Abstract
Well-defined cuprous oxides (Cu2O) with different morphologies were successfully deposited on fluorine-doped tin oxide (FTO)-covered glass substrates by potentiostatic deposition. The effect of the pH values on the crystal morphologies of Cu2O was studied. The as-prepared products were characterized by means of X-ray diffraction (XRD), scanning electron microscopy (SEM), linear sweep voltammetry (LSV), electrochemical impedance spectroscopy (EIS), and UV-visible spectroscopy. The typical XRD patterns revealed that all Cu2O thin films were pure Cu2O phase. Different crystal morphologies of Cu2O could be obtained by varying pH. The Cu2O thin film was composed of a large number of star-like shape grains with the pH of 4.8. With a further increase of the pH, the Cu2O film showed the uniform dendrite morphology. LSV uncovered that E R (reduction potential of Cu2+ to Cu+) increased with the pH value enlargement. According to the EIS, diffusion coefficient of Cu2+ ion was reduced with the increase of the pH. The UV-vis absorption spectra revealed that the broad absorption peaks were from 300 to 500 nm and the evaluated band gap of the films was tunable in the range of 2.02–2.67 eV, with the variation of the pH values. According to the experiment and relevant literatures, the probable electrodeposition mechanism of the Cu2O was discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a non-stoichiometric semiconductor [1], with a direct band gap around 2.09 eV, cuprous oxide (Cu2O) has advantages of good gas sensitivity, non-toxicity, and low costs. Therefore, it is widely used in solar cells, gas sensors, magnetic storage, lithium ion batteries, biological sensors, microdevices, and negative electrodes [2–9]. The utilization efficiency of sunlight by Cu2O thin films can reach 20 % in theory [10], so most of research work has been done in solar energy conversion. However, the present report on the highest energy conversion efficiency is only 2.1 % [11], whose main reason is that it is difficult to control Cu2O surface morphology, particle size, and other semiconducting properties.
So far, many deposited methods have been intensively focused on the synthesis of Cu2O with a variety of morphologies, such as thermal oxidation [12], chemical vapor deposition [13], reactive sputtering [14], wet chemical reduction [15–17], chemical oxidation [18], electrodeposition [19–24], and so on. Among these techniques, electrodeposition is one of the most attractive ways to manufacture semiconductor oxide films over conductive substrates. It provides advantages such as the ability to use a low costs, low synthesis temperature, and large area deposition. Also, the thickness and morphology of the films can be controlled by adjusting the parameters such as the additive, substrate, electrolyte concentration, solution temperature, pH, and so on [25–28].
Shao et al. [29] reported the electrochemical deposition of Cu2O microcrystals on the FTO glass substrates. When varying the bath pH, Cu2O nanocrystalline changed from dendritic structure to quasi-cuboid structure. Paracchino et al. [7] studied the effects of solution temperature, pH, and deposition current density on the photoelectrochemical properties of Cu2O, and they found that the highest photocurrent obtained was −2.4 mA cm−2 at 0.25 V vs. reversible hydrogen electrode (RHE) for a film thickness of 1.3 μm.
According to the above, we can draw a conclusion that the deposition conditions play an important part in the microstructure and properties of Cu2O thin films. The bath pH can also affect the shape formation of crystals in various ways [30]. Its effect can interfere with diffusion limited growth. In this paper, a series of Cu2O films were electrodeposited using cupric acetate solutions with bath pH ranging from 4.8 to 5.8. The effects of bath pH on the morphological, microstructural, and electrochemical properties of the as-deposited Cu2O films were investigated in detail.
Experiment section
Materials
All of the chemical reagents were analytical grade and used without further purification. All the solutions in this study were prepared from deionized water which was further purified by a Millipore Milli-Q purification system (resistivity ≥18.2 MΩ·cm). Fluorinated tin oxide-covered glass substrates (10 Ω·cm−2, Xiang Town Technologies Ltd., China) were used as substrates and cleaned in four 10-min steps of cleaning in ultrasonicated acetone, ethanol, 1:1 H2O2/ammonia, and finally ultrapure water.
Preparation of Cu2O films
The electrodeposition was performed on a IM6 electrochemical workstation (Zahner, GER). Growth of Cu2O particles was carried out in a conventional three-electrode cell equipped with a carbon rod as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode. The working electrode was a FTO glass with a surface area of 30 × 20 mm2 exposed to the electrolyte. The electrolytes are 0.02 M Cu(Ac)2 and 0.1 M NaAc aqueous solution. The pH values of the electrolytes were modulated by adding acetic acid. The temperature of the electrolytes was maintained at 50 °C during the experiments. Electrodeposition was carried out under potentiostatic condition of −250 mV against the SCE for 300 s. The samples are deposited in the acidic electrolyte system. If the acidity is too strong, such as the pH smaller than 4, H+ would prompt Cu2O into Cu. When the pH is too high, such as the pH more than 6, it is difficult to deposit Cu2O on the conductive glass. Therefore, the samples were prepared at different bath pH (4.8, 5.0, 5.2, 5.4, 5.6, and 5.8). After deposition, the films were rinsed with copious amounts of double-distilled water, dried at room temperature.
Characterization
To determine the phase of the products, the crystallographic properties were carried out on a Philips X’Pert PRO X-ray diffractometer, using Cu Kα radiation, at a scanning rate of 2°/min in the 2θ range of 20–65°. The surface morphology of the Cu2O thin films were observed by scanning electron microscopy (SEM, Fei Quanta 200, USA). The transmission spectra of the thin films were measured with a UV-2550 UV-vis spectrophotometer (Shimadzu, Japan) ranging from 300 to 650 nm.
The electrochemical properties of the deposited samples were characterized by linear sweep voltammogram (LSV) and electrochemical impedance spectroscopy (EIS) performed on an IM6 electrochemical workstation system. The LSV measurements were performed in an aqueous solution of 0.1 M sodium acetate and 0.02 M cupric acetate with different pH in a standard three-electrode configuration coupled with the FTO (working electrode), a saturated calomel electrode (reference electrode), and a carbon rod (counter electrode). The EIS measurements were performed in an aqueous solution of 0.1 M sodium acetate and 0.02 M cupric acetate with different pH in a standard three-electrode configuration coupled with the sample films (working electrode), a saturated calomel electrode (reference electrode), and a carbon rod (counter electrode). The LSV measurements were operated at 10 mV·s−1 between −0.8 and 0.2 V (vs. SCE), and the EIS analyses were performed at the amplitude of 5 mV and at the frequency from 10 mHz to 100 kHz.
Results and discussion
Structural characterization of Cu2O thin films
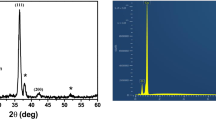
The XRD patterns for Cu2O thin films deposited on FTO conductive glass with different pH values are shown in Fig. 1. From Fig. 1a, no impurities such as cupric oxides are found in the XRD pattern besides the substrate, which means that single-phase Cu2O crystals can be obtained through electrodeposition method in this system. Figure 1b displays the enlarged peaks corresponding to (111) for Cu2O samples. According to JCPDS card No. 78-2076, the diffraction peak appearing at 2θ = 36.46° corresponds to the (111) plane of cubic Cu2O. The diffraction peak (111) shifted toward the region of small theta when the pH value was 4.8. According to Bragg equation, 2dsinθ = nλ (where d is the lattice plane distance value, θ the value of diffraction theta, and λ the wavelength of X-ray), it is obvious that as θ turns to be smaller, d will become larger accordingly. It indicated the crystalline lattice inflation. When the pH was greater than 4.8, the diffraction peak (111) shifted toward the region of larger theta and d turns to be smaller which lead to the crystalline lattice contraction.
To determine the preferred orientation of the Cu2O films, the intensity ratio I(111)/I(200) for the two most intense peaks is plotted as a function of the pH, and it is shown in Fig. 2. The relative ratio I(111)/I(200) value corresponding to a polycrystalline pure Cu2O sample without preferential orientation is indicated by a solid horizontal line, and it has a value of 2.88. Hence, if the ratio is smaller than 2.88, the Cu2O films deposited are formed in a [100] preferred orientation. Otherwise, the films reveal a [111] texture [31, 32]. Thus, it can be seen that pure Cu2O films as-prepared displayed a [100] preferential orientation. This is maybe caused by anisotropic growth of the dendritic crystal films [33].
Surface morphology analysis
SEM images of Cu2O crystals deposited at E = −0.25 V for 5 min in solutions containing 0.02 M copper acetate and 0.1 M sodium acetate at pH changing from 4.8 to 5.8 modulated with acetic acid are shown in Fig. 3.
It can be seen from Fig. 3a that particles of Cu2O with star-like shape are dispersed on FTO and the grain sizes are heterogeneous. The Cu2O crystalline grains are not completely covered on FTO conductive glass. When the pH increases to 5.0, the crystal particles developed along the apexes of the star-like morphology and many different branches were formed over a large area of the FTO (see Fig. 3b). With a further increase of the pH to 5.2–5.4, we found that the degree of branching gradually increased and more pronounced multiple branches were observed and formed a physically continuous crystal body (see Fig. 3c, d), which is consistent with the results reported by Jinbo Xue [34]. At a higher pH of 5.6–5.8, smaller grain size and dendrites were observed (Fig. 3e, f) and the dendrite morphology Cu2O film was compact on the upper part of FTO. The results indicate that the morphologies of the Cu2O films can be affected by the pH in electrolyte.
The EDX of Cu2O film deposited at E = −0.25 V for 5 min in solution containing 0.02 M copper acetate and 0.1 M sodium acetate at a pH of 4.8 is shown in Fig. 4a. We can see the elements of Cu, O, Sn, and Ca in the EDX. Sn and Ca elements are from the conductive layer and the glass layer. According to the analysis of XRD and EDX, we can determine that the thin film is Cu2O. Figure 4b is the macroscopic image of the Cu2O obtained at a pH of 4.8. The entire substrates really covered by Cu2O.
According to the relevant literatures and in combination with our experimental phenomenon, the possible formation process of the Cu2O thin films is proposed as follows. Electrodeposition of Cu2O involves two steps: the first step is reduction of Cu2+ ions to Cu+ ions (Eq. (R1)) and the second step is precipitation of Cu+ ions to Cu2O on the working electrode. (Eq. (R2)) [34, 35]:
The overpotential was generated for the cathodic deposition of Cu2O when a deposition potential was more negative than the reduction potential of Cu2+ to Cu+ ions (Eq. (R1)). The overpotential (η) is defined as the difference between the deposition potential (E D) and the reduction potential (E R) [36–38]:
The reduction potential of Cu2+ to Cu+ is described by the Nernst equation [37]:
where E° = 0.159 V and 2.303RT/nF = 0.05916 V at T = 298.15 K for the Cu2+/Cu+ redox couple. The solubility of Cu+ ions in an aqueous solution at T = 323.15 K is [36]
Thus, Eq. (2) at T = 323.15 K is reduced to
Therefore, the overpotential for Eq. (R1) is controlled by several factors including the deposition potential, solution pH, solution temperature, and concentration of Cu2+ in the solution. In this paper, we make sure that electrodeposition potential and electrodeposition temperature remained unchanged. According to Eq. (4), the reduction is determined by pH of the solution and the concentration of Cu2+ ion at the interfacial region. Since the concentration of Cu2+ precursor is constant, E R is linear correlation with the pH values [34]. In our experiment, E D is constant. Therefore, we can come to a conclusion that a larger overpotential (η) is established at higher solution pH, which is consistent with the discovery of Han et al. [39]. According to Eq. (3) and Eq. (R1), the high solubility of Cu+ ions at low pH values (<5.0) allows more Cu2+ ions to present in solution and promote the mass transport. Meanwhile, under the low pH, the reduction rate of Cu2+ ions is relatively slow and the consumption of Cu2+ ions at the interface can be quickly compensated by mass transport [40]. Under these conditions, crystals grow surrounded by sufficient Cu2+ ions and are allowed to form a shape that can reach a minimum surface energy, so faceted shapes were obtained. With the increase of pH, the increased overpotential facilitated the nucleation process in the electrodeposition. Cu2+ ions were consumed faster than the transport rate of Cu2+ ions to the interfacial region, so the concentration of Cu2+ ions were very low at the interface, which created a depletion zone around the growing Cu2O crystal [40, 41]. Once the depletion layer is formed, the apexes of a polyhedral crystal protrude further into the region of higher concentration and they can grow faster than the central parts of the facets; thus, dendritic branches are formed [34, 37, 40]. Therefore, branched crystals were produced at large pH because of the limitation of the transportation of Cu2+ ions (Fig. 3b). In this case, the degree of branching was expected to become more pronounced as the pH increases (Fig. 3c, d) [33]. When the pH reached 5.6 and 5.8, the primary branches of the dendrite grew together to form a surface coverage on the FTO and secondary branches of the dendrite were developed on the primary ones. In addition, the solubility of Cu2+ ions was extremely limited at high pH values, and Cu2+ ions rapidly precipitated out of solution as soon as they were generated electrochemically, which meant that they would quickly crystallize even if the morphology were not thermodynamically favorable (such as formation of branches).
Electrochemical properties
To better understand the relationship between reduction potential and pH values, the linear sweep voltammograms of Cu2O were further applied for studying the reduction potential of the cathode. The RHE scale compensates for pH and would be more helpful in the discussions vs. the pH-sensitive SCE scale. Therefore, we referenced the potential to the RHE. RHE was converted through the following relationship [42].
Figure 5 shows the linear sweep voltammograms of Cu2O films electrodeposited on FTO at room temperature. The scan was performed in the cathodic direction. The cathodic peak is associated with the Cu(II) to Cu(I) restored (chemical equation in Fig. 4). It can be observed that as the pH values varied from 4.8 to 5.8, the reduction potential peak increased from 0.07 V to 0.33 V. An increase in solution pH produces a more positive reduction potential and we can get the same result according to Eq. (4).
To gain further insight on the electrochemical performance, EIS is used to evaluate the Cu2+ diffusion coefficient and the dynamic process on the interface of working electrodes. Figure 6 exhibits the typical EIS (presented in the form of the Nyquist plot) of the samples. The typical Nyquist plot includes a semicircle portion (known as capacity reactance arc) in the high-frequency range and a straight line in the low-frequency range. The high-frequency semicircle is related to the migration of the Cu2+ ions at the electrode/electrolyte interface and charge transfer process. The shape and semidiameter of the semicircle is linked to charge-transfer resistance at the interface between the electrolyte and the cathode [43–46]. A line in the low-frequency range is associated with the Warburg diffusion of the Cu2+ ions in the electrolyte [47]. To better understand the dynamic process on the interface of working electrodes, we plotted the electrical equivalent circuit. The equivalent circuit parameters are the reflection of EIS data. The simulation circuit and the simulation results are shown in the inset of Fig. 6. According to the impedance spectrum, we can calculate the equivalent circuit parameters. The calculated equivalent circuit parameters for the different pH values in solution are presented in Table 1.
The electrochemical impedance plot of Cu2O in an electrolyte containing 0.1 M sodium acetate and 0.02 M cupric acetate with different pH values. Inset: the equivalent circuit model used in the fitting of the impedance data of the Cu2O films, R L = solution resistance, R P = charge transfer resistance of the electrical double layer (EDL), W = Warburg impedance element, and C d = double-layer capacitance
Figure 6a shows a small capacitive arc in the high frequency range, indicating that the electrode process is carried out quickly. In addition, the capacitive arc is not a complete semicircle: its center is located in the fourth quadrant of the complex plane impedance, indicating there is a serious “dispersion effect.” In terms of experience, diffusion effect is usually caused by the inhomogenous surface of the electrode [48]. The phenomenon indicates that Cu2O film deposited on FTO surface is very uneven. This is in agreement with the results observed in Fig. 3a.
With increasing the pH from 5.0 to 5.8, EIS showed a new feature. As shown in Fig. 6b–f, the capacitive arc diameters in the high-frequency range gradually increase, which indicates that the charge transfer resistance R p increases. This fact is consistent with the calculated equivalent circuit parameters in Table 1. We may safely draw the conclusion that electrochemical polarization increases, which is advantageous to the refinement of Cu2O crystallization and improves Cu2O adhesion strength on the FTO-coated glass [49]. In addition, the capacitive reactance arc in the high-frequency range is closer to semicircle, indicating a decrease of the dispersion effect. Hence, Cu2O films in the electrode surface become smooth, which is consistent with surface morphology observed in Fig. 3b–f. This means that it is easy to obtain a dense and a uniform Cu2O thin film in a high pH solution.
Diffusion coefficient plays an important role in the analysis of the electrodeposited process of Cu2O film. We can calculate the diffusion coefficient in different bath pH values by EIS. The inclined lines in the low-frequency range in Fig. 6 are attributed to a Warburg impedance. From the inclined lines in the Warburg region, the Cu2+ ion diffusion coefficient may be estimated using the following equation [49]:
where R is the gas constant, T is the absolute temperature, A is the surface area of the cathode, n is the number of transferred electrons per molecule during deoxidization, F is the Faraday constant, C 0 is the Cu2+ ion concentration in electrode material, σ is the Warburg coefficient, and D 0 is the Cu2+ diffusion coefficient. In addition, σ is related to Z″ (imaginary part of impedance, reactive resistance ), as shown in Eq. (7) [48–52].
Z″ and ω could be measured by the EIS. Therefore, the relationship between Z″ and reciprocal square root of frequency (ω −1/2) in the low-frequency region is shown in Fig. 7. From Eq. (7) and Fig. 7, the Warburg coefficient could be obtained.
Thus, the ratio of D 0/B could be obtained by Eq. (6), as is shown in Fig. 8, where B is a constant (B = R 2 T 2/2A 2 n 4 F 4 C 0 2).
Figure 8 shows the value of D 0/B decreases gradually with the increase of pH, which means that the diffusion coefficient D 0 also decreases. When the pH value is equal to 4.8, the diffusion coefficient D 0 is the largest and the transport capacity is the strongest. It is advantageous to fill Cu2+ ions into the clearance position. However, under the large pH (≥5.0) values, the Cu2+ ion diffusion transport speed becomes slow. And the rate of Cu2O deposition is greater than that of the Cu2+ diffusion; it will form a depletion region around the Cu2O nuclei. So, the dendritic branching growth in electrodeposition can be induced and result in the formation of dendritic branching Cu2O films.
Optical properties
Cu2O is a typical direct band gap semiconductor. The optical band gap E g was obtained from Eq. (8) [53, 54]:
Here, α is the absorption coefficient, A is a constant, hʋ is the discrete photon energy, and E g is the band gap energy. The absorption coefficient α can be obtained by the following formula:
where T is the transmittance and d is thickness of film, respectively. The thickness of Cu2O films is shown in Fig. 9.
Figure 10 illustrates the transmittance spectrum of the Cu2O film grown on FTO glass under the conditions of different pH 4.8, 5.0, 5.2, 5.4, 5.6, and 5.8. It can be seen that all films show a low transmission (smaller than 15 %) in the wavelength between 300 and 650 nm. Therefore, this phenomenon demonstrates a strong adsorption in the wavelength range from 300 to 650 nm, which is the characteristic absorption of Cu2O.
The band gap E g is obtained by extrapolation the linear section of the plot of (αhʋ)2 vs. hʋ, and the estimated direct band gaps of Cu2O films are listed in Table 2. It can be found that the absorption edges show redshift then blueshift in comparison with that of bulk Cu2O (2.09 eV), with the increasing of pH. The optical band gap of semiconductors could be severely affected by the defects, disorder at the grain boundaries, crystallinity, as well as the stress of the crystals, which could decrease the energy band gap with the presence of tensile stress in crystals [55–57]. According to the analysis of XRD, tensile stress existed in the crystals when the pH value was 4.8. It resulted in the band gap smaller according to the theory of quantum mechanics periodic potential [58, 59]. Therefore, it generated redshift in the spectrum of the Cu2O film when the pH value was 4.8. The band gaps of the samples for pH value of 5.0, 5.2, 5.4, 5.6, and 5.8 shows blueshift. The possible reason is the following. The crystalline lattice of Cu2O became shrunk, resulting in the decrease of the distance between the atoms, which indicated Cu2O crystals were under compressive stress. In this case, blueshift phenomenon would appear according to the theory of quantum mechanics periodic potential [58].
Conclusions
In summary, Cu2O with different morphologies and sizes were easily obtained on fluorine-doped tin oxide (FTO) covered glass substrates by potentiostatic deposition. LSV showed that reduction potential slightly increased with increasing pH. The impedance spectra indicated that the electrochemical polarization was become large and diffusion coefficient became small with increasing pH. Thus, we can get different morphologies and excellent performance of Cu2O thin films by changing the pH of the solution. With controlling the pH, the optical transmittance of the Cu2O was under 15 % in the wavelength range of 300–650 nm, and the optical band gaps of Cu2O thin films calculated from the transmission spectra were changing from 2.06–2.84 eV.
References
Orel ZC, Anzlovar A, Drazic G, Zigon M (2007) Cuprous oxide nanowires prepared by an additive-free polyol process. Cryst Growth Des 7:453–458
Kuo CH, Huang MH (2010) Morphologically controlled synthesis of Cu2O nanocrystals and their properties. Nano Today 5:106–116
Park JC, Kim J, Kwon H, Song H (2009) Gram-scale synthesis of Cu2O nanocubes and subsequent oxidation to CuO hollow nanostructures for lithium-ion battery anode materials. Adv Mater 21:803–807
Miyake M, Chen YC, Braun PV, Wiltzius P (2009) Fabrication of three-dimensional photonic crystals using multibeam interference lithography and electrodeposition. Adv Mater 21:3012–3015
Huang L, Peng F, Wang HJ, HaO Y, Li Z (2009) Preparation and characterization of Cu2O/TiO2 nano-nano heterostructure photocatalysts. Catal Commun 10:1839–1843
Wei HM, Gong HB, Chen L, Zi M, Cao BQ (2012) Photovoltaic efficiency enhancement of Cu2O solar cells achieved by controlling homojunction orientation and surface microstructure. J Phys Chem C 116:10510–10515
Paracchino A, Brauer JC, Moser JE, Thimsen E, Graetzel M (2012) Synthesis and characterization of high-photoactivity electrodeposited Cu2O solar absorber by photoelectrochemistry and ultrafast spectroscopy. J Phys Chem C 116:7341–7350
Osherov A, Zhu CQ, Panzer MJ (2013) Role of solution chemistry in determining the morphology and photoconductivity of electrodeposited cuprous oxide films. Chem Mater 25:692–698
McShane CM, Siripala WP, Choi KS (2010) Effect of junction morphology on the performance of polycrystalline Cu2O homojunction solar cells. J Phys Chem Lett 1:2666–2670
Cui JB, Gibson UJ (2010) A simple two-step electrodeposition of Cu2O/ZnO nanopillar solar cells. J Phys Chem C 114:6408–6412
Mittiga A, Salza E, Sarto F, Tucci M, Vasanthi R (2006) Heterojunction solar cell with 2 % efficiency based on a Cu2O substrate. Appl Phys Lett 88:163502 (2pp)
Lamberti A, Destro M, Bianco S, Quaglio M, Chiodoni A, Pirri CF, Gerbaldi C (2012) Facile fabrication of cuprous oxide nanocomposite anode films for flexible Li-ion batteries via thermal oxidation. Electrochim Acta 86:323–329
Bergerot L, Jiménez C, Pluchery OC, Rapenne L, Deschanvres JL (2015) Growth and characterization of Sr-doped Cu2O thin films deposited by metalorganic chemical vapor deposition. Phys Status Solidi A 212:1735–1741
Chu CL, Lu HC, Lo CY, Lai CY, Wang YH (2009) Physical properties of copper oxide thin films prepared by dc reactive magnetron sputtering under different oxygen partial pressures. Physica B 404:4831–4834
Xu HL, Wang WZ (2007) Template synthesis of multishelled Cu2O hollow spheres with a single-crystalline shell wall. Angew Chem Int Ed 46:1489–1492
Lu CH, Qi LM, Yang JH, Wang XY, Zhang DY, Xie JL, Ma JM (2005) One-pot synthesis of octahedral Cu2O nanocages via a catalytic solution route. Adv Mater 17:2562–2567
Huang L, Peng F, Yu H, Wang HJ (2009) Preparation of cuprous oxides with different sizes and their behaviors of adsorption, visible-light driven photocatalysis and photocorrosion. Solid State Sci 11:129–138
Ai ZH, Zhang LZ, Lee SC, Ho W (2009) Interfacial hydrothermal synthesis of Cu@Cu2O core-shell microspheres with enhanced visible-light-driven photocatalytic activity. J Phys Chem C 113:20896–20902
Siegfried MJ, Choi KS (2005) Directing the architecture of cuprous oxide crystals during electrochemical growth. Angew Chem Int Ed 44:3218–3223
Siegfried MJ, Choi KS (2006) Elucidating the effect of additives on the growth and stability of Cu2O surfaces via shape transformation of pre-grown crystals. J Am Chem Soc 128:10356–10357
Paracchino A, Laporte V, Sivula K, Grätzel M, Thimsen E (2011) Highly active oxide photocathode for photoelectrochemical water reduction. Nat Mater 10:456–461
Liau LCK, Lin YC, Peng YJ (2013) Fabrication pathways of p-n Cu2O homojunction films by electrochemical deposition processing. J Phys Chem C 117:26426–26431
Jeong YS, Kim H, Lee HS (2013) Growth and characterization of p-Cu2O/n-ZnO nanorod heterojunctions prepared by a two-step potentiostatic method. J Alloys Compd 573:163–169
Wei HM, Gong HB, Wang YZ, Hu XL, Chen L, Xu HY, Liu P, Cao BQ (2011) Three kinds of Cu2O/ZnO heterostructure solar cells fabricated with electrochemical deposition and their structure-related photovoltaic properties. CrystEngComm 13:6065–6070
Li Q, Xu MM, Fan HQ, Wang HR, Peng BL, Long CB, Zhai YC (2013) Electrical charge conductivity behavior of electrodeposited Cu2O/ZnO heterojunction thin films on PET flexible substrates by impedance spectroscopy analysis. J Mater Sci 48:3334–3340
Sun F, Guo YP, Song WB, Zhao JZ, Tang LQ, Wang ZC (2007) Morphological control of Cu2O micro-nanostructure film by electrodeposition. J Cryst Growth 304:425–429
Tang YW, Chen ZG, Jia ZJ, Zhang LS, Li JL (2005) Electrodeposition and characterization of nanocrystalline cuprous oxide thin films on TiO2 films. Mater Lett 59:434–438
Ghadimkhani G, de Tacconi NR, Chanmanee W, Janaky C, Rajeshwar K (2013) Efficient solar photoelectrosynthesis of methanol from carbon dioxide using hybrid CuO-Cu2O semiconductor nanorod arrays. Chem Commun 49:1297–1299
Shao F, Sun J, Gao L, Luo JQ, Liu YQ, Yang SW (2012) High efficiency semiconductor-liquid junction solar cells based on Cu/Cu2O. Adv Funct Mater 22:3907–3913
Liu XM, Zhou YC (2005) Electrochemical deposition and characterization of Cu2O nanowires. Appl Phys A Mater Sci Process 81:685–689
Bijani S, Martnez L, Gabas M, Dalchiele EA, Ramos-Barrado J-R (2009) Low-temperature electrodeposition of Cu2O thin films: modulation of micro-nanostructure by modifying the applied potential and electrolytic bath pH. J Phys Chem C 113:19482–19487
Bao M, Wang D, Liu SY, Kuang LL, Sun J, Wang F, Wen YX (2012) Electrodeposition and electrocatalytic activity of Cu2O film on stainless steel substrate. Appl Surf Sci 258:8008–8014
Zhao WY, Fu WY, Yang HB, Tian CJ, Li MH, Li YX, Zhang L, Sui YM, Zhou XM, Chen H, Zou GT (2011) Electrodeposition of Cu2O films and their photoelectrochemical properties. CrystEngComm 13:2871–2877
Xue JB, Liang W, Liu XG, Shen QQ, Xu BS (2012) Crystallization behavior and formation mechanism of dendrite Cu2O crystals. CrystEngComm 14:8017–8022
Pourbaix M (1974) Atlas of electrochemical equilibria in aqueous solutions. National Association of Corrosion Engineers, Houston, pp. 384–392
McShane CM, Choi KS (2009) Photocurrent enhancement of n-type Cu2O electrodes achieved by controlling dendritic branching growth. J Am Chem Soc 131:2561–2569
Siegfried MJ, Choi K-S (2008) Elucidation of an overpotential-limited branching phenomenon observed during the electrocrystallization of cuprous oxide. Angew Chem Int Ed 47:368–372
Huang L, Peng F, Wang HJ, Yu H, Geng W, Yang J, Zhang SQ, Zhao HJ (2011) Controlled synthesis of octahedral Cu2O on TiO2 nanotube arrays by electrochemical deposition. Mater Chem Phys 130:316–322
Han K, Kang F, Han XF, Tao M (2012) Enhanced crystal grain size by bromine doping in electrodeposited Cu2O. Thin Solid Films 520:5239–5244
Lopez CM, Choi K-S (2006) Electrochemical synthesis of dendritic zinc films composed of systematically varying motif crystals. Langmuir 22:10625–10629
Brady RM, Bal RC (1984) Fractal growth of copper electrodeposits. Nature 309:225–229
Wong EM, Searson PC (1999) ZnO quantum particle thin films fabricated by electrophoretic deposition. Appl Phys Lett 74:2939–2941
Huang S, Luo WJ, Zou ZG (2013) Band positions and photoelectrochemical properties of Cu2ZnSnS4 thin films by the ultrasonic spray pyrolysis method. J Phys D Appl Phys 46:235108 (6pp)
Li LX, Tang XC, Liua HT, Qu Y, Lua ZG (2010) Morphological solution for enhancement of electrochemical kinetic performance of LiFePO4. Electrochim Acta 56:995–999
Wang L, Wang HB, Liu ZH, Xiao C, Dong SM, Han PX, Zhang ZY, Zhang XY, Bi CF, Cui GL (2010) A facile method of preparing mixed conducting LiFePO4/graphene composites for lithium-ion batteries. Solid State Ionics 181:1685–1689
Wang YR, Yang YF, Yang YB, Shao HX (2010) Enhanced electrochemical performance of unique morphological LiMnPO4/C cathode material prepared by solvothermal method. Solid State Commun 150:81–85
Pasquale MA, Gassa LM, Arvia AJ (2008) Copper electrodeposition from an acidic plating bath containing accelerating and inhibiting organic additives. Electrochim Acta 53:5891–5904
Jia Z, Dai CS, Chen L (2006) Electrochemical measurement methods. Chemical industry press, Peking, pp. 170–173
Zhang JQ (2011) Electrochemical measurement technology. Chemical industry press, Peking, pp. 220–225
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York, pp. 381–385
Hu HL (2007) Electrochemical measurement. National Defence Industry Press, Peking, pp. 212–229
Zhou YK, Wang J, Hu YY, O’Hayreb R, Shao ZP (2010) A porous LiFePO4 and carbon nanotube composite. Chem Commun 46:7151–7153
Tauc J, Menth A (1972) States in the gap. J Non-Cryst Solids 8(10):569–585
Liu YL, Liu YC, Mu R, Yang H, Shao CL, Zhang JY, Lu YM, Shen DZ, Fan XW (2005) The structural and optical properties of Cu2O films electrodeposited on different substrates. Semicond Sci Technol 20:44–49
Dow JD, Redfield D (1972) Toward a unified theory of Urbach’s rules and exponential absorption edges. Phys Rev B 5:594–610
Revathi N, Prathap P, Subbaiah YPV, Reddy KTR (2008) Substrate temperature dependent physical properties of In2S3 films. J Phys D Appl Phys 41:155404 (10pp)
Dong GB, Zhang M, Wang M, Li YZ, Li CR, Li H, Huang AP, Yan H (2012) Effect of substrate temperature on the structure and properties of Cu2O thin rilms. J Synth Cryst 41:457–460
Qiao ZG (2005) Semiconductor physics. Zhejiang university press, Zhejiang, pp. 56–64
Kittel C (1996) Introduction to solid state physics. Wiley, New York, pp. 121–125
Acknowledgments
The authors thank the ‘863’ project of China (No. 2009AA03Z427) for providing research grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, G., Huang, Y., Fan, Q. et al. Effects of bath pH on structural and electrochemical performance of Cu2O. Ionics 22, 2213–2223 (2016). https://doi.org/10.1007/s11581-016-1734-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1734-x