Abstract
Pr-doped Li4Ti5O12 in the form of Li4−x/3Ti5−2x/3PrxO12 (x = 0, 0.01, 0.03, 0.05, and 0.07) was synthesized successfully by an electrospinning technique. ICP shows that the doped samples are closed to the targeted samples. XRD analysis demonstrates that traces of Pr3+ can enlarge the lattice parameter of Li4Ti5O12 from 8.3403 to 8.3765 Å without changing the spinel structure. The increase of lattice parameter is beneficial to the intercalation and de-intercalation of lithium-ion. XPS results identify the existence form of Ti is mainly Ti4+ and Ti3+ in minor quantity in Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) samples due to the small amount of Pr3+. The transition from Ti4+ to Ti3+ is conducive to the electronic conductivity of Li4Ti5O12. FESEM images show that all the nanofibers are well crystallized with a diameter of about 200 nm and distributed uniformly. The results of electrochemical measurement reveal that the 1D Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) nanofibers display enhanced high-rate capability and cycling stability compared with that of undoped nanofibers. The high-rate discharge capacity of the Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) samples is excellent (101.6 mAh g−1 at 50 °C), which is about 58.48 % of the discharge capacity at 0.2 °C and 4.3 times than that of the bare Li4Ti5O12 (23.5 mA g−1). Even at 10 °C (1750 mA g−1), the specific discharge capacity is still 112.8 mAh g−1 after 1000 cycles (87.9 % of the initial discharge capacity). The results of cyclic voltammograms (CV) and electrochemical impedance spectroscopy (EIS) illustrate that the Pr-doped Li4Ti5O12 electrodes possess better dynamic performance than the pure Li4Ti5O12, further confirming the excellent electrochemical properties above.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Owing to recent requirements for electrical energy storage devices, increasing attention has been paid to spinal lithium-ion batteries (LIBs) with high power density and long cycle life [1, 2]. Currently, graphite, as a commercialized anode material, has been used as an active material of negative electrode (anode) for rechargeable lithium-ion batteries. However, it has a lot of problems in safety and life, which limits its practical applications seriously. Therefore, it is important to search for alternative anode materials with better safety and rate capability [3, 4].
Compared with graphite or other materials [5], spinel-type Li4Ti5O12 shows some advantages in long life, low cost, and high security, which is largely due to the following three key characteristics. First, it has outstanding safety with a high insertion potential at around 1.55 V (vs. Li/Li+), which can avoid the formation of SEI layers [6–9]. Second, its zero-strain structure makes it a promising material with a long cycle life during lithium-ion intercalation and de-intercalation [10]. Last but not the least, the rich content of Ti sources makes it a relatively cost-effective material [11]. However, Li4Ti5O12 has a low electrical conductivity and Li ionic conductivity, resulting in poor full capacity at high charge-discharge rates and an initial capacity loss [12]. To improve the electrochemical properties in high current density, tremendous efforts have been made to enhance the electrical and ionic conductivity of Li4Ti5O12. The most efficient methods are morphology altering [13, 14], surface coating [15–17], and element doping [18–20]. Surface coating can improve the electrical conductivity between Li4Ti5O12 particles without enhancing the intrinsic electronic and Li ionic conductivities. Morphology altering and element doping can enhance them fundamentally. 1D nanostructures are beneficial to the electrochemical properties because the morphology can shorten Li+ diffusion pathways and improve electron transport in lithium batteries [21]. What is more, the 1D nanostructures show advantages in uniformity and mass production compared with other methods, such as solid-state reaction, sol-gel method, or hydrothermal [22]. Large particle size element doping can also improve the electrochemical properties, such as La3+ [23], Ru4+ [24], and Sm3+ [25].
In this paper, we used rare earth element Pr3+ as the doping element (never been mentioned) and Li4−x/3Ti5−2x/3PrxO12 (x = 0, 0.01, 0.03, 0.05, and 0.07) compounds were synthesized through electrospinning method. The electrochemical performance and structure were systematically investigated, and we found such composites with Pr doping are advantageous for enhancing the electronic conductivity and Li ionic conductivity, resulting in excellent rate capability and cycling stability.
Experimental
Sample preparation
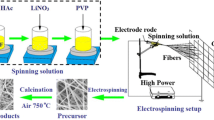
Li4−x/3Ti5−2/3xPrxO12 (x = 0, 0.01, 0.03, 0.05, and 0.07) compounds were synthesized by an electrospinning technique. Scheme 1 illustrates the brief synthesis route of the nanofibers. To get the bare Li4Ti5O12, 0.03 mol of titanium (IV) isopropoxide and 0.0247 mol of lithium acetate were blended with 10 ml of ethanol and 21 ml of acetic acid. After magnetic stirring for 30 min, 0.9 g of polyvinylpyrrolidone (PVP) was added. Then the mixture was stirred for 1 h to obtain the precursor solution. To get the Pr-doped Li4Ti5O12, different contents of praseodymium (III) nitrate with the ratio of Li4−x/3Ti5−2/3xPrxO12 (x = 0, 0.01, 0.03, 0.05, and 0.07) were added to the precursor solution. The buff transparent solution was immediately put into a plastic syringe which linked to a high-voltage power supply. In order to increase the electrospinning production, the feeding rate for the precursor was set to 2.5 ml h−1, which is five times than that of the average rate [26]. The distance between the tip of the needle and collector was 13 cm, and the voltage of the high-voltage power supply was 20 kV. After electrospinning, the nanowires were taken off from the collector. Ultimately, the nanofibers were calcined at 600 °C for 2 h and 750 °C for 5 h with a slow heating rate of 2 °C min−1 in air.
Material characterization
The relative contents of Li, Ti, and Pr in the prepared samples were monitored by inductively coupled plasma-atomic emission spectrometry (ICP, Perkin Optima 7000DV) analysis. The phase analysis of the prepared powders were performed by powder X-ray diffraction (XRD, Rigaku RINT2400, Japan) with Cu Kα radiation in 2θ degree ranging from 10 to 80° at a scanning rate of 10°/minute. The morphology and microstructure of the prepared powders were observed by field emission scanning electron microscopy (FESEM, Zeiss SuprATM 55, Germany) equipped with energy dispersive X-ray spectroscopy (EDS). The Brunauer–Emmett–Teller (BET, ASAP2010) model was adopted to calculate specific surface area and the density functional theory (DFT) method to determine pore-size distribution from the adsorption-desorption data. The surface chemistry composition and valence variation of all samples were investigated by X-ray photoelectron spectroscopy (XPS, Shimadzu Kratos Axis Ultra DLD) with Al K α radiation (1486.7 eV).
Electrode preparation
CR2025 coin cells were assembled to research the electrochemical performance of the work electrodes by galvanostatic charge-discharge test. The slurry was formed with 75 wt% as-prepared samples, 15 wt% acetylene black, and 10 wt% polyvinylidene fluoride (PVDF, as a binder), then, it was coated on the aluminum foil to form the work electrodes. After coating, the electrode was dried at 100 °C in vacuum oven for 12 h. 1.0 mol L−1 LiPF6 was dissolved in a mixture—consist of 50 % ethylene carbonate (EC) and 50 % dimethyl carbonate (DMC)—as the electrolyte. The coin cells were assembled in an argon-filled glove box ([O2] <1 ppm, [H2O] <1 ppm) using the prepared electrode as cathode, pure lithium foil as anode and a Celgard 2400 as the separator.
Electrochemical characterization
The galvanostatic charge-discharge tests were carried out using a Land battery test system (LANHE CT2001A) between a cut-off voltage of 1 and 2.5 V. The rate capabilities of the as-prepared samples were measured at different rates of 0.2 °C (35 mA g−1), 1 °C (175 mA g−1), 5 °C (875 mA g−1), 10 °C (1750 mA g−1), 20 °C (3500 mA g−1), 30 °C (5250 mA g−1), 40 °C (7000 mA g−1), and 50 °C (8750 mA g−1). The cycling tests were conducted at 10 °C. The cyclic voltammetry (CV) curves were measured on a VMP2 electrochemical workstation under a scanning rate of 0.5 mV s−1 between 1.0 and 2.5 V (vs. Li/Li+). Electrochemical impedance spectroscopy (EIS, Princeton Applied Research VersaSTAT3) at open-circuit voltage was performed with an AC voltage of 10 mV between 100 kHz and 10 MHz.
Results and discussions
According to inductively coupled plasma-atomic emission spectrometry (ICP), the doped samples were closed to the targeted samples, as shown in Table 1.
The XRD patterns of Li4−x/3Ti5−–2x/3PrxO12 (x = 0, 0.01, 0.03, 0.05, 0.07, and 0.10) samples are displayed in Fig. 1a. The major diffraction peaks of all annealed samples are in alignment to the standard diffraction pattern of Li4Ti5O12 (JCPDS No. 49-–0207), which indicates that a small amount of Pr3+ doping does not change the cubic spinel structure of Li4Ti5O12 with the space group of Fd3 m. No impurity peak can be detected in all samples, indicating that a small quantity of the dopant Pr3+ ions have successfully entered the lattice structure of Li4Ti5O12 without causing any changes in structural characteristics. However, some impurities are detectable when x = 0.10, denoting that the excess Pr cannot be doped into the LTO and it exists in the form of Pr6O11. In order to certify that the lattice distortion of Pr-doped Li4Ti5O12 has taken place, the peak positions of (111) planes of all samples are enlarged and shown in Fig. 1b. It is easy to find a slight structural change of the XRD patterns between pristine Li4Ti5O12 and Pr-doped Li4Ti5O12 in close inspection. Compared with that of the pristine Li4Ti5O12, the (111) peaks of the Li4−x/3Ti5−2x/3PrxO12 (x = 0.01, 0.03, 0.05, 0.07, and 0.10) shift to smaller angles after Pr doping, revealing that the lattice parameter increases gradually with increasing Pr doping amount. What is more, Li4−x/3Ti5−2x/3PrxO12 (x = 0.05, 0.07, and 0.10) shows similar (111) peaks, indicating that when x = 0.07, a small amount of Pr is excess, which hinders the improvement of electrochemical properties. The lattice parameters are calculated and the data obtained from XRD are listed in Table 2. The results show that the lattice parameter increases as the increase of the doping amount of Pr (NO)3. The reasons for the changes of lattice parameter after Pr modifying in all samples may be the following fact: Pr3+ ion (0.113 nm [27]) has a lager ionic radius than Li+ (0.076 nm [28]) and Ti4+ (0.0605 nm [28]) in the octahedral 16d sites and in the structure, and every two Ti4+ and one Li+ may be replaced with three Pr3+ [29]. Ultimately, the lattice parameter is slightly enlarged, which is beneficial for lithium batteries in lithium-ion intercalation and de-intercalation. It indicates that the fast Li+ diffusion caused by Pr doping will improve the electrochemical performance.
X-ray photoelectron spectroscopy (XPS) was carried out to prove the existence of Pr and confirm the valence variation of Ti in Li4−x/3Ti5−2x/3PrxO12 (x = 0.05). Figure 2a shows the XPS spectrum of Pr 3d. The XPS spectrum of Pr 3d exhibits two characteristic binding energy peaks of 933.5 and 954 eV, corresponding to Pr 3d3/2 and Pr 3d5/2 [30], indicating that Pr3+ has embedded into the Li4Ti5O12 lattice and no Pr2O3 phase appears. The high-resolution XPS spectrum of Ti 2p in Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) is shown in Fig. 2b. In Fig. 2b , two pairs of peaks can be found. One pair at 458.2 and 464.2 eV stands for Ti 2p3/2 and Ti 2p1/2 of Ti4+ and the other pair at 457.1 and 462.4 eV stands for Ti 2p3/2 and Ti 2p1/2 of Ti3+. It is vigorously confirmed that some oxygen vacancies have generated in order to maintain the charge balance from Ti4+ to Ti3+ due to Pr doping. The valence state change of Ti ion from Ti4+ to Ti3+ or the oxygen vacancies are conducive to electrochemical properties, which has been mentioned many times [31, 32].
To meet the requirements of 1D morphology and excellent electrical conductivity, the smooth PVP/inorganic composite nanofibers were synthesized through electrospinning method and the morphology was characterized by SEM, as shown in Fig. 3a. After calcination and grinding, the morphologies of the as-prepared Li4−x/3Ti5−2x/3PrxO12 (x = 0, 0.01, 0.03, 0.05, and 0.07) samples are shown in Fig. 3b–f. It is not hard to find that the images of the Pr-doped and undoped Li4Ti5O12 powders are similar, and they are all composed of nanorods with a uniform size distribution. The diameters of the nanofibers are about 0.2 μm. Figure 4a shows the FESEM image of Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) and the rest of Fig. 4 are the corresponding EDS element mapping of O (Fig. 4b), Ti (Fig. 4c), and Pr (Fig. 4d) of Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) particles. It is obvious to find that the shape of the FESEM image and the EDS element mapping images are similar, which indicates that the Pr element distributes uniformly in Pr-doped Li4Ti5O12 (x = 0.05).
Figure 5 shows the N2 adsorption-desorption isotherms and the DFT pore-size distribution curve (inset) of Li4−x/3Ti5−2x/3PrxO12 (x = 0 and x = 0.05). The N2 adsorption-desorption isotherms are identified as the type IV (IUPAC classification) [33]. From the pore-size distribution curves depicted in the insets, the pore size of the bare LTO is 29.4 nm and the surface area is 7.9 m2 g−1 (Fig. 5a). The Pr doping also gives rise to uniformity of pore-size distribution. Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) has a pore size of 23.6 nm and a surface area of 10.6 m2 g−1 (Fig. 5b). The nanoparticles with large surface area are conducive to accelerating the electrode reaction, shortening the diffusion path of Li ions, and facilitating charge transfer.
In order to certify the effect of Pr doping on improving the rate capability, different doping contents of Li4Ti5O12 at different rates of 0.2, 1, 5, 10, 20, 30, 40, and 50 °C were tested, and the results are shown in Fig. 6. Each stage of the charge-discharge processes were tested for 3 cycles. It is obvious that the discharge capacity decreases as the discharge current rate increases from 0.2 to 50 °C. Meanwhile, it is not difficult to find that the discharge capacity of Pr-doped samples decreased less than that of undoped Li4Ti5O12 samples when the discharge current increased, especially at a high rate. The button batteries assembled with undoped materials show a bad performance with the discharge capacity of 23.5 mAh g−1, which indicates that the insertion/de-insertion progress proceeds unsmoothly. However, when using the doping ratio of x = 0.05 materials, the discharge capacity is much better (101.6 mAh g−1, 4.3 times of the undoped samples and 58 % of the theoretical specific capacity). Table 3 shows the initial discharge capacity at different rates of Li4−x/3Ti5−2x/3PrxO12 (x = 0 and 0.05).
In order to compare the insertion/de-insertion progress between the bare Li4Ti5O12 and Li4−x/3Ti5−2x/3PrxO12 (x = 0.05), the initial charge-discharge curves of them are shown in Fig. 7. The cycling rates are from 0.2 to 50 °C and the potential window is between 1.0 and 2.5 V. From the figure, it is easy to observe that the charge-discharge curves have distinct potential plateaus around 1.5 V (vs. Li/Li+) at 0.2 and 1 °C for both of the two electrodes, which is consistent with the two-phase insertion reaction between Li4Ti5O12 and Li7Ti5O12. With the increase of the charge-discharge current density for both of the two samples, the charge plateau increases and discharge plateau decreases, indicating that the overpotential between the electrode and the electrolyte increases. Compared with the Pr-doped samples, the discharge plateaus can be hardly found in pure Li4Ti5O12 samples at 50 °C. The high polarization resistance of the pure samples is the main reason for this phenomenon [34]. The Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) shows an obvious discharge plateau at 30, 40, and 50 °C, and there is only a small reduction from 30 to 50 °C, demonstrating that the polarization resistance decreases due to the doping element of Pr. Nevertheless, among all of the Pr-doped samples, the Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) shows the best performance in rate capability. The main reason may be the fact that excess dopant of Pr3+, in Li+, and Ti4+ sites will gradually increase the transfer resistance (R ct ).
Cyclic performance of Li4−x/3Ti5−2x/3PrxO12 (x = 0, 0.01, 0.03, 0.05, and 0.07) at 10 °C rate is shown in Fig. 8. Compared with the bare Li4Ti5O12, Pr-doped Li4Ti5O12 batteries exhibit higher reversible capacities. Among all the Pr-doped electrodes, the initial discharge capacity of Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) is 128.3 and 112.8 mAh g−1 through 1000 cycles at 10 °C. The capacity retention rate is 87.9 %. However, the bare Li4Ti5O12 has a relatively inferior discharge capacity from 105.9 to 53 mAh g−1. The capacity retention rate is only 50.05 %. The reason why a small amount of Pr is beneficial to the cycling performance is that the lattice constant is enlarged due to the doping of Pr, which makes it easier for the insertion and extraction of Li+ ions and enhances the stability of the structure. The result is consistent with the rate capabilities. Hence, the Pr-doped Li4Ti5O12 is considered to be an excellent electrode material, especially when x = 0.05.
Cyclic voltammograms (CV curves) of Li4−1/3xTi5−2/3xPrxO12 (x = 0, 0.01, 0.03, 0.05, and 0.07) samples as active materials at a scanning rate of 0.5 mV s−1 between 1.0 and 2.5 V are shown in Fig. 9. They have similar appearance with only a pair of reversible redox peaks, suggesting that the electrochemical reaction processes of them are similar and the redox reaction of Li4Ti5O12 was not affected by Pr doping. The cathodic peak is located at about 1.45 V (vs. Li/Li+), corresponding to the voltage plateau of the first discharge process of Li+ intercalated into Li4Ti5O12 anode [35]. There is also an anodic peak at about 1.75 V (vs. Li/Li+), which corresponds to the voltage plateau of the first charge process of Li+ de-intercalated from the anode [35]. The differences between anodic peaks and cathodic peaks are given in Table 4. The potential differences of Pr-doped Li4Ti5O12 sample are smaller than those of undoped Li4Ti5O12, indicating that Pr doping has improved the electrochemical properties. From Table 4, we can also find the anodic peak and cathodic peak of Li4−1/3xTi5−2/3xPrxO12 (x = 0.05) are located at 1.697 and 1.491 V. The difference between them is 206 mV, which is the smallest in all samples. The cyclic voltammograms are in accordance with the results of the tests, further illustrating that the Pr-doped Li4Ti5O12 electrodes possess better kinetics than the pure Li4Ti5O12.
To compare the differences in electrochemical properties between Li4−x/3Ti5−2x/3PrxO12 (x = 0, 0.01, 0.03, 0.05, and 0.07) electrodes, the electrochemical impedance spectroscopy (EIS) measurement is conducted at open-circuit voltages after 300 cycles of charge and discharge at 10 °C. The corresponding Nyquist plots are displayed in Fig. 10 and they are composed of one semicircle at high frequency and a sloping straight line at low frequency. The diameter of the semicircle stands for the meaning of charge transfer resistance (R ct ) and the sloping straight line is related to the Warburg impedance [36]. From Fig. 9a, it is easy to find that the diameter of the semicircle of Pr-doped Li4Ti5O12 is shorter than that of bare Li4Ti5O12, suggesting that a small amount of Pr doping is beneficial to the electronic conductivity of Li4Ti5O12. The experimental result is fitted using an equivalent circuit in the inset of Fig. 10. In the inset, R s is the ohmic resistance of electrolyte, R ct is the charge transfer resistance, W is the Warburg impedance of solid-phase diffusion, and CPE is the constant phase element [37]. The results of the EIS for Li4−x/3Ti5−2x/3PrxO12 (x = 0, 0.01, 0.03, 0.05, and 0.07) are shown in Table 5, indicating that a small amount of Pr3+ doping can result in a higher lithium-ion diffusion coefficient and a lower electrode polarization.
Conclusions
In this paper, well-structured cubic spinel Li4−x/3Ti5−2x/3PrxO12 (x = 0, 0.01, 0.03, 0.05, and 0.07) nanofibers were successfully synthesized through electrospinning method. ICP shows that the doped samples are close to the targeted samples and a small amount of Pr doping does not change the bulk structure of Li4Ti5O12. Among all the Pr-doped samples, Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) exhibits the best properties in high-rate capability and cyclic performance. The high-rate discharge capacity of the Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) sample is excellent (101.6 mAh g−1 at 50 °C), which is about 58.48 % of the discharge capacity at 0.2 °C and 4.3 times than that of the bare Li4Ti5O12. Even at 10 °C (1750 mA g−1), the specific discharge capacity is still 112.8 mAh g−1 after 1000 cycles. Compared with the bare Li4Ti5O12, the Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) shows smaller electrochemical impedance and electrochemical polarization at high-rate charge-discharge processes. All the results suggest that the outstanding electrochemical properties benefit from Pr doping and the Li4−x/3Ti5−2x/3PrxO12 (x = 0.05) nanofibers make it a promising anode material for lithium-ion battery.
References
Armand M, Tarascon J-M (2008) Building better batteries. Nature 451:652–657
Yuan T, Cai R, Gu P, Shao Z (2010) Synthesis of lithium insertion material Li4Ti5O12 from rutile TiO2 via surface activation. J Power Sources 195:2883–2887
Zhu W, Huang H, Gan Y et al (2014) Mesoporous cobalt monoxide nanorods grown on reduced graphene oxide nanosheets with high lithium storage performance. Electrochim Acta 138:376–382
Yi T-F, Yang S-Y, Xie Y (2015) Recent advances of Li4Ti5O12 as a promising next generation anode material for high power lithium-ion batteries. J Mater Chem A 3:5750–5777
Wen W, Wu J, Jiang Y et al (2015) Anatase TiO2 ultrathin nanobelts derived from room-temperature-synthesized titanates for fast and safe lithium storage. Sci Rep 5:11804
Lu X, Zhao L, He X et al (2012) Lithium storage in Li4Ti5O12 spinel: the full static picture from electron microscopy. Adv Mater 24:3233–3238
Pan H, Zhao L, Hu YS et al (2012) Improved Li-storage performance of Li4Ti5O12 coated with C–N compounds derived from pyrolysis of urea through a low-temperature approach. Chem Sus Chem 5:526–529
Pan H-L, Hu Y-S, Li H, Chen L-Q (2011) Significant effect of electron transfer between current collector and active material on high rate performance of Li4Ti5O12. Chinese Phys B 20:118202-1–118202-4
Jia Z, Zhou Q, Li X et al (2015) Effect of rigidity of porous structure on electrochemical behavior of pristine Li4Ti5O12 microspheres. Electrochim Acta 156:216–222
Chang L, Luo S, Zhang H et al (2014) Synthesis and performance of Li4Ti5O12 anode materials using the PVP-assisted combustion method. Chinese Chem Lett 25:1569–1572
Ohzuku T, Ueda A, Yamamoto N (1995) Zero-strain insertion material of Li [Li1/3Ti5/3]O4 for rechargeable lithium cells. J Electrochem Soc 142:1431–1435
Ouyang CY, Zhong ZY, Lei MS (2007) Ab initio studies of structural and electronic properties of Li4Ti5O12 spinel. Electrochem Commun 9:1107–1112
Ma Y, Ding B, Ji G, Lee JY (2013) Carbon-encapsulated F-doped Li4Ti5O12 as a high rate anode material for Li+ batteries. ACS Nano 7:10870–10878
Wang J, Shen L, Li H et al (2014) Mesoporous Li4Ti5O12/carbon nanofibers for high-rate lithium-ion batteries. J Alloys Compd 587:171–176
Peng J, Zuo Y-T, Li G, Wang G (2016) Preparation of few-layer reduced graphene oxide-wrapped mesoporous Li4Ti5O12 spheres and its application as an anode material for lithium-ion batteries. Chinese Chem Lett:10–13
Shen L, Yuan C, Luo H et al (2011) In situ growth of Li4Ti5O12 on multi-walled carbon nanotubes: novel coaxial nanocables for high rate lithium ion batteries. J Mater Chem 21:761–767
Liu G-QG-Y, Wang H-Y, Liu G-QG-Y et al (2013) Synthesis and electrochemical performance of high-rate dual-phase Li4Ti5O12-TiO2 nanocrystallines for Li-ion batteries. Electrochim Acta 87:218–223
Li F, Zeng M, Li J et al (2016) Sb doped Li4Ti5O12 hollow spheres with enhanced lithium storage capability. RSC Adv 6:26902–26907
Bai Y-J, Gong C, Lun N, Qi Y-X (2013) Yttrium-modified Li4Ti5O12 as an effective anode material for lithium ion batteries with outstanding long-term cyclability and rate capabilities. J Mater Chem A:89–96
Zhang Q, Liu Y, Lu H et al (2016) Ce3+-doped Li4Ti5O12 with CeO2 surface modification by a sol-gel method for high-performance lithium-ion batteries. Electrochim Acta 189:147–157
Kim J, Shi D, Kong K et al (2013) Structurally and electronically designed TiO2Nx. ACS Appl Mater Interfaces 5:691–696
Huang S, Wen Z, Zhu X, Gu Z (2004) Preparation and electrochemical performance of Ag doped Li4Ti5O12. Electrochem Commun 6:1093–1097
Yang S-Y, Yuan J, Zhu Y-R et al (2015) Structure and electrochemical properties of Sc3+-doped Li4Ti5O12 as anode materials for lithium-ion battery. Ceram Int 41:7073–7079
Jhan YR, Lin CY, Duh JG (2011) Preparation and characterization of ruthenium doped Li4Ti5O12 anode material for the enhancement of rate capability and cyclic stability. Mater Lett 65:2502–2505
Li Z, Li J, Zhao Y et al (2016) Structure and electrochemical properties of Sm-doped Li4Ti5O12 as anode material for lithium-ion batteries. RSC Adv 6:15492–15500
Jo MR, Jung YS, Kang Y-M (2012) Tailored Li4Ti5O12 nanofibers with outstanding kinetics for lithium rechargeable batteries. Nanoscale 4:6870
Su W, Chen J, Wu L et al (2008) Visible light photocatalysis on praseodymium (III)-nitrate-modified TiO2 prepared by an ultrasound method. Appl Catal B Environ 77:264–271
Yi T-F, Liu H, Zhu Y-R et al (2012) Improving the high rate performance of Li4Ti5O12 through divalent zinc substitution. J Power Sources 215:258–265
Zhang Q, Verde MG, Seo JK et al (2015) Structural and electrochemical properties of Gd-doped Li4Ti5O12 as anode material with improved rate capability for lithium-ion batteries. J Power Sources 280:355–362
Hsieh PT, Chen YC, Kao KS, Wang CM (2008) Luminescence mechanism of ZnO thin film investigated by XPS measurement. Appl Phys A Mater Sci Process 90:317–321
Qi Y, Huang Y, Jia D et al (2009) Preparation and characterization of novel spinel Li4Ti5O12-xBrx anode materials. Electrochim Acta 54:4772–4776
Wolfenstine J, Allen JL (2008) Electrical conductivity and charge compensation in Ta doped Li4Ti5O12. J Power Sources 180:582–585
Liu G-Y, Wang H-Y, Liu G-Q et al (2012) Facile synthesis of nanocrystalline Li4Ti5O12 by microemulsion and its application as anode material for Li-ion batteries. J Power Sources 220:84–88
Wang D, Zhang CM, Zhang YY, Wang J, He DN (2013) Synthesis and electrochemical properties of La-doped Li4Ti5O12 as anode material for Li-ion battery. Ceram Int 39:5145–5149
Zhang Y, Zhang C, Lin Y et al (2014) Influence of Sc3+ doping in B-site on electrochemical performance of Li4Ti5O12 anode materials for lithium-ion battery. J Power Sources 250:50–57
Zhu Yang H, Zhang W, Huang H, Tao X, Xia Y, Gan Y, Guo XW (2015) Synthesis and electrochemical performance of Li4Ti5O12/TiO2/C nanocrystallines for high-rate lithium ion batteries. RSC Adv 5:74774–74782
Shenouda AY, Murali KR (2008) Electrochemical properties of doped lithium titanate compounds and their performance in lithium rechargeable batteries. J Power Sources 176:332–339
Acknowledgments
The work was supported by the Science and Technology Project of State Grid Corporation of China (DG71-15-042) and the National Natural Science Foundation of China (nos. 51572024 and 51172023).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, Y., Li, J., Li, Z. et al. Pr-modified Li4Ti5O12 nanofibers as an anode material for lithium-ion batteries with outstanding cycling performance and rate performance. Ionics 23, 597–605 (2017). https://doi.org/10.1007/s11581-016-1851-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1851-6