Abstract
N-doped graphene/SnS composite as high-performance anode materials has been synthesized by a simultaneous solvothermal method using ethylene glycol as solvent. The morphology, structure, and electrochemical performance of N-doped graphene/SnS composite were investigated by transmission electron microscope (TEM), X-ray diffraction (XRD), Raman spectra, Fourier transform infrared (FTIR) spectra, X-ray photoelectron spectroscopy (XPS), and electrochemical measurements. The SnS nanoparticles with sizes of 3–5 nm uniformly distribute on the N-doped graphene matrix. The N-doped graphene/SnS composite exhibits a relatively high reversible capacity and good cycling stability as anode materials for lithium ion batteries. The good electrochemical performance can be due to that the N-doped graphene as electron conductor improves the electronic conductivity of composite and elastic matrix accommodates the large volume changes of SnS during the cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium ion batteries with high energy and powder densities have been wildly applied in various portable electronic and storage devices. Carbon material as anode materials for lithium ion batteries is far below in meeting the demands of high-performance lithium ion batteries due to the low theoretical capacity of 372 mAh g−1. Sn-based materials have been considered as potential candidate as anode materials in lithium ion batteries due to their high theoretical capacities [1]. However, the huge volume changes of Sn-based materials during lithium ions insertion and extraction process cause the rapid capacity fading, which impedes the practical utilization of Sn-based materials as anode materials [2]. During the recent years, SnO2 and SnO2-based composite have been widely investigated in various Sn-based materials [3–7]. In addition, SnS and SnS2 materials have also been synthesized and exhibited good electrochemical performance as anode materials for lithium ion batteries [8–10]. SnS as anode materials for lithium ion batteries has high theoretical specific capacity, and low lost and suitable working potential. To solve the problem of capacity fading for SnS electrode caused by large volume changes during cycles, one effective way is preparation of the SnS/C composite to enhance the cycling stability [11–13]. SnS/C composite can combine the advantages of carbon (good cycling stability and long life) and SnS (high specific capacity) because carbon as a buffer can alleviate the volume stress and maintain the structural integrity of electrode materials during the discharge-charge process.
Among various carbon materials, graphene with excellent conductivity, good chemical stability, and large surface area has been widely studied in lithium ion batteries to improve the electronic conductivity and cycling stability of the composite electrodes [4, 14–20]. In addition, graphene is an effective nanoscale block for preparation of composite materials with metal oxide or metal sulfur nanoparticles. Therefore, graphene is deemed to an ideal matrix to improve the electrochemical performance of SnS-based materials. Recently, SnS/graphene and SnS2/graphene composites have been synthesized to exhibit the improved electrochemical performance by hydrothermal method [21, 22].
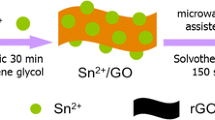
Herein, we report the preparation of N-doped graphene/SnS composite by one-step solvothermal route using the ethylene glycol as solvent. Compared with the hydrothermal method using the deionized water as solvent [21], ethylene glycol acts not only as solvent but also as reducing agent during the solvothermal process, which can ensure the effective reducing of graphene oxide (GO) to graphene during the solvothermal process [23]. The formation of SnS nanoparticles and the reducing GO to graphene occur simultaneously, which ensures the uniform distribution of SnS nanoparticles on the graphene matrix. The N-doped graphene/SnS composite delivers an enhanced electrochemical performance as anode materials for lithium ion batteries. The N-doped graphene in the composite improves the electronic conductivity and prevents the aggregation of SnS nanoparticles. Moreover, the N-doped graphene as elastic matrix can accommodate the large volume change of SnS during lithium ions insertion/extraction and maintain the structural integrity of composite electrode. The introduction of N atoms during solvothermal process can decrease the energy barrier for Li+ penetration and increase the defect of graphene, which increases the lithium ions storage capacity.
Experimental
Synthesis of N-doped graphene/SnS composite
Graphite oxide was synthesized by modified Hummers method using natural graphite [24]. Graphite oxide was exfoliated by ultrasound to form GO solution. In a typical preparation of N-doped graphene/SnS composite process, 5-mL GO suspension (25 mg/mL) was firstly dispersed in ethylene glycol (35 mL). SnCl2 · 2H2O (5 mmol) and thiourea (5 mmol) were added into this solution under vigorous stirring for 60 min. The mixed solution was transferred into the 50-mL Teflon-lined stainless steel autoclave and then heated in oven at 180 °C for 24 h. The black precipitate was washed with deionized water and ethyl alcohol repeatedly for several times. The product was dried at 80 °C under vacuum for 24 h. For comparison, pure SnS was prepared under the same condition without adding the GO suspension.
Structural characterization
The microstructure and morphology of the samples were characterized by field emission scanning electron microscopy (FESEM, JSM-7500F), transmission electron microscopy (TEM, JEM-2100F), and high-resolution TEM (HRTEM). The composites were characterized by X-ray diffraction (XRD) patterns on a Rigaku D/max-2500 diffractometer with Cu Ka irradiation (0.1542 nm). Raman spectra were collected on a Horiba Jobin-Yvon T6400 Raman spectrometer. X-ray photoelectron spectroscopy (XPS) measurements were carried out with on an AXIS-ULTRA DLD-600W spectrometer. Fourier transform infrared (FTIR) spectra were measured on a NEXUS FTIR spectrometer from 500 to 4,000 cm−1. Thermogravimetric analysis (TGA, STA449C) was carried out in air atmosphere from 35 to 1,000 °C.
Electrochemical characterization
The electrochemical properties of the composite were measured using the coin cells. In a typic fabrication process of the electrodes, the working electrodes were prepared by mixing active materials (SnS and N-doped graphene/SnS composite), carbon black, and polyvinylidene fluoride (PVDF) at a weight ratio of 70:20:10 and then pasted on a copper foil. The electrolyte is 1 mol L−1 LiPF6 dissolved in a mixture of ethylene carbonate and dimethyl carbonate with a volume ratio of 1:1. Lithium metal was used as the counter electrode, and the separator was a microporous polypropylene film. The cells were assembled in a glove box filled with pure argon atmosphere. The active material was measured to be about 2 mg/cm2. Galvanostatic discharge/charge measurements were performed at a current density of 50 mA g−1 over a voltage range of 0.01–3 V vs. Li/Li+. The rate performance of N-doped graphene/SnS composite was carried out at current densities of 100, 200, and 500 mA g−1. Cyclic voltammetry (CV) measurements were carried out using a CHI660C electrochemical workstation (Shanghai Chenhua) between 0.01 and 3 V with a scan rate of 0.2 mV s−1. Electrochemical impedance spectroscopy (EIS) measurements were tested on a CHI660C electrochemical workstation (Shanghai Chenhua) with amplitude of 5 mV in the frequency range from 0.01 Hz to 100 kHz. Full cells were further assembled using commercial LiCoO2 as cathode and N-doped graphene/SnS as anode. The cathode was prepared by mixing commercial LiCoO2, carbon black, and PVDF at a weight ratio of 80:10:10. The molar mass ratio of LiCoO2 to N-doped graphene/SnS composite in the full cells is about 9:1. Galvanostatic charge/discharge tests of full cells were carried out at a rate of 20 mA g−1 between 2.0 and 4.3 V. All of electrochemical tests were carried out at 25 °C.
Results and discussion
A schematic sketch of the preparation of N-doped graphene/SnS composite is shown in Fig. 1. Firstly, Sn2+ was adsorbed on the GO sheets by electrostatic interaction due to the presence of O-containing functional group. During the solvothermal process, the Sn2+ was converted into SnS nanoparticles, and GO was reduced to graphene. The SnS nanoparticles attach to the graphene nanosheets during the solvothermal process, which provides the intimate contact between SnS and graphene and ensures the unique structure of composite.
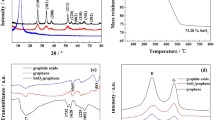
The crystallographic structures of the SnS and N-doped graphene/SnS composite were identified by XRD as shown in Fig. 2. There is a peak at about 10.6° for the graphite oxide, suggesting the successful oxidation of graphite. For the SnS, all the diffraction peaks are in good agreement with the standard data for pure phase of orthorhombic SnS structure (JCPDS Card No. 014-0620). The characteristic (110), (120), (021), (101), (111), (040), and (131) planes at about 22.1°, 26.2°, 27.6°, 30.6°, 31.9°, 33.8°, and 39.2° are observed in the SnS. In the case of N-doped graphene/SnS composite, the similar XRD patterns to pure SnS are observed, indicating that the introduction of GO does not influence the fabrication of SnS nanostructure during the solvothermal process. No diffraction patterns for graphene are observed in N-doped graphene/SnS composite, indicating the low intensity and low amount of graphene in the composite.
FTIR spectra of GO and N-doped graphene/SnS were also characterized as shown in Fig. 3a. There are some O-containing stretches, such as -OH stretch at 3,415 cm−1, carboxy C-O stretch at 1,380 cm−1, and alkoxy C-O stretch at 1,060 cm−1 in the GO, but these stretches disappeared in the N-doped graphene/SnS composite, indicating the successful reduction process of GO to graphene during the solvothermal process. The reduction of GO during the solvothermal process is further confirmed by Raman spectra. In the Raman spectrum of GO, the broad peaks displayed at about 1,352 and 1,585 cm−1 are attributed to the D band and G band of carbon materials. The Raman spectrum for N-doped graphene/SnS composite presents D band and G band located at 1,347 and 1,590 cm−1. Besides the similarity of Raman spectra between GO and N-doped graphene/SnS composite, it is noted that the intensity ratio of D band to G band for N-doped graphene/SnS is 1.12. In comparison with the value of 0.91 for GO, the increased intensity of D band to G band indicates a decrease in the size of the in-plane sp2 domains and a partially ordered crystalline structure of graphene [25]. This result is agreement with the FTIR spectra, indicating the successful reduction of GO to graphene during the solvothermal process.
XPS spectra were used to characterize the elemental composition of N-doped graphene/SnS composite. The XPS spectrum displays the presence of Sn, S, C, O, and N atoms (Fig. 4a). The peaks at about 495.5 and 487.1 eV are corresponding to the binding energy of Sn3d3/2 and Sn3d5/2 (Fig. 4b), and the peak at about 162.9 eV is attributed to the binding energy of S2p3/2 (Fig. 4c). The high-resolution C1s XPS spectrum of N-doped graphene/SnS composite shows two peaks at about 284.5 and 285.5 eV after fitting, which are corresponding to the C-C or C = C bonds and the C-O or C-N bonds, respectively [26] (Fig. 4d). The high-resolution N1s XPS spectrum of N-doped graphene/SnS composite displays two peaks at about 400.0 and 401.1 eV, which can be attributed to the pyrrolic N and graphitic N, respectively [27] (Fig. 4e).
The pure SnS particles with sizes of hundreds of nanometer agglomerate each other (Fig. 5a). Figure 5b shows the TEM images of N-doped graphene/SnS composite, which displays a typical lamellar structure. A large amount of nanoparticles are uniformly distributed on the graphene, as shown in the inset of Fig. 5b. The nanoparticles with sizes of 3–5 nm are uniformly distributed on the graphene sheets (Fig. 5c). Some nanoparticles show clear lattice fringes, and the lattice fringes with a distance of 0.34 nm were clearly observed, which can be indexed as the (120) plane of orthorhombic SnS (Fig. 5d).
TGA was carried out to determine the carbon content in the composite. From Fig. 6, the initial weight loss can be due to the evaporation of adsorbed water from room temperature to 200 °C. The weight loss can be mainly attributed to the transformation from SnS to SnO2 and the removal of N-doped graphene. The weight content of N-doped graphene in the composite can be calculated to be 17.8 %.
In order to demonstrate the improved electrochemical performance of N-doped graphene/SnS composite, galvanostatic discharge (lithium ions insertion) and charge (lithium ions extraction) measurements were carried out on both SnS and N-doped graphene/SnS composite. Figure 7 depicts the 1st, 2nd, 10th, 20th, and 30th discharge/charge typical voltage profiles of the SnS and N-doped graphene/SnS electrodes at a rate of 50 mA g−1 between 0.01 and 3.0 V. The capacity values are calculated according to the total weight of composite. The first discharge curve for SnS electrode shows an obvious plateau at about 1.3 V, which corresponds to the decomposition of SnS and the formation of SEI layer [11]. While the plateau decreases to about 1.0 V for the N-doped graphene/SnS electrode during the first discharge process, indicating that the N-doped graphene can cause the decreased voltage for the formation of SEI layer and the decomposition of SnS. The SnS electrode shows a discharge capacity of 1,031 mAh g−1 and reversible capacity of 688 mAh g−1 during the first cycle, respectively. The reversible capacity for SnS electrode is only about 492 mAh g−1 during the second cycle. The N-doped graphene/SnS composite delivers a high initial discharge capacity of 1,250 mAh g−1 with a coulombic efficiency of 56 % in the first discharge/charge cycle. The large area of N-doped graphene/SnS composite and defect of N-doped graphene consume more lithium ions than SnS during the first cycle, which causes the low coulombic efficiency of composite. During the second cycle, the reversible capacity of 682 mAh g−1 is obtained, and the corresponding coulombic efficiency is 90 % during the second cycle. The initial capacity loss is mainly due to the formation of SEI layer and decomposition of electrolyte [28].
Figure 8a shows the cycling performance of SnS and N-doped graphene/SnS composite at a current density of 50 mA g−1. The SnS electrode displays an initial reversible capacity of 688 mAh g−1, which decreases to 64 mAh g−1 after 30 cycles. In comparison, the initial charge capacity of N-doped graphene/SnS composite remains at around 699 mAh g−1, which decreases to 592 mAh g−1 after 10 cycles. During the following cycles, the reversible capacity keeps relatively stable. After 60 cycles, the reversible capacity of N-doped graphene/SnS composite remains at 466 mAh g−1, indicating the good cycling stability and reversibility of the electrochemical reactions. The rate performance of N-doped graphene/SnS composite at different current densities is presented in Fig. 8b. The reversible capacities are about 450, 320, and 230 mAh g−1 at current densities of 100, 200, and 500 mA g−1. The improved electrochemical performance can be attributed to the N-doped graphene, which improves the electronic conductivity of the composite and prevents the agglomeration of SnS nanoparticles. In addition, the spacing between graphene can be used as channels for lithium ion transportation and also facilitates the penetration of electrolyte in electrode material. The residual oxygen-containing functional groups of graphene may enhance the adhesion between SnS nanoparticle and graphene sheets, which maintains the structural integrity of electrode.
The first three CV curves of N-doped graphene/SnS composite are shown in Fig. 9. The CV shows a cathodic peak at about 1.0 V during the first cycle. According the reported literature [29], the peak is due to the formation of SEI layer and the decomposition of SnS, SnS + xLi+ + xe−1 ↔ Li x S + Sn. Since the reaction is irreversible, SEI layer is formed in the first cycle. Therefore, the cathodic peak at about 1.0 V disappears during the following cycles. During the more negative scanning direction, the second cathodic peak appears from 0.3 to 0 V. This peak repeatedly appears at the same potential for the following cycles presented in the curves. This peak is attributed to the intercalation of lithium ions into Sn, Sn + xLi+ + xe−1 ↔ Li x Sn. During the anodic scanning process, the de-intercalation of lithium ions from the LixSn emerges at about 0.75 V, Li x Sn ↔ Sn + xLi+ + xe−1. The cathodic peak at about 1.3 V during the second cycle and the anodic peak at about 1.9 V during the first cycle are due to the reaction of polysulfides [30].
To further understand the improved electrochemical performance, EIS measurements on SnS and N-doped graphene/SnS composite were performed after 3 cycles in the charged state. The Nyquist plots of both SnS and N-doped graphene/SnS composite show a depressed semicircle at high frequency and an incline line at low frequency (Fig. 10a). The high-frequency semicircle is related to the overlap of between SEI layer and charge-transfer resistance, and the low frequency incline line is ascribed to the Li ion diffusion process. The equivalent circuit model was shown in Fig. 10b to simulate the kinetic process for the N-doped graphene/SnS composite as anode materials [31]. R e is related to the resistance for lithium ion transport in electrolyte. R f and CPE1 are associated with the resistance for lithium ion migration through the SEI layer and SEI capacitance. R ct, CPE2, and Warburg impedance Z w are corresponding to charge-transfer resistance at the electrode and electrolyte interface, double-layer capacitance, and the diffusion of lithium ions in electrode materials, respectively. According the fitting results, the resistances R f and R ct of the cells decrease from 13.5 and 61.5 Ω for the SnS to 5.7 and 28.7 Ω for the N-doped graphene/SnS composite, indicating a better electrical conductivity of the N-doped graphene/SnS electrodes (Table 1). The improved electrochemical performance may be due to the increased electric conductivity of N-doped graphene/SnS composite and enhanced interfacial stability of between composite electrode and electrolyte during the cycling process.
The electrochemical performance of full cell using commercial LiCoO2 as cathode and N-doped graphene/SnS composite as anode was further investigated. During the charge process, lithium ions are removed from the LiCoO2 lattice, transferred into the electrolyte, and intercalated into the SnS lattice and the interlayer of graphene at the anode. This process is reversed during discharge. Therefore, the lithium ions shuttle between the two electrodes, while the electrodes flow through the external circuit during the charge/discharge process. Figure 11 shows the first 5-V profiles of charge and discharge at a current density of 20 mA g−1, exhibiting a reversible capacity of 625 mAh g−1 (based on the N-doped graphene/SnS composite) for the first cycle. The full cell also shows slightly decreasing galvanostatic charge-discharge performance during the first 5 cycles. The full cell achieves a high discharge-specific capacity of 458 mAh g−1 after 5 cycles, indicating that the N-doped graphene/graphene composite is a promising high-capacity anode material in the full cell.
Conclusion
In summary, a one-step and simple solvothermal method is employed to synthesize N-doped graphene/SnS composite as anode materials for lithium ion batteries. The N-doped graphene/SnS composite displays good reversibility and excellent capacity stability. The resulting N-doped graphene/SnS composite shows a high capacity of 466 mAh g−1 over 60 cycles. The N-doped graphene with good conductivity and high surface area provides an excellent electron transport network. The N-doped graphene matrix also accommodates the volume change of the SnS-based electrode, and the SnS also prevents aggregation of graphene nanosheets. The same strategy can be developed and explored to other materials with large volume change during electrochemical reaction or graphene-based composite for lithium ion batteries.
References
Idota Y, Kubota T, Matsufuji A, Maekawa Y, Miyasaka T (1997) Tin-based amorphous oxide: a high-capacity lithium-ion-storage material. Science 276:1395–1397
Vaughey JT, O’Hara J, Thackeray MM (2000) Intermetallic insertion electrodes with a zinc zlende‐type structure for Li batteries: a study of LixInSb (0 ≤ x ≤ 3). Electrochem Solid State Lett 3:13–16
Lin J, Peng Z, Xiang C, Ruan G, Yan Z, Natelson D, Tour JM (2013) Graphene nanoribbon and nanostructured SnO2 composite anodes for lithium ion batteries. ACS Nano 7:6001–6006
Guo Q, Zheng Z, Gao H, Ma J, Qin X (2013) SnO2/graphene composite as highly reversible anode materials for lithium ion batteries. J Power Sources 240:149–154
Wang X, Zhou X, Yao K, Zhang J, Liu Z (2011) A SnO2/graphene composite as a high stability electrode for lithium ion batteries. Carbon 49:133–139
Paek SM, Yoo EJ, Honma I (2009) Enhanced cyclic performance and lithium storage capacity of SnO2/Graphene nanoporous electrodes with three-dimensionally delaminated flexible structure. Nano Lett 9:72–75
Zhou X, Wang LJ, Guo YG (2013) Binding SnO2 nanocrystals in nitrogen-doped graphene sheets as anode materials for lithium-ion batteries. Adv Mater 25:2152–2157
Lu J, Nan CY, Li L, Peng Q, Li YD (2013) Flexible SnS nanobelts: Facile synthesis, formation mechanism and application in Li-ion batteries. Nano Res 6:55–64
Seo JW, Jang JT, Park SW, Kim C, Park B, Cheon J (2008) Two-dimensional SnS2 nanoplates with extraordinary high discharge capacity for lithium ion batteries. Adv Mater 20:4269–4273
Kim TJ, Kim C, Son D, Choi M, Park B (2007) Novel SnS2-nanosheet anodes for lithium-ion batteries. J Power Sources 167:529–535
Li Y, Tu JP, Huang XH, Wu HM, Yuan YF (2007) Net-like SnS/carbon nanocomposite film anode material for lithium ion batteries. Electrochem Commun 9:49–53
Cai J, Li Z, Shen PK (2012) Porous SnS nanorods/carbon hybrid materials as highly stable and high capacity anode for Li-ion batteries. ACS Appl Mater Inter 4:4093–4098
Li Y, Tu JP, Huang XH, Wu HM, Yuan YF (2006) Nanoscale SnS with and without carbon-coatings as an anode material for lithium ion batteries. Electrochim Acta 52:1383–1389
Wang X, Cao XQ, Bourgeois L, Guan H, Chen S, Zhong Y, Tang DM, Li HQ, Zhai TY, Li L, Bando Y, Golberg D (2012) N-doped graphene-SnO2 sandwich paper for high-performance lithium-ion batteries. Adv Funct Mater 22:2682–2690
Xu CH, Sun J, Gao L (2012) Direct growth of monodisperse SnO2 nanorods on graphene as high capacity anode materials for lithium ion batteries.J Mater Chem 22:975–979
Tao HC, Fan LZ, Yan XQ, Qu XH (2012) In situ synthesis of TiO2–graphene nanosheets composites as anode materials for high-power lithium ion batteries. Electrochim Acta 69:328–333
Pan Q, Xie J, Liu SY, Cao GS, Zhu TJ, Zhao XB (2013) Facile one-pot synthesis of ultrathin NiS nanosheets anchored ongraphene and the improved electrochemical Li-storage properties. RSC Adv 3:3899–3906
Fei L, Lin Q, Yuan B, Chen G, Xie P, Li Y, Xu Y, Deng SG, Smirnov S, Luo HM (2013) Reduced graphene oxide wrapped FeS nanocomposite for lithium-ion battery anode with improved performance. ACS Appl Mater Inter 5:5330–5335
Huang Y, Huang XL, Lian JS, Xu D, Wang LM, Zhang XB (2012) Self-assembly of ultrathin porous NiO nanosheets/graphene hierarchical structure for high-capacity and high-rate lithium storage. J Mater Chem 22:2844–2847
Wang ZL, Xu D, Wang HG, Wu Z, Zhang XB (2013) In situ fabrication of porous graphene electrodes for high-performance energy storage. ACS Nano 7:2422–2430
Tao HC, Yang XL, Zhang LL, Ni SB (2014) One-step in situ synthesis of SnS/graphene nanocomposite with enhanced electrochemical performance for lithium ion batteries. J Electroanal Chem 728:134–139
Chang K, Wang Z, Huang G, Li H, Chen WX, Lee JY (2012) Few-layer SnS2/graphene hybrid with exceptional electrochemical performance as lithium-ion battery anode. J Power Sources 201:259–266
Liu Y, Zhang Y, Ma G, Wang Z, Liu K, Liu H (2013) Ethylene glycol reduced graphene oxide/polypyrrole composite for supercapacitor. Electrochim Acta 88:519–525
Hummers W, Ofleman R (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Stankovich S, Dikin DA, Piner RD, KohIhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Xiang XX, Liu E, Huang Z, Shen S, Tian Y, Xiao C, Yang J, Mao Z (2011) Microporous carbon derived from polyaniline base as anode material for lithium ion secondary battery. Mater Res Bull 46:1266–1271
Lin Z, Waller GH, Liu Y, Liu M, Wong CP (2013) 3D Nitrogen-doped graphene prepared by pyrolysis of graphene oxide with polypyrrole for electrocatalysis of oxygen reduction reaction. Nano Energ 2:241–248
Liu Y, Hanai K, Yang J, Imanishi N, Hirano A, Takeda Y (2004) Silicon/carbon composites as anode materials for Li-ion batteries. Electrochem Solid State Lett 7:A369–A372
Arakawa M, Yamaki J (1987) The cathodic decomposition of propylene carbonate in lithium batteries. J Electroanal Chem 219:273–280
Wang J, Yang J, Wan C, Du K, Xie J, Xu N (2003) Sulfur composite cathode materials for rechargeable lithium batteries. Adv Funct Mater 13:487–492
Su LW, Zhou Z, Ren MM (2010) Core double-shell Si@SiO2@C nanocomposites as anode materials for Li-ion batteries. Chem Commun 46:2590–2592
Acknowledgments
The authors acknowledge financial supports from the Natural Science Foundation of China (NSFC, 51272128, 51302152, 51302153, 51402168) and Excellent Youth Foundation of Hubei Scientific Committee (2011CDA093). The authors are grateful to Dr. Jianlin Li at China Three Gorges University for his kind support to our research.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhu, SC., Tao, HC., Yang, XL. et al. Synthesis of N-doped graphene/SnS composite and its electrochemical properties for lithium ion batteries. Ionics 21, 2735–2742 (2015). https://doi.org/10.1007/s11581-015-1490-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1490-3