 2, 3, and 4. That peak can be attributed to the formation of LiF. This hypothesis was supported by the occurrence of LiF (56.8 eV) in the Li1s spectrum (Fig. 6f). Moreover, the intensity of the LiF peak in sample 2 was much taller than that in sample 3 and sample 4, which implies that LiF was the major component of the film on the surface of the cycled cathode at 60 °C. The higher LiF peak intensity of sample 2 also indicates that LiBF4-based electrolyte was more likely to decompose and create a higher amount of LiF than the LiODFB-based electrolyte system. The fact that the amount of LiF decreased significantly when a small amount of LiODFB was added to the electrolyte confirmed that LiODFB inhibits the decomposition of the electrolyte. Three peaks were identified in the C1s spectrum of sample 1: 284.8, 286.5, and 291.3 eV (Fig. 6c). These peaks correspond to the C–C bond in the conductive carbon, the –CH2 bond and the –CF2 bond in the adhesive (PVDF), respectively. In samples 3 and 4, both the C–O peak (287 eV) and the C=O peak (289.5 eV) were visible. By contrast, in sample 2, only the C–O peak was detected. The C=O peak was not observed in the C1s spectrum of sample 2 because it overlaps with the –CF2 peak. It was, however, detected in the O1s spectrum of the sample (Fig. 6d). Both the O=C peak (533.5 eV) and the O–C peak (534.3 eV) in the O1s spectrum were strengthened significantly. This implied that the electrolyte decomposition took place on the electrode and that a polymer containing C–O and C=O bond was generated in the process. The B1s spectra indicated the appearance of a B–O peak (193.6 eV) on the surface of the electrode after the cycling process (Fig. 6e). This new peak was the strongest in sample 2, which indicates that both lithium salts, LiBF4 and LiODFB, participate in the interfacial reaction at high temperature. Even though both lithium salts decomposed to generate the compound containing the detected B–O bond, the decomposition process of and LiBF4 appeared stronger. Ultimately, the decomposition products deposited on the cathode surface formed a thick and loose interfacial film. Due to the relatively poor film-forming performance of the LiBF4-based electrolyte, the electrolyte decomposed continuously at high temperature. The addition of LiODFB caused the formation of a dense interfacial film, which prevented the further oxidation and decomposition of the electrolyte. Thus, the introduction of a certain amount of LiODFB into LiBF4 may improve the cycling stability of the cell at high temperature.
Conclusion
This paper systematically investigates the temperature range of an electrolyte system based on a mixture of LiBF4/LiODFB lithium salts in a 1 M PC/EC/EMC (1:1:3) + FEC(5%) and their application in LiCoO2/Li cells.
Electrolyte cells based on pure LiBF4 or LiODFB showed poor performance in a wide range of temperature. However, the addition of LiODFB as a co-salt to LiBF4 improved the conductivity of the electrolyte. Also, it caused the formation of a dense and stable interfacial film on the cathode surface, which suppressed the decomposition of the LiBF4-based electrolyte. As a result, Li/LiCoO2 cells using LiBF4/LiODFB(8:2)-based electrolytes exhibited high discharge capacity and excellent cycling stability.
However, the rate performance of the LiCoO2/Li cell was very poor at lower temperature (− 20 °C). This study proves that mixed lithium salts used in LIB electrolytes have great potential. However, the electrolyte system presented in this paper needs to be optimized further in order to improve the rate performance. Moreover, electrochemical mechanism of the synergistic effect at high and low temperatures should be investigated.
References
Goodenough JB, Kim Y (2011) Challenges for rechargeable batteries. J Power Sources 196(16):6688–6694. https://doi.org/10.1016/j.jpowsour.2010.11.074
Scrosati B, Garche J (2010) Lithium batteries: status, prospects and future. J Power Sources 195(9):2419–2430. https://doi.org/10.1016/j.jpowsour.2009.11.048
Liu H, Miao C, Meng Y, He Y-B, Xu Q, Zhang X, Tang Z (2014) Optimized synthesis of nano-sized LiFePO4/C particles with excellent rate capability for lithium ion batteries. Electrochim Acta 130:322–328. https://doi.org/10.1016/j.electacta.2014.03.034
Li S, Li X, Liu J, Shang Z, Cui X (2014) A low-temperature electrolyte for lithium-ion batteries. Ionics 21(4):901–907
Azeez F, Fedkiw PS (2010) Conductivity of libob-based electrolyte for lithium-ion batteries. J Power Sources 195(22):7627–7633. https://doi.org/10.1016/j.jpowsour.2010.06.021
Li S, Zhao W, Zhou Z, Cui X, Shang Z, Liu H, Zhang D (2014) Studies on electrochemical performances of novel electrolytes for wide temperature range lithium ion batteries. Appl Mater Interfaces 6(7):4920–4926. https://doi.org/10.1021/am405973x
Lai Y, Peng B, Zhang Z, Li J (2014) A wide operating temperature range electrolyte containing lithium salts mixture and a co-solvent for the LiFePO4 cathode. J Electrochem Soc 161(6):A875–A879. https://doi.org/10.1149/2.023406jes
Zhang Z, Chen X, Li F, Lai Y, Li J, Liu P, Wang X (2010) LiPF6 and lithium oxalyldifluoroborate blend salts electrolyte for LiFePO4/artificial graphite lithium-ion cells. J Power Sources 195(21):7397–7402
Zhou H, Xiao K, Li J (2016) Lithium difluoro(oxalate)borate and LiBF4 blend salts electrolyte for LiNi0.5Mn1.5O4 cathode material. J Power Sources 302:274–282
Wang DY, Xiao A, Wells L, Dahn JR (2015) Effect of mixtures of lithium hexafluorophosphate (LiPF6) and lithium bis(fluorosulfonyl)imide (LiFSI) as salts in li[Ni1/3Mn1/3Co1/3]O2/graphite pouch cells. J Electrochem Soc 162(1):A169–A175
Zhang SS, Xu K, Jow TR (2002) Study of LiBF4 as an electrolyte salt for a li-ion battery. J Electrochem Soc 149(5):A586. https://doi.org/10.1149/1.1466857
Xu K, Zhang S, Jow TR, Xu W, Angell CA (2002) LiBOB as salt for lithium-ion batteries: a possible solution for high temperature operation. Electrochem Solid-State Lett 5(1):A26. https://doi.org/10.1149/1.1426042
Agubra VA, Fergus JW (2014) The formation and stability of the solid electrolyte interface on the graphite anode. J Power Sources 268:153–162. https://doi.org/10.1016/j.jpowsour.2014.06.024
Zhang SS (2007) Electrochemical study of the formation of a solid electrolyte interface on graphite in a LiBC2O4F2-based electrolyte. J Power Sources 163(2):713–718. https://doi.org/10.1016/j.jpowsour.2006.09.040
Zygadło-Monikowska E, Florjańczyk Z, Kubisa P, Biedroń T, Tomaszewska A, Ostrowska J, Langwald N (2010) Mixture of LiBF4 and lithium difluoro(oxalato)borate for application as a new electrolyte for lithium-ion batteries. J Power Sources 195(18):6202–6206
Lu Z, Yang L, Guo Y (2006) Thermal behavior and decomposition kinetics of six electrolyte salts by thermal analysis. J Power Sources 156(2):555–559. https://doi.org/10.1016/j.jpowsour.2005.05.085
Aravindan V, Gnanaraj J, Madhavi S, Liu HK (2011) Lithium-ion conducting electrolyte salts for lithium batteries. Chemistry 17(51):14326–14346. https://doi.org/10.1002/chem.201101486
Fu MH, Huang KL, Liu SQ, Liu JS, Li YK (2010) Lithium difluoro(oxalato)borate/ethylene carbonate+propylene carbonate+ethyl(methyl) carbonate electrolyte for LiMn2O4 cathode. J Power Sources 195(3):862–866. https://doi.org/10.1016/j.jpowsour.2009.08.042
Li J, Xie K, Lai Y, Za Z, Li F, Hao X, Chen X, Liu Y (2010) Lithium oxalyldifluoroborate/carbonate electrolytes for LiFePO4/artificial graphite lithium-ion cells. J Power Sources 195(16):5344–5350. https://doi.org/10.1016/j.jpowsour.2010.03.038
Xu MQ, Zhou L, Hao LS, Xing LD, Li WS, Lucht BL (2011) Investigation and application of lithium difluoro(oxalate)borate (LiDFOB) as additive to improve the thermal stability of electrolyte for lithium-ion batteries. J Power Sources 196(16):6794–6801. https://doi.org/10.1016/j.jpowsour.2010.10.050
Gao HQ, Zhang ZA, Lai YQ, Jie L, Liu YX (2008) Structure characterization and electrochemical properties of new lithium salt LiODFB for electrolyte of lithium ion batteries. J Cent South Univ 15(6):830–834. https://doi.org/10.1007/s11771-008-0153-1
Liao L, Cheng X, Ma Y, Zuo P, Fang W, Yin G, Gao Y (2013) Fluoroethylene carbonate as electrolyte additive to improve low temperature performance of LiFePO4 electrode. Electrochim Acta 87(1):466–472
Bordes A, Eom K, Fuller TF (2014) The effect of fluoroethylene carbonate additive content on the formation of the solid-electrolyte interphase and capacity fade of li-ion full-cell employing nano Si–graphene composite anodes. J Power Sources 257:163–169
Zugmann S, Moosbauer D, Amereller M, Schreiner C, Wudy F, Schmitz R, Schmitz R, Isken P, Dippel C, Müller R, Kunze M, Lex-Balducci A, Winter M, Gores HJ (2011) Electrochemical characterization of electrolytes for lithium-ion batteries based on lithium difluoromono(oxalato)borate. J Power Sources 196(3):1417–1424. https://doi.org/10.1016/j.jpowsour.2010.08.023
Ding MS, Jow TR (2004) How conductivities and viscosities of PC-DEC and PC-EC solutions of LiBF4, LiPF6, LiBOB, Et4NBF4, and Et4NPF6 differ and why. J Electrochem Soc 151(12):A2007–A2015. https://doi.org/10.1149/1.1809575
Shui Zhang S (2006) An unique lithium salt for the improved electrolyte of li-ion battery. Electrochem Commun 8(9):1423–1428. https://doi.org/10.1016/j.elecom.2006.06.016
Li F, Gong Y, Jia G, Wang Q, Peng Z, Fan W, Bai B (2015) A novel dual-salts of LiTFSI and LiODFB in LiFePO4-based batteries for suppressing aluminum corrosion and improving cycling stability. J Power Sources 295:47–54
Schedlbauer T, Rodehorst UC, Schreiner C, Gores HJ, Winter M (2013) Blends of lithium bis(oxalato)borate and lithium tetrafluoroborate: useful substitutes for lithium difluoro(oxalato)borate in electrolytes for lithium metal based secondary batteries? Electrochim Acta 107(3):26–32. https://doi.org/10.1016/j.electacta.2013.05.130
Egashira M, Sawada N, Ueda K, Yoshimoto N, Morita M (2010) Capacitance of porous carbon electrode in mixed salt non-aqueous electrolytes. J Power Sources 195(6):1761–1764
Mun J, Kim S, Yim T, Ryu JH, Kim YG, Oh SM (2010) Comparative study on surface films from ionic liquids containing saturated and unsaturated substituent for LiCoO2. J Electrochem Soc 157(2):A136–A141. https://doi.org/10.1149/1.3265476
Acknowledgements
This work is supported by the Joint Funds of the National Natural Science Foundation of China (Nos. U1407105 and U1407106), the Applied Basic Research Program of Qinghai Province (No.2015-ZJ-740), and Qinghai Provincial Thousand Talents Program for High-level Innovative Professionals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Sun, Y., Zhou, Y. et al. Investigation of the synergetic effects of LiBF4 and LiODFB as wide-temperature electrolyte salts in lithium-ion batteries. Ionics 24, 2995–3004 (2018). https://doi.org/10.1007/s11581-018-2470-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2470-1