Abstract
A new type of gel polymer electrolyte (GPE) based on poly(butyl acrylate) (PBA) semi-interpenetrating polymer networks (IPNs) and polyvinylidene fluoride (PVDF) was prepared in different molar ratios ranging from 1:0.5 to 1:1. A series of structure characterizations of PBA/PVDF had been measured using FTIR, XRD, and SEM. The electrolyte uptake test revealed that when the semi-IPNs were swollen with the commercial liquid electrolyte solutions, they showed an outstanding electrolyte uptake of 120% with a chemically cross-linked structure. All results indicated that the GPE exhibited the best performance when the molar ratio of BA and PVDF was 1:0.5. The prototype cell assembled with LiFePO4 as cathode, lithium metal as anode, and GPE as the electrolyte as well as separator retained 94% of its initial specific capacity after 100 charge-discharge cycles, showing an excellent cycling stability and a high electrochemical window (up to 4.5 V against Li+/Li) at room temperature. Compared with the liquid electrolyte, the GPE exhibited a similar stable cycling performance and was suitable for practical application in Li-ion batteries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, lithium-ion batteries have aroused great interest due to their outstanding characteristics such as light weight, high specific capacity, non-toxic, low cost, etc. [1]. Typically, the liquid electrolyte is used to transport the Li+ in current lithium-ion batteries systems. However, it may have the risk of leakage in some ways. Polymer electrolyte has been studied by many researchers due to the outstanding characteristics such as variable shapes, desirable sizes, and no leakage [2]. Unfortunately, its low ionic conductivity (only 10−8–10−5 S cm−1 at room temperature) and poor interfacial properties weaken the high rate performance and prevent it from large-scale application [3–5]. As a kind of solid-state electrolyte, gel polymer electrolyte (GPE) could penetrate liquid contained alkali salt into solid organics to form a stable gel structure [6], which has higher ionic conductivity (10−5–10−3 S cm−1) and better compatibility than the traditional solid polymer electrolyte [7].
Initial works on GPE were mainly related to polyethylene oxide (PEO) [8–11], but the high degree of crystallization of PEO restricted the working temperature of the lithium batteries. In recent years, many kinds of polymeric hosts such as polyacrylonitrile (PAN) [12–14], polymethyl methacrylate (PMMA) [15, 16], polyimide (PI) [17–19], polyvinylidene fluoride (PVDF) [20–24], and its derivatives poly(vinylidene fluoride-hexafluoropylene) (PVDF-HFP) [25–29] have been proposed as the matrix materials for GPEs. Nevertheless, these polymers are obviously far from the standard of large scale lithium-ion batteries and other electrochemical devices. Thus, seeking for new polymer matrix with good mechanical strength, high ionic conductivity and favorable interfacial compatibility are still of great significance for the development of GPE.
Owing to the good compatibility with the electrolyte, we chose PBA as the raw material [30–34]. The polymer electrolyte based on PBA had a high uptake of the liquid electrolyte which could ensure a good transportation of Li+ between the positive and negative materials as well as a stable inner structure during a charge/discharge process. Meanwhile, PVDF was chosen as the film-forming material because of its high dielectric constant (ε = 7.25) and good ionic conductivity [12, 24]. A special kind of polymer blend—full interpenetrating polymer network (IPN) or semi-IPN—was formed by the combination of two polymers, which had been used to improve compatibility between immiscible phases [3, 4]. In this work, PBA was synthesized in the immediate presence of PVDF to form a novel PBA-based semi-IPN electrolyte system; more significantly, it is believed that an ideal gel polymer electrolyte with good ion conductivity and low interfacial polarization could be achieved by the method employed in the present work.
Experimental section
Materials
BA monomer and N-methyl-2-pyrrolidone (NMP) were obtained from Sinopharm Chemical Reagent Co., Ltd. PVDF (MW 130,000) was purchased from Shanghai 3F New Material Co., Ltd. Triethylene glycol dimethacrylate (TEDME) and 2,2-azobisisobutyronitrile (AIBN) were purchased from Aldrich Chemistry and Shanghai No. 4 Reagent & H. V. Chemical Co., Ltd., respectively. The liquid electrolyte (1 M LiPF6 in the mixed solvent of ethylene carbonate (EC), diethyl carbonate (DEC), and dimethyl carbonate (DMC) with the volume ratio of 1:1:1) was obtained from Guangzhou Tinci Material Technology Co., Ltd.
Preparation of semi-IPN films
The semi-IPN films of PBA/PVDF were prepared via free radical polymerization. BA monomer, PVDF, and TEDME used as the cross-linking agents and 80 mL of NMP were sequentially added into a 150-mL round-bottomed flask followed by stirring for half an hour until the polymer powder was completely dissolved. Meanwhile, the molar ratios of BA and PVDF were 1:0.5, 1:0.7, 1:0.9, and 1:1, and the amount of the TEDME accounted for 2.5 wt% in the whole reactants. After that, a mixture of AIBN and NMP was dropwise added into the flask at 80 °C under nitrogen atmosphere. The mixed solution was stirred and heated continuously until the liquid became a homogeneous viscous solution. The PBA/PVDF semi-IPN films thus obtained were subsequently dried in a vacuum oven at 120 °C for at least 12 h. Finally, the semi-IPN films with a thickness of 100 μm were obtained by open mill and hot press, and then punched into circular disks with a diameter of 16 mm for standby.
The semi-IPN films were soaked in the liquid electrolytes until saturated in the glove box to obtain the PBA/PVDF GPEs which were then used to evaluate the properties.
Characterization of GPEs
The tests of internal groups were carried out on a Fourier transform infrared measurement spectrometer (FTIR; Nexus, American) in a wavenumber range of 400–4000 cm−1. The crystalline phases of the films were analyzed by X-ray diffractometer (XRD; Bruker, Germany) with Cu Kα radiation in the range of 10°–80° with a sweep speed of 6° min−1. The morphologies of semi-IPN films were observed on scanning electron microscopy (SEM; ULTRA PLUS-43-13, Germany).
The circular disks of polymer films were taken out from the electrolyte at different times to measure the electrolyte uptake, which was calculated according to the following Eq. (1)
where W i and W f are the weights of the dry and wet membrane, respectively.
The electrochemical stability window of the GPE was evaluated by means of linear sweep voltammetry (LSV), which was carried out using stainless steel (SS) as the working electrode, lithium metal electrodes as the counter and the reference electrodes at a scanning rate of 1.0 mV s−1 between 0 and 5 V.
The semi-IPN films soaked with liquid electrolyte were sandwiched between two stainless steel blocking electrodes and assembled in a model cell. The AC impedance measurement was performed using CHI660 frequency response analyzer (Shanghai Chenhua, China) over a frequency range from 0.01 to 100 kHz. Ionic conductivity was calculated from the bulk resistance obtained from the impedance spectra
where R d. is the bulk resistance, and d and S are the thickness and known area of the polymer electrolyte membrane, respectively. It was worth noting that all of the electrochemical tests were carried out in a dry box where the temperature was controlled at 25 °C.
Electrochemical measurements of GPE-based cells
CR2032-type coin cells were assembled in an Ar-filled glove box. The composite cathode slurry was simply prepared by mixing 80 wt% LiFePO4 with 10 wt% acetylene black and 10 wt% PVDF, and NMP was used as the solvent. The mixture was coated on a piece of aluminum foil with doctor blade, and then dried under vacuum at 60 °C for 12 h. Lithium sheet was employed as the counter electrode and the GPE was used as the liquid electrolyte as well as a separator. For comparison, we assembled the same cells with liquid electrolyte.
The charge/discharge cycling tests were measured galvanostatically on a BTS high-precision serial version battery testing system (Shenzhen Neware Electronic Co. Ltd., China). The charge and discharge curves at different rates (0.1–0.5 C) were obtained to evaluate the rate properties of the LiFePO4/GPE/Li cells.
Results and discussion
Structure of the semi-IPN films
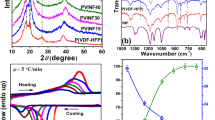
The FTIR spectra of pure BA, pure PVDF, and PBA/PVDF semi-IPN films are displayed in Fig. 1. As shown from the spectrum of pure PVDF, the band at 1185 cm−1 is assigned to the symmetry stretching band of -CF2 group and the peak at 1400 cm−1 is the in-plane bending vibration of -CH2 group [35]. It is clearly observed that the peak at about 1700 cm−1 that represents for -C=O in the monomer BA was blue shifted to 1730 cm−1 in the infrared spectra of the semi-IPNs films, indicating that the inductive effect plays the major role and the polarity of the -C=O groups is enhanced [35]. Meanwhile, the peaks at 1620 and 1300 cm−1 corresponding to -C=C disappear in the IPNs membranes, suggesting that the addition reaction has been carried out completely [14]. In addition, compared with the curve of PVDF, the bands observed at 762 and 1672 cm−1 corresponding to the crystalline phase disappear in the copolymer and the typical band at 882 cm−1 corresponding to the amorphous phase is enhanced, implying that the crystallinity of the copolymer is reduced after copolymerization [35]. Therefore, due to the enhancement of the polarity, the ionic conductivity of polymer electrolyte can be improved [35]. More significantly, the unique internal structure of the semi-IPNs provides a wider channel for the transport of lithium-ions [14].
The XRD patterns of pure PVDF and PBA/PVDF films with different molar ratios are demonstrated in Fig. 2. For pure PVDF, three distinct peaks appear at 18°, 20°, and 26.5°, respectively. Compared with pure PVDF, the XRD data of the semi-IPN films present two relatively weak peaks at 18° and 20° and the peak at 26.5° almost disappears, indicating that the addition of PVDF would increase the degree of crystallinity of the copolymer, which is consistent with the result of FTIR spectra.
According to the results of FTIR (Fig. 1) and XRD (Fig. 2), the crystallinity of the sample was the lowest at the molar ratio of 1:0.5 and the polarity of the -C=O groups was enhanced at the same time, which might have the best performance [35]. Hence, the sample with molar ratio of 1:0.5 was selected to investigate the microstructure. As illustrated in Fig. 3a, b, the PBA/PVDF semi-IPN film presents an irregular wavelike uneven surface and a relatively homogeneous porous structure, owing to the interpenetration between the two networks. The size of these pores is approximately dozens of microns owing to the long and interlaced PVDF molecular chain, which absorbs abundant NMP in the polymerization. Notably, the evaporation of NMP would generate pores, which are essential for absorbing liquid electrolyte and transmitting the lithium-ions. After swelling, the interpenetrating cross-linked polymer network was full of the liquid electrolyte solution. Due to the different degrees of swelling of the PVDF and PBA, the PBA absorbed with liquid electrolyte separated from the homogeneous porous structure, and the microphase separation took place [3] (Fig. 3c, d). Such a chemically cross-linked structure ensures a well distribution of LiPF6 in the PBA/PVDF GPE, which guarantees a smooth transfer of Li+.
Properties of GPE
Figure 4 shows the absorption behavior of the various semi-IPN films. Obviously, the electrolyte uptake reaches 120% at the molar ratio 1:0.5, which is in accordance with the XRD studies (Fig. 3). The possible reason is that PBA and the main solvent in the liquid electrolyte (EC, DEC, DMC) all belong to aliphatic esters. Due to their similar structure (−COO ester groups), PBA has a good compatibility with the commercial liquid electrolyte. According to the result of XRD, with a small amount of PVDF, the interpenetrating cross-linked copolymer of PBA/PVDF is partially crystallized, which is a benefit for the stability of the GPE. Moreover, the porous structure of PVDF is conductive to the absorption of electrolyte. However, due to the low liquid absorption rate of PVDF, the electrolyte uptake is bound to decrease when the content of PVDF increased.
The electrochemical stability is an important parameter for the characterization of GPE, which determines its operating voltage range. Linear sweep voltammogram curve of gel polymer membrane with BA/PVDF = 1:0.5 is presented in Fig. 5. It can be observed from the curve that no electrochemical reactions occur below 4.5 V vs. Li+/Li. When the voltage exceeds 4.5 V, the current increases rapidly. Generally, the charge and discharge voltage of lithium-ion battery is around 3.8 V [36]. In this regard, the GPE prepared by PBA/PVDF can meet the application requirements of lithium-ion batteries.
The electrochemical impedance spectroscopy (EIS) is a powerful method to study the interfacial resistance. It is consisted of two parts, a semi-circle at high frequency and medium frequency representing the charge transfer resistance (R ct) and a inclined line at low frequency indicating the Warburg impedance (W o) [8, 36]. The intercept on the real axis corresponds to the resistance of the bulk electrolyte resistance [37]. The diameter of the semi-circle gives the overall interfacial resistance, the smaller the diameter is, the easier transfer of Li-ion will be [33]. Figure 6 presents the EIS of the cell with PBA/PVDF GPE. It can be seen that the impedance increases firstly and then decreases as the content of PVDF build up in the copolymer. When the PBA/PVDF ratio is 1:0.5, the impedance is smaller than that of PBA/PVDF with ratios of 1:0.7, 1:0.9, and 1:1. The more the electrolyte is absorbed, the higher the ionic conductivity of the gel polymer electrolyte will be [35].The impedance result is in good agreement with the previous result of the electrolyte uptake test. The ionic conductivity at room temperature was calculated to be 0.81 mS cm−1 from the electrolyte resistance with thickness and surface area of the GPE. Compared to the solid-state electrolyte [2–5], the ionic conductivity of the gel polymer electrolyte is significantly enhanced. It is attributed to the semi-IPN structure of the GPE, which helps trap liquid electrolyte and contributes to ion conduction [33].
Performances of the LiFePO4/GPE/Li cell
In order to explore the rate capability of the LiFePO4/GPE/Li cell, the charge and discharge tests at different rates were conducted. As given in Fig. 7, the cell delivers a reversible discharge capacity of 88 mAh g−1 at the rate of 0.1 C and a flat charge and discharge plateau at the reversible redox potential of around 3.51 and 3.36 V (vs. Li/Li+), corresponding to the typical redox reaction of Fe2+/Fe3+ [38]. With the increase of the current density, the specific capacity of charge and discharge reduces. A reversible capacity of 68 mAh g−1 is obtained at the rate of 0.5 C, which is 74% of the capacity obtained at 0.1 C, which is related to the decreasing ionic conductivity. The lower ionic conductivity of the GPE leads to a higher ohmic polarization so that the voltage margin between the working and cutoff potentials is reduced [39]. Meanwhile, concentration polarization follows during cycling, which is primarily associated to the migration and depletion of Li-ion [34].
The cycle performances of the batteries assembled with GPE and liquid electrolyte are demonstrated in Fig. 8, while the molar ratio of GPE is 1:0.5. It is worth noting that we have used the pure LiFePO4 as the cathode without any modification. In the process of charge/discharge, the specific capacities of GPE and the traditional liquid electrolyte are approximately 95 and 90 mAh g−1, respectively. After 100 cycles at the rate of 0.1 C, the cell with GPE exhibits excellent capacity retention of 85 mAh g−1, only 0.05% capacity loss per cycle, while the liquid electrolyte remains 97% of its initial capacity. The batteries with these two kinds of electrolyte both exhibit excellent cycle stability. Compared to the conventional liquid electrolytes, the GPE prepared by PBA/PVDF can not only ensure the transmission of Li+ and provide a relatively high and stable discharge capacity but also satisfy flexibility and safety requirements. This result shows that the PBA/PVDF GPE allows the cell to function normally and has a satisfying cycling performance.
Conclusions
In this work, a new type of cross-linked PBA/PVDF semi-interpenetrating polymer network electrolyte was successfully prepared by soaking the semi-IPN films with liquid electrolyte solution. Besides the enhancement on mechanical strength, the special semi-IPN structure can facilitate the liquid electrolyte absorption and prevent the battery from electrolyte leakage. According to the electrolyte uptake analysis, while the molar ratio of BA/PVDF is 1:0.5, the absorption rate is up to 120%, which leads to an ionic conductivity of 0.81 mS cm−1 at room temperature. The electrochemical stable window of PBA/PVDF GPE could reach 4.5 V (vs. Li/Li+), and the battery exhibits a good cycling stability. This novel PBA-based semi-IPN not only provides a new polymer matrix material for the study of GPE but also has the potential for the application in other electronic devices such as supercapacitor, lithium sulfur battery, and fuel cell.
References
Wang X, Kato M, Naito H, Yamada C, Segami G, Kibe K (2006) A feasibility study of commercial laminated lithium-ion polymer cells for space applications. J Electrochem Soc 153(1):A89–A95
Cao J, Wang L, He X, Fang M, Gao J, Li J (2013) In situ prepared nano-crystalline TiO2-poly(methyl methacrylate) hybrid enhanced composite polymer electrolyte for li-ion batteries. J Mater Chem A 1(19):5955–5961
Hou X, Siow KS (2001) Novel interpenetrating polymer network electrolytes. Polymer 42(9):4181–4188
Rocco AM, Fonseca CP, Loureiro FAM, Pereira RP (2004) A polymeric solid electrolyte based on a poly(ethylene oxide)/poly(bisphenol a-co-epichlorohydrin) blend with LiClO4. Solid State Ionics 166(1–2):115–126
Kim HS, Shin JH, Moon SI, Kim SP (2003) Preparation of gel polymer electrolytes using PMMA interpenetrating polymeric network and their electrochemical performances. Electrochim Acta 48(11):1573–1578
Choudhury NA, Sampath S, Shukla AK (2009) Hydrogel-polymer electrolytes for electrochemical capacitors: an overview. Energy Environ Sci 2(1):55–67
Kim HS, Shin JH, Doh CH, Moon SI, Kim SP (2002) Preparation and electrochemical performance of gel polymer electrolytes using tri(ethylene glycol) dimethacrylate. J Power Sources 112(2):469–476
Song JY, Wang YY, Wan CC (1999) Review of gel-type polymer electrolytes for lithium-ion batteries. J Power Sources 77(2):183–197
Zhao M, Zuo X, Wang C, Xiao X, Liu J, Nan J (2016) Preparation and performance of the polyethylene-supported polyvinylidene fluoride/cellulose acetate butyrate/nano-SiO2, particles blended gel polymer electrolyte. Ionics 22(11):2123–2132
Sannier L, Bouchet R, Santinacci L, Grugeon S, Tarascon JM (2004) Lithium metal batteries operating at room temperature based on different FEO-PVdF separator configurations. J Electrochem Soc 151(6):A873–A879
Kang Y, Cho N, Noh KA, Kim JS, Lee C (2005) Improvement on cycling efficiency of lithium by PEO-based surfactants in cross-linked gel polymer electrolyte. J Power Sources 146(1):171–175
Subramania A, Sundaram NTK, Kumar GV, Vasudevan T (2006) New polymer electrolyte based on (PVA–PAN) blend for Li-ion battery applications. Ionics 12(2):175–178
Wang Q, Song WL, Fan LZ, Song Y (2015) Facile fabrication of polyacrylonitrile/alumina composite membranes based on triethylene glycol diacetate-2-propenoic acid butyl ester gel polymer electrolytes for high-voltage lithium-ion batteries. J Membrane Sci 486:21–28
Wang Q, Song WL, Fan LZ, Shi Q (2015) Effect of polyacrylonitrile on triethylene glycol diacetate-2-propenoic acid butyl ester gel polymer electrolytes with interpenetrating crosslinked network for flexible lithium ion batteries. J Power Sources 295:139–148
Jung HR, Lee WJ (2011) Electrochemical characteristics of electrospun poly(methyl methacrylate)/polyvinyl chloride as gel polymer electrolytes for lithium ion battery. Electrochim Acta 58(5):674–680
Rao M, Geng X, Liao Y, Hu S, Li W (2012) Preparation and performance of gel polymer electrolyte based on electrospun polymer membrane and ionic liquid for lithium ion battery. J Membrane Sci 399-400(3):37–42
Wang Q, Song WL, Wang L, Song Y, Shi Q, Fan LZ (2014) Electrospun polyimide-based fiber membranes as polymer electrolytes for lithium-ion batteries. Electrochim Acta 132(3):538–544
Lv YY, Wu J, Wan LS, Xu ZK (2008) Novel porphyrinated polyimide nanofibers by electrospinning. J Phys Chem C 112(29):10609–10615
Li YH, Wu XL, Kim JH, Xin S, Su J, Yan Y (2013) A novel polymer electrolyte with improved high-temperature-tolerance up to 170 °C for high-temperature lithium-ion batteries. J Power Sources 244(4):234–239
Xiao Q, Li Z, Gao D, Zhang H (2009) A novel sandwiched membrane as polymer electrolyte for application in lithium-ion battery. J Membrane Sci 326(2):260–264
Wu N, Cao Q, Wang X, Chen Q (2011) Study of a novel porous gel polymer electrolyte based on TPU/PVDF by electrospinning technique. Solid State Ionics 203(1):42–46
Liu J, Li W, Zuo X, Liu S, Li Z (2013) Polyethylene-supported polyvinylidene fluoride-cellulose acetate butyrate blended polymer electrolyte for lithium ion battery. J Power Sources 226(6):101–106
Zhu Y, Wang F, Liu L, Xiao S, Chang Z, Wu Y (2013) Composite of a nonwoven fabric with poly(vinylidene fluoride) as a gel membrane of high safety for lithium ion battery. Energy Environ Sci 6(2):618–624
Huang X, Zeng S, Liu J, He T, Sun L, Xu D (2015) High-performance electrospun poly(vinylidene fluoride)/poly(propylene carbonate) gel polymer electrolyte for lithium-ion batteries. J Phys Chem C 119(50):27882–27891
Kim KS, Park SY, Choi S, Lee H (2006) Ionic liquid-polymer gel electrolytes based on morpholinium salt and PVDF(HFP) copolymer. J Power Sources 155(2):385–390
Jeong HS, Kim JH, Lee SY (2010) A novel poly(vinylidene fluoride- hexafluoropropylene)/poly(ethylene terephthalate) composite nonwoven separator with phase inversion-controlled microporous structure for a lithium-ion battery. J Mater Chem 20(41):9180–9186
Ferrari S, Quartarone E, Mustarelli P, Magistris A, Fagnoni M, Protti S (2010) Lithium ion conducting PVdF-HFP composite gel electrolytes based on N-methoxyethyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)-imide ionic liquid. J Power Sources 195(2):559–566
Wongchitphimon S, Wang R, Jiraratananon R, Shi L, Loh CH (2011) Effect of polyethylene glycol (PEG) as an additive on the fabrication of polyvinylidene fluoride-co-hexafluropropylene (PVDF-HFP) asymmetric microporous hollow fiber membranes. J Membrane Sci 369(1–2):329–338
Aravindan V, Vickraman P, Madhavi S, Sivashanmugam A, Thirunakaran R, Gopukumar S (2011) Improved performance of polyvinylidenefluoride-hexafluoropropylene based nanocomposite polymer membranes containing lithium bis(oxalato)borate by phase inversion for lithium batteries. Solid State Sci 13(5):1047–1051
Zeng Z, Yu J, Guo Z, Li Y (2006) Preparation and application of cross-linked core-shell PBA/PS and PBA/PMMA nanoparticles. Front Chem China 1(4):459–464
Mani S, Khabaz F, Godbole RV, Hedden RC, Khare R (2015) Structure and hydrogen bonding of water in polyacrylate gels: effects of polymer hydrophilicity and water concentration. J Phys Chem B 119(49):15381–15393
Xu X, Liu B, Zhang M, Liu S, Zhu F, Wang J (2016) Electrolytes on governing particle coagulation and size distribution in the emulsion polymerization of butyl acrylate. J Polym Res 23(1):1–9
Wang Q, Song WL, Fan LZ, Song Y (2015) Flexible, high-voltage and free-standing composite polymer electrolyte membrane based on triethylene glycol diacetate-2-propenoic acid butyl ester copolymer for lithium-ion batteries. J Membrane Sci 492:490–496
Wang Q, Song WL, Fan LZ, Shi Q (2015) Effect of alumina on triethylene glycol diacetate-2-propenoic acid butyl ester composite polymer electrolytes for flexible lithium ion batteries. J Power Sources 279:405–412
Ran Y, Yin Z, Ding Z, Guo H, Yang J (2013) A polymer electrolyte based on poly(vinylidene fluoride-hexafluoropropylene)/hydroxypropyl methyl cellulose blending for lithium-ion battery. Ionics 19(5):757–762
Wang G, Lai Y, Zhang Z, Li J, Zhang Z (2015) Enhanced rate capability and cycle stability of lithium-sulfur batteries with a bifunctional MCNT@PEG-modified separator. J Mater Chem A 3(13):7139–7144
Quartarone E, Mustarelli P (2011) Electrolytes for solid-state lithium rechargeable batteries: recent advances and perspectives. Chem Soc Rev 40(5):2525–2540
Wang Q, Fan H, Fan LZ, Shi Q (2013) Preparation and performance of a non-ionic plastic crystal electrolyte with the addition of polymer for lithium ion batteries. Electrochim Acta 114:720–725
Cho YG, Kim YS, Sung DG, Seo MS, Song HK (2014) Nitrile-assistant eutectic electrolytes for cryogenic operation of lithium ion batteries at fast charges and discharges. Energy Environ Sci 7(5):1737–1743
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51673154, 51503159).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., Liu, Y., Yang, Q. et al. Properties of gel polymer electrolytes based on poly(butyl acrylate) semi-interpenetrating polymeric networks toward Li-ion batteries. Ionics 23, 2319–2325 (2017). https://doi.org/10.1007/s11581-017-2083-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2083-0