Abstract
Focusing on additive-free electrodes, thin films are of typical interest as electrodes for lithium ion battery application. Herein, we report the fabrication of TiO2 thin films by spray pyrolysis deposition technique. X-ray diffraction and transmission electron microscopic analysis confirms the formation of anatase TiO2. Electrochemical evaluation of these sub-micron TiO2 thin films exhibits high-rate performance and long cycling stability. At 1C rate (1C = 335 mA/g), the electrode delivered discharge capacity of 247 mAh/g allowing about 0.74 lithium into the structure. The electrodes also delivered specific capacities of 122 and 72 mAh/g at 10 and 30C rates, respectively. Without conductive additives, this excellent performance can be attributed to the nanosize effect of TiO2 particles combined with the uniform porous architecture of the electrode. Upon cycling at high rates (10 and 30C), the electrode exhibited excellent cycling stability and retention, specifically only < 0.6% capacity loss per cycle over 2500 cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thin-film battery research were receiving momentous attention due to its wide spread applications ranging from RFID tags, sensors, medical devices, and micro batteries [1, 2]. This is principally due to benefit associated with the formation of additive-free electrode minimizing dead mass and maximizing utilization of entire active electrode [3]. Fabrication process for such thin-film electrodes ranges from dip-coating [4], spray pyrolysis process [3], ink-jet printing [5], physical vapor deposition [6], and chemical vapor deposition [7]. Numerous transition metal oxides, insertion-based lithium-containing compounds, and alloying electrodes were deposited by the aforementioned processes [8,9,10]. Among the transition metal oxides, TiO2 is an interesting negative electrode for lithium ion battery applications. Out of the different polymorphs of TiO2, anatase phase synthesized at low temperature emerges as an anode material with a high theoretical capacity (335 mAh/g), fairly less volume expansion (3–4%), and better lithium kinetics [11]. Yet, the innate problem of the materials is in its kinetic limitation allowing only 0.5 Li into the lattice. This can easily be tackled by nanostructuring thus reducing the diffusion lengths and improving the practical capacity values [12].
TiO2 thin films as battery electrodes are well investigated through different fabrication techniques [3, 13, 14]. However, the intricate processes parameters and the requirement of sophisticated equipment with high vacuum conditions complicate the fabrication processes, posing serious challenges to the scale-up of the films. In order to address the aforementioned issues here, we demonstrate the fabrication and electrochemical characterization of TiO2 thin films through a simple and scalable spray pyrolysis deposition technique. The process involves the atomization of a colloidal sol solution and deposition of the same onto a heated substrate. Since the sol was synthesized prior, control in TiO2 particle size can easily be achieved. Further, the spraying process does not need any high vacuum condition and can be carried at low temperature conditions. The same technique can be utilized to fabricate well-compacted films over large area demonstrating the potential of same to be extended to large scale applications as well. An added advantage is in its capacity to tune film thickness (even up to 100 s of microns) by varying the duration of deposition [3]. In this work, we report the high-rate electrochemical performance of additive/binder less TiO2 thin films for lithium ion battery applications. The SPD-TiO2 film crystallized in anatase phase was observed to have interlinked nanosized TiO2 particles forming a porous electrode structure which attributes to the excellent electrochemical and cycling performances. The same rendered high-specific capacity of 274 mAh/g at 1C and ≤ 0.6% capacity loss per cycle for over 2500 cycles at both 10 and 30C rate.
Materials and methods
Titanium isopropoxide, ethanol, Triton-X 100, and acetic acid were purchased from Sigma-Aldrich. Stainless steel substrate (SS 316 with 0.1 mm thick) used for deposition was obtained from MTI (USA). The synthesis and spray pyrolysis deposition has been described by Haridas et al. [3]. Prior to deposition, a colloidal technique was carried out to synthesis TiO2 nanoparticles wherein 10 ml titanium isoproxide dissolved in mixture of 15 ml isopropanol and 1.5 ml acetic acid was allowed to precipitate by passing steam. The resulting thick mass was diluted with distilled water and allowed to stir until formation of a proper dispersion. Later stabilization of the colloidal mass was done through a hydrothermal technique maintaining the solution at 180 °C for 3 h. Further, 10 ml of this solution was diluted with 10 ml isopropyl alcohol, 2.5 ml of acetic acid, and 3 drops of Triton-X 100 and used as such for spray deposition. Optimized spraying conditions to obtain films of 0.7 mg/cm2 loadings were temperature 110 °C, flow rate 6 ml/min, and post annealing at 400 °C for 3 h in air.
Thin-film X-ray diffraction analysis was conducted in Rigaku (Ultimate IV Japan) diffractometer with scan rate of 1°/min. Surface and cross sectional images were performed in FEI Nova NanoSEM while transmission electron microscopic imaging were carried out in transmission electron microscopic (TEM) Technai FEI-G2. Surface analyses were done using XPS Kratos (Axis Ultra UK) with Al-Kα as X-ray source. Obtained annealed films were used as such for cell fabrication in Swagelok setup with lithium metal as reference/counter electrode and 1 M LiPF6 (EC/DMC = 1:1) as electrolyte. All electrochemical measurements were conducted in battery cycler Arbin (BT 2000) in 1 ≤ V ≤ 3 potential window.
Results and discussions
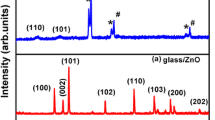
Annealing the SPD-TiO2 films at 400 C results in crystallization of the sample in anatase phase. Figure 1 shows the thin-film XRD pattern of the SPD-TiO2 film deposited on stainless steel substrate. This is in concurrence with data available in literature with 100% intensity peak indexed to (101) of anatase TiO2 [11, 12, 15].
Scanning electron microscopy images depicted in Fig. 2a shows the surface image of the as-deposited film. As can be visualized, the films were composed of interconnected TiO2 nanoparticles with pores in-between. It is well established that such porous films favor better electrolyte access throughout the entire film leading to better lithium ion transport [3, 16]. Porous nature of the film can even be confirmed from cross-sectional image shown in Fig. 2b, and the imaging was done by spraying the TiO2 colloidal solution on silicon substrate. The sample was then cleaved to get a clear image of the cross-section of SPD-TiO2 film. Spray pyrolysis deposition was optimized in order to obtain an electrode active loading of 0.7 mg/cm2 which was observed to have a thickness of around 3 μm.
TEM image of the colloidal TiO2 nanoparticles used for SPD deposition are shown in Fig. 3a. TEM image shows uniformly sized nanoparticles of average 12–15 nm in size, while HRTEM image (Fig. 3b) shows crystalline particles of lattice spacing 0.35 nm which corresponds to (101) plane of anatase phase of TiO2.
XPS spectra of the SPD electrode are presented in Fig. 4. Figure 4a displays the survey spectra and the absence of any parasitic signals except that of Ti, O, and C confirm the purity of the sample. Curve fitting the high-resolution Ti 2p spectra (Fig. 4b) shows Ti in 4+ oxidation state which was confirmed from the Ti 2p3/2 and Ti 2p½ binding energies and from the spin-orbit separation value of 5.7 eV [17, 18]. Deconvoluting the high-resolution O 1 s spectrum (Fig. 4c) results in two curves peaking at around 529.4 and 531.2 eV. Former can be related to the presence of lattice oxygen Ti-O while later one with lesser intensity to adsorbed hydroxyl ions [19].
Electrochemical characterizations
Electrochemical performance of SPD-TiO2 thin-film electrodes are presented in Fig. 5. Figure 5a shows the charge-discharge profile of SPD-TiO2 films at three different C-rates (1C = 335 mAh/g) from 3 to 1 V voltage window. As can be seen, the electrode showed typical anatase TiO2 profile with discharge plateau at 1.7 V and charge at 2.1 at 1C rate, and the electrode was capable of rendering first discharge capacity of 247 mAh/g. The nanosized TiO2 particles facilitate the lithiation of more than 0.5 Li into the lattice which is kinetically restricted in bulk particles. At 3C rate, the electrode delivered discharge capacity of 175 mAh/g and charge capacity of 149 mAh/g. Upon increasing the C-rate to 10C, the electrode was observed to have slightly polarized voltage profiles yet delivering 122 and 115 mAh/g as discharge and charge capacity, respectively.
Further, to understand the high rate and cycling stability of SPD-TiO2 electrodes, we have cycled electrodes at 10 and 30C rates for 2500 cycles. Figure 5b shows 10C charge-discharge profiles at different cycle numbers, wherein the initial cycle capacity was observed to be 122 mAh/g and with a Coulombic efficiency of 94% refer Fig. 6a. Subsequently, the Coulombic efficiency value increases and attains almost 99.2 and 99.7% at 10 and 30C respectively till the end of 2500 cycles. This capacity/retention values are better than several reports on TiO2 nanostructures [20, 21]. The SPD-TiO2 electrode displayed negligible capacity loss per cycle (0.6%) while maintaining a capacity of 106 mAh/g at the end of 2500 cycles. Both 10 and 30C long cycling performance is depicted in Fig. 5c. The electrode was capable of rendering excellent cycling stability even at a high rate of 30C. Even after 2500 cycles, the electrode retained 95.6% of its initial capacity indicating the electrodes potential for high-power, long-life applications. As a comparison, we have investigated the electrochemical performance of synthesized TiO2 nanoparticles casted via the conventional slurry casting method with mass ratio 70:20:10 (active material: conductive carbon: PVdF). From the electrochemical results (Fig. 6b), it is confirmed that even with a high amount of conductive carbon (20%) in the composite electrode was able to deliver similar yet slightly less capacity at 10C rate. This improved performance emerges from the deposition of nanosized TiO2 particles as interconnected porous film through spray pyrolysis deposition leading to better electrolyte percolation and kinetics. It may be noted that the SPD technique is capable of fabricating additive-free electrodes in large scale and with high rate of deposition. Thus, the excellent performance of TiO2 thin films fabricated via SPD technique validates the advantages of having additive-free, high energy density electrodes for lithium ion batteries, which can even be extended to other electrode materials.
Conclusions
In this report, we have demonstrated fabrication of additive-free TiO2 thin films through spray pyrolysis deposition technique. Uniqueness of this technique is in its capability to fabricate large scale films of uniform and tunable thickness with excellent particle-particle electrical contact and good porosity. Since the particles are synthesized prior to deposition, control over the particle size is also feasible. Utilizing these additive-free electrodes as lithium ion battery electrodes, we have demonstrated excellent electrochemical performance in terms of its cycling stability (2500 cycles) and high-rate capability (30C). The electrode delivered 274 mAh/g at 1C rate while at high rates of both 10 and 30 C; the electrode delivered stable capacity with negligible loss (< 0.6% per cycle) for almost 2500 cycles. Our results ascertain spray pyrolysis deposition technique as an effective technique in fabricating additive-free thin-film electrodes and can be extended to 3D micro batteries, flexible energy storage, and conversion devices.
References
Navone C, Pereira-Ramos JP, Baddour-Hadjean R, Salot R (2010) Lithiated c-V2O5 thin-film as positive electrode for rocking-chair solid-state lithium microbattery. Ionics (Kiel) 16(7):577–580. https://doi.org/10.1007/s11581-010-0460-z
Li J, Daniel C, Wood D (2011) Materials processing for lithium-ion batteries. J Power Sources 196(5):2452–2460. https://doi.org/10.1016/j.jpowsour.2010.11.001
Haridas AK, Gangaja B, Srikrishnarka P, Unni GE, Nair AS, Nair SV, Santhanagopalan D (2017) Spray pyrolysis-deposited nanoengineered TiO2 thick films for ultra-high areal and volumetric capacity lithium ion battery applications. J Power Sources 345:50–58. https://doi.org/10.1016/j.jpowsour.2017.01.136
Bai Y, Knittlmayer C, Gledhill S (2009) Preparation and characterization of Li2CoMn3O8 thin film cathodes for high energy lithium batteries. Ionics (Kiel) 15(1):11–17. https://doi.org/10.1007/s11581-008-0287-z
Chi K, Zhang Z, Xi J, Huang Y, Xiao F, Wang S, Liu Y (2014) Freestanding graphene paper supported three-dimensional porous graphene-polyaniline nanocomposite synthesized by inkjet printing and in flexible all-solid-state supercapacitor. ACS Appl Mater Interfaces 6(18):16312–16319. https://doi.org/10.1021/am504539k
Bates JB, Dudney NJ, Neudecker B et al (2000) Thin-film lithium and lithium-ion batteries. Solid State Ionics 135(1-4):33–45. https://doi.org/10.1016/S0167-2738(00)00327-1
Magasinski A, Dixon P, Hertzberg B, Kvit A, Ayala J, Yushin G (2010) High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat Mater 9(5):461–461. https://doi.org/10.1038/nmat2749
Chou SL, Wang JZ, Liu HK, Dou SX (2008) Electrochemical deposition of porous Co3O4 nanostructured thin film for lithium-ion battery. J Power Sources 182(1):359–364. https://doi.org/10.1016/j.jpowsour.2008.03.083
Takahashi M, Tani J, Kido H, Hayashi A, Tadanaga K, Tatsumisago M (2011) Thin film electrode materials Li4Ti5O12 and LiCoO2 prepared by spray pyrolysis method. IOP Conf Ser Mater Sci Eng 18(12):122004. https://doi.org/10.1088/1757-899X/18/12/122004
Demirkan MT, Trahey L, Karabacak T (2016) Low-density silicon thin films for lithium-ion battery anodes. Thin Solid Films 600:126–130. https://doi.org/10.1016/j.tsf.2016.01.029
He B-L, Dong B, Li H-L (2007) Preparation and electrochemical properties of Ag-modified TiO2 nanotube anode material for lithium–ion battery. Electrochem Commun 9(3):425–430. https://doi.org/10.1016/j.elecom.2006.10.008
Wang D, Choi D, Li J, Yang Z, Nie Z, Kou R, Hu D, Wang C, Saraf LV, Zhang J, Aksay IA, Liu J (2009) Self-assembled TiO2-graphene hybrid nanostructures for enhanced li-ion insertion. ACS Nano 3(4):907–914. https://doi.org/10.1021/nn900150y
Aslan S, Guler MO, Cevher O, Akbulut H (2012) Nano crystalline TiO2; thin films as negative electrodes for lithium ion batteries. J Nanosci Nanotechnol 12(12):9248–9253. https://doi.org/10.1166/jnn.2012.6749
Chiu K-F, Lin KM, Leu HJ, Chen CL, Lin CC (2012) Fabrication and characterization of nano-crystalline TiO2 thin film electrodes for lithium ion batteries. J Electrochem Soc 159(3):A264–A268. https://doi.org/10.1149/2.055203jes
Wang J, Zhou Y, Hu Y (2011) Facile synthesis of nanocrystalline TiO2 mesoporous microspheres for lithium-ion batteries. J Phys Chem 115(5):2529–2536. https://doi.org/10.1021/jp1087509
Yu Y, Gu L, Dhanabalan A, Chen CH, Wang C (2009) Three-dimensional porous amorphous SnO2 thin films as anodes for Li-ion batteries. Electrochim Acta 54(28):7227–7230. https://doi.org/10.1016/j.electacta.2009.07.028
Göpel W, Anderson JA, Frankel D, Jaehnig M, Phillips K, Schäfer JA, Rocker G (1984) Surface defects of TiO2 (110): a combined XPS, XAES AND ELS study. Surf Sci 139(2-3):333–346. https://doi.org/10.1016/0039-6028(84)90054-2
Pan D, Huang H, Wang X, Wang L, Liao H, Li Z, Wu M (2014) C-axis preferentially oriented and fully activated TiO2 nanotube arrays for lithium ion batteries and supercapacitors. J Mater Chem A 2(29):11454–11464. https://doi.org/10.1039/c4ta01613k
Madhusudanan SP, Gangaja B, Shyla AG, Nair AS, Nair SV, Santhanagopalan D (2017) Sustainable chemical synthesis for phosphorus-doping of TiO2 nanoparticles by upcycling human urine and impact of doping on energy applications. ACS Sustain Chem Eng 5(3):2393–2399. https://doi.org/10.1021/acssuschemeng.6b02722
Acevedo-Peña P, Haro M, Rincón ME, Bisquert J, Garcia-Belmonte G (2014) Facile kinetics of Li-ion intake causes superior rate capability in multiwalled carbon nanotube@ TiO2 nanocomposite battery anodes. J Power Sources 268:397–403. https://doi.org/10.1016/j.jpowsour.2014.06.058
Zheng H, Ncube NM, Raju K, Mphahlele N, Mathe M (2016) The effect of polyaniline on TiO2 nanoparticles as anode materials for lithium ion batteries. Spring 5(1):630. https://doi.org/10.1186/s40064-016-1908-z
Acknowledgements
Infrastructural support from Amrita Vishwa Vidyapetham is greatly acknowledged. We also thank Dr. A. Sreekumaran Nair for his help. DS acknowledges SERB, India, for the award of Ramanujan fellowship (Ref: SB/S2/RJ-100/2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Gangaja, B., Haridas, A.K., Nair, S. et al. Spray pyrolysis-deposited TiO2 thin films as high-performance lithium ion battery anodes. Ionics 24, 2193–2198 (2018). https://doi.org/10.1007/s11581-017-2389-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2389-y