Abstract
A new carbon coating method for improving the performance of anode material of lithium ion battery is proposed in this paper. In this method, cetyl trimethyl ammonium bromide (CTAB) is used as dispersant and phenolic resin formed in situ on Li4Ti5O12-TiO2 is used as carbon precursor; thus, uniform coated sample with low carbon content is achieved. Three samples with different carbon contents are prepared, and their carbon content, morphology, structure, and electrochemical performance are investigated by thermogravimetry, scanning electron microscopy, transmission electron microscopy, X-ray diffraction, electrochemical impedance spectroscopy, and charge-discharge test. It is found that the sample with a carbon content of as low as 2.5 wt.% exhibits superior electrochemical performance. It delivers an initial capacity of 162.4 mAh g−1 with capacity retention of 95 % after 200 cycles at 1 C rate. When cycled at a higher rate of 5 C, the sample delivers a capacity of 148 mAh g−1 with no apparent capacity decaying after 90 cycles. The superior performance of the developed anode can be attributed to the uniform carbon coating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium ion battery is believed to be a promising alternative power source for vehicles to reduce the emission from conventional power sources such as gasoline- or diesel-powered internal combustion engines [1–4]. As the power source of electric vehicles, the safety and rate performance of lithium ion battery need to be improved [5–10]. The conventional lithium ion battery based on graphite anode cannot meet these requirements, because graphite has a lithium insertion potential as low as metal lithium, at which the organic liquid electrolyte is unstable, and exhibits a low lithium insertion/deinsertion kinetics due to its layered structure [11, 12].
As an alternative anode material, spinel lithium titanate (Li4Ti5O12) is attracting more and more attentions, because it has stable structure and higher lithium insertion potential (about 1.55 V vs. Li+/Li) than graphite [13–18]. However, Li4Ti5O12 has a poor electronic conductivity and presents a catalytic activity toward the electrolyte decomposition [19, 20]. Carbon coating is believed to be an effective way for improving the electronic conductivity and reducing the catalytic activity of Li4Ti5O12 anode [21–28].
Many methods have been proposed for carbon coating of Li4Ti5O12. Zhao et al. used ionic liquid as the carbon precursor to prepare the Li4Ti5O12 coated with N-doped carbon and showed that the sample with 7.0 wt.% carbon exhibited good rate capability and cycling performance [19]. Guo et al. used amphiphilic carbonaceous material as carbon precursor to prepare the coated Li4Ti5O12 with different carbon contents and found that the optimal carbon content was 5.7 wt.% [29]. Li et al. prepared Li4Ti5O12/carbon/carbon nano-tubes by solid-state method using 6 wt.% as the optimal carbon content [30]. Jayaprakash et al. used pitch as carbon precursor and prepared the carbon-coated Li4Ti5O12 [31]. Thermogravimetric analysis (TGA) showed that the sample contained 20 % of carbon but delivers an initial discharge capacity of only 166 mAh g−1 at 0.1 C. Zheng et al. synthesized Li4Ti5O12/C composite via solid-state reaction using lithium citrate as lithium source and carbon source [32]. The obtained sample with carbon content of 5.5 % delivered an initial discharge capacity of 162 mAh g−1 at 1 C with capacity retention of 92 % after 100 cycles.
Besides, anatase TiO2 has also attracted much attention since its high voltage platform of ~1.7 V (vs. Li/Li+) for lithium ion insertion/extraction, low volume expansion of 3–4 % during cycling [33–39]. Since the anatase-type TiO2 is formed symbiotically in the synthesis process of Li4Ti5O12, the ratio of Li4Ti5O12 to TiO2 is not under consideration when we evaluate the effect of preparation method on the performances of Li4Ti5O12-TiO2 anode. Rahman et al. [40, 41] used a new route of simple basic molten salt process to synthesize nanocrystalline Li4Ti5O12-TiO2 and perform carbon coating by using citric acid as carbon precursor. The obtained product had a carbon content of about 4.2 wt.% and delivered a capacity of 166 mAh g−1 at 0.5 C.
However, all the methods reported in literatures can improve the performance of Li4Ti5O12 or TiO2 anode to some extent; the obtained products usually have high carbon contents that the specific capacity of anode is reduced. Furthermore, carbon-coated anode is usually prepared by directly mixing the carbon precursors with pristine anode, followed by a violent stirring or ball milling. The disadvantage of this traditional method is difficult to ensure a uniform carbon coating during the preparation processes; thus, the resulting products usually have higher carbon content.
In this paper, we reported a new method for uniformly coating Li4Ti5O12-TiO2 with low carbon content. In this method, the carbon coating is performed directly on Li4Ti5O12-TiO2 rather than in the formation process of Li4Ti5O12-TiO2 by using phenolic resin as carbon precursor and cetyl trimethyl ammonium bromide (CTAB) as dispersant. Phenolic resin is formed in situ on Li4Ti5O12-TiO2, instead of adding during coating process, which favors coating uniformly and reducing the formation of independent carbon phase and thus reducing the carbon content in the products. The CTAB helps disperse uniformly the solid particles to ensure the complete coating of all particles.
Experimental
Material preparation
All the chemicals were used as received without further purification. A certain amount of pristine Li4Ti5O12-TiO2 anode (Tianjiao Corporation) and cetyl trimethyl ammonium bromide (CTAB) were dispersed in deionized water. With the treatment under ultrasonication and mechanical stirring, the slurry turned to be milky and homogeneous. Resorcinol and formaldehyde with a molar ratio of 1:3 and Na2CO3 as the catalyst were added into the slurry under stirring. The resultant slurry was refluxed at 90 °C for 1 h under continuous stirring, and then the resultant brick-red solution was centrifuged. The as-prepared precursor was washed with deionized water several times, dried at 80 °C overnight, and calcined at 700 °C for 2 h under N2 atmosphere with a heating rate of 3 °C min−1. Three Li4Ti5O12-TiO2/C composites with different carbon contents were prepared, denoted as S1, S2, and S3. The carbon content was controlled by using different amount of resorcinol and formaldehyde.
Material characterization
To determine the carbon content, a Perkin-Elmer TGA7 was used for thermogravimetric analysis (TGA) in a heating rate of 10 °C min−1 from 30 to 800 °C under an air atmosphere. Crystalline phase information of the materials was obtained by a powder X-ray diffraction (XRD, Bruker D8 Advance, Germany) with a Cu Ka radiation in the range from 10 to 90°. The morphology of the as-prepared samples was observed with a scanning electron microscope (SEM, JEOL JSM-6510, Japan) and transmission electron microscope (TEM, JEM-2100HR, USA).
The electrochemical measurements were performed with coin-type cells (CR2025). A lithium metal foil was used as the counter electrode as well as reference electrode. The working electrode was prepared by mixing active materials of Li4Ti5O12-TiO2 anode (80 wt.%), conductive agent of Super-P (10 wt.%), and binder of polyvinylidene difluoride (PVDF, 10 wt.%) and then coating the mixture onto a copper foil. The average mass loading of the electrode for the electrochemical tests was 4.095 mg cm−2, in which contained 3.276 mg cm−2 of active material. The copper foil was chosen as the current collector because it exhibited better rate and cycle performance than that of Al current collector [42].
The electrolyte was 1.0 M LiPF6 in a 1:1 (w/w) mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC). A Celgard 2300 microporous membrane was used as the separator. The cells were assembled in a glove box filled with argon. Electrochemical impedance spectra (EIS) was carried out in an Autolab (PGSTAT302N, Switzerland) with the frequency range from 106 to 0.01 Hz. Constant current charge-discharge tests were conducted on a battery test system (LAND CT2001A, China) with a voltage range of 1.0~2.5 V (vs. Li/Li+) at different rates.
Results and discussion
TGA was used to estimate the carbon content of the prepared samples. Figure 1 shows the TGA curves of the pristine sample and three carbon-coated samples, S1, S2, and S3. As can be seen from Fig. 1, there is a slight weight loss for all the samples before 200 °C, which is attributed to the evaporation of trace absorbed water. For pristine Li4Ti5O12-TiO2, its weight maintains almost constant with the further increase of temperature. Differently, carbon-coated composites exhibit apparent weight loss over 210 °C, which obviously results from the decomposition of carbon. When the temperature is over 600 °C, the weights of the samples retain constant, indicating that the carbon in the samples has decomposed completely. Based on the result of weight loss, the carbon contents of the as-prepared samples can be calculated, whose value is 2.27 wt.% for S1, 2.5 wt.% for S2, and 3.34 wt.% for S3.
Figure 2 shows the SEM and TEM images of Li4Ti5O12-TiO2 anode before and after carbon coating. It can be seen from Fig. 2a that the uncoated sample has a spherical morphology with an average grain size of about 5 μm. As shown in Fig. 2b, the spheres are composed of about 30-nm-sized nanoparticles, which are beneficial for enhancing the kinetics of lithium intercalation into the Li4Ti5O12-TiO2 host structure. After carbon coating, the spherical morphology and microstructure remain unchanged, as shown in Fig. 2c. Comparing Fig. 2b with Fig. 2d, it can be seen that a continuous and uniform carbon layer has been successfully formed on the surface of Li4Ti5O12-TiO2 (Fig. 2d).
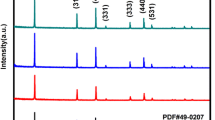
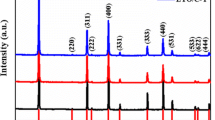
XRD was performed to identify the crystal structure of the prepared sample. Figure 3 displays the XRD patterns of the pristine sample and carbon-coated composites. It is found that all the samples are composed of spinel Li4Ti5O12 (PDF no.49-0207) with some diffraction peaks of anatase TiO2 as the secondary phase. It also can be noted that there is no change in the phase composition, but the diffraction peaks attributed to anatase TiO2 become stronger after carbon coating, suggesting that the carbon coating process results in partial evaporation of Li2O but does not change the crystal structure of Li4Ti5O12. In addition, no diffraction peaks related to carbon can be observed, suggesting that there is no independent carbon phase and confirming that the carbon content in the sample is low.
The charge-discharge profiles of uncoated and coated different contents of carbon at 0.5 C rate are presented in Fig. 4. Obviously, carbon coating hardly affects the charge-discharge characteristics at such low rate. All the samples display similar charge-discharge profiles, but the S2 sample with carbon content of 2.5 wt.% has the highest discharge capacity, which is 179.2 mAh g−1, contributed from both Li4Ti5O12 and TiO2, a little higher than the theoretical capacity of Li4Ti5O12 (175 mAh g−1). The uncoated sample has a discharge capacity of 155.6 mAh g−1, indicative of the improved effect of the carbon coating. Besides, the lithiation/delithiation processes of TiO2 can be observed at the short voltage platform of 1.7 and 2.0 V, conforming the sample is composed of Li4Ti5O12 and TiO2.
To understand the rate capability of the prepared samples, charge-discharge tests were performed at various rates. Figure 5 presents the rate performances of all the samples in the voltage range of 1.0~2.5 V. The S2 sample has the highest specific capacity in various C rates, followed by S1. It is worth noticing that the S3 with carbon content of 3.34 wt.% performs the worst rate performance when the C rate is higher than 5 C. The possible reason is that the rate performance of the prepared samples is determined by both the ionic conductivity and electronic conductivity. Although carbon has a superior electronic conductivity, it does not contribute to ionic conductivity. The sample with 2.5 wt.% carbon exhibits excellent rate performance, indicating that the carbon coating is effective. To our knowledge, this is the minimum carbon content among the values that have been reported to be effective for the carbon coating of Li4Ti5O12-related anodes [18, 28–30].
EIS was used to confirm the difference in the kinetics of lithiation/delithiation of the prepared samples. Figure 6 presents the Nyquist plots of different samples. It can be seen from Fig. 6 that the plots are characteristic of a semicircle at high frequency region, which is attributed to the charge transfer resistance (Rct), and an oblique line at low frequency region, which is the Warburg behavior related to the diffusion of ion in electrode. The Rct of pristine sample is quite higher than that of the coated samples, indicating that the carbon coating significantly improves the kinetics of lithiation/delithiation of Li4Ti5O12. The S2 sample has the smallest Rct among three coated samples, which further confirms the optimal carbon content.
Figure 7 presents the comparison of cycling stability between pristine and S2 samples. As seen from Fig. 7a, the S2 sample exhibits better cycling stability than the pristine one. At 1 C rate, S2 delivers an initial charge capacity of 162.4 mAh g−1 and its capacity retention is 95 % after 200 cycles. The uncoated sample has an initial charge capacity of 96.5 mAh g−1 and its capacity achieves a maximum value of 153.5 mAh g−1 at 23rd cycle and remains 76 % of the maximum after 200 cycles. The appearance of the maximum capacity in the pristine one suggests that there exist poor electronic contacts among the Li4Ti5O12-TiO2 particles in the sample without carbon coating.
The carbon layer in S2 sample has sp2-hybridized carbon-containing conductive networks originated from the carbonation of benzene ring that acts as a bridge function between particles, making it easier for electron to transfer into the bulk of the sample and providing the sample with a higher specific capacity and a superior rate and cycle performance. The cycling performance of S2 sample at high rate is also excellent. As shown in Fig. 7b, the S2 sample at 5 C rate delivers a capacity as high as 148 mAh g−1 with negligible capacity loss after 90 cycles, showing its superior cycling performance. Thus, the cycle and the rate performances of S2 sample with low carbon coating content are as good as those of the samples with larger carbon content reported in literatures [30, 31], indicating that the developed carbon coating method in this paper is a good alternative to prepare the high performance Li4Ti5O12-TiO2 anode.
Conclusions
In this paper, we reported a new carbon coating method for improving the performance of Li4Ti5O12-TiO2 as anode of lithium ion battery. In this method, cetyl trimethyl ammonium bromide is used as dispersant and phenolic resin formed in situ on Li4Ti5O12-TiO2 is used as carbon precursor. With this method, carbon can be successfully coated on Li4Ti5O12-TiO2 anode and the uniformly coated carbon with low content is achieved. The resulting composite with only 2.5 wt.% carbon delivers excellent cycle and rate performances.
References
Suryakala K, Marikkannu KR, Paruthimal Kalaignan G, Vasudevan T (2007) Ionics 13:41
Ren J, He X, Wang K, Pu W (2010) Ionics 16:503
Liao YH, Li XP, Fu CH, Xu R, Zhou L, Tan CL, Hu SJ, Li WS (2011) J Power Sources 196:2115
Zhu GN, Wang YG, Xia YY (2012) Energy Environ Sci 5:6652
Wu M, Wang C, Chen J, Wang F, Yi B (2013) Ionics 19:1341
Hassoun J, Lee K, Sun Y, Scrosati B (2011) J Am Chem Soc 133:3139
Dalavi S, Xu MQ, Ravdel B, Zhou L, Lucht BL (2010) J Electrochem Soc 157:A1113
Zhang D, Cai R, Zhou YK, Shao ZP, Liao XZ, Ma ZF (2010) Electrochim Acta 55:2653
Xie H, Liao Y, Sun P, Chen T, Rao M, Li W (2014) Electrochim Acta 127:327
Sun P, Liao Y, Xie H, Chen T, Rao M, Li W (2014) J Power Sources 269:299
Lee ML, Li YH, Yeh JW, Shih HC (2012) J Power Sources 214:251
Yu ZJ, Zhang XF, Yang GL, Liu J, Wang JW, Wang RS, Zhang JP (2011) Electrochim Acta 56:611
Wang J, Zhao H, Yang Q, Zhang T, Wang J (2013) Ionics 19:415
Wang X, Shen L, Li H, Wang J, Dou H, Zhang X (2014) Electrochim Acta 129:283
Wu D, Cheng Y (2013) Ionics 19:395
Wang Y, Rong H, Li B, Xing L, Li X, Li W (2014) J Power Sources 246:213
Lu X, Zhao L, He XQ, Xiao RJ, Gu L, Hu YS, Li H, Wang ZX, Duan XF, Chen LQ, Maier JC, Ikuhara YC (2012) Adv Mater 24:3233
Sun Y, Zhao L, Pan H, Lu X, Gu L, Hu Y-S, Li H, Armand M, Ikuhara Y, Chen L, Huang X (2013) Nat Commun 4:1870
Zhao L, Hu YS, Li H, Wang ZX, Chen LQ (2011) Adv Mater 23:1385
Ding ZJ, Zhao L, Suo LM, Jiao Y, Meng S, Hu YS, Wang ZX, Chen LQ (2011) Phys Chem Chem Phys 13:15127
Kang E, Jung YS, Kim GH, Chun J, Wiesner U, Dillon AC, Kim JK, Lee J (2011) Adv Funct Mater 21:4349
Kim HK, Bak SM, Kim KB (2011) Electrochem Commun 12:1768
Yuan T, Yu X, Cai R, Zhou YK, Shao ZP (2010) J Power Sources 195:4997
Nugroho A, Chung KY, Kim J (2014) J Phys Chem C 118:183
Shen LF, Yuan CZ, Luo HJ, Zhang XG, Chen L, Li HS (2011) J Mater Chem 21:14414
Ma Y, Ding B, Ji G, Lee JY (2013) ACS Nano 7:10870
Wang YG, Liu HM, Wang KX, Eiji H, Wang YR, Zhou HS (2009) J Mater Chem 19:6789
Shen LF, Li HS, Uchaker E, Zhang XG, Cao GZ (2012) Nano Lett 12:5673
Guo XF, Wang CY, Chen MM, Wang JZ, Zheng JM (2012) J Power Sources 214:107
Li X, Qu MZ, Huai YJ, Yu ZL (2010) Electrochim Acta 55:2978
Jayaprakash N, Moganty SS, Lou XW, Archer LA (2011) Appl Nanosci 1:7
Zheng SW, Xu YL, Zhao CJ, Liu HK, Qian XZ, Wang JH (2012) Mater Lett 68:32
Lei JF, Li WS, Li XP, Cairns EJ (2012) J Mater Chem 22:22022
Lei JF, Li WS, Li XP, Zeng LZ (2013) J Power Sources 242:838
Lei JF, Li LB, Shen XH, Du K, Ni J, Liu CJ, Li WS (2013) Langmuir 29:13975
Yi J, Liu YL, Wang Y, Li XP, Hu SJ, Li WS (2012) Int J Miner Metall Mater 19:1058
Yi J, Lu DS, Li XP, Li WS, Hu SJ, Lei JF, Wang Y (2012) J Solid State Electrochem 16:443
Lei JF, Li XP, Li WS, Sun FQ, Lv DS, Lin YL (2012) J Solid State Electrochem 16:625
Lei JF, Sun FQ, Li WS, Zhou ZH, Yue L, Yi J (2011) Int J Hydrogen Energy 36:8167
Rahman MM, Wang JZ, Hassan MF, Wexler D, Liu HK (2011) Adv Energy Mater 1:212
Rahman MM, Wang J-Z, Hassan MF, Chou S, Wexler D, Liu H-K (2010) J Power Sources 195:4297
Pan H-L, Hu Y-S, Li H, Chen L-Q (2011) Chin Phys B 20:118202
Acknowledgments
The authors are highly grateful for the financial support from the joint project of National Natural Science Foundation of China and Natural Science Foundation of Guangdong Province (Grant No. U1134002), National Natural Science Foundation of China (Grant No. 21273084), Natural Science Foundation of Guangdong Province (Grant No. 10351063101000001), and the scientific research project of Department of Education of Guangdong Province (Grant No. 2013CXZDA013).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Y., Liao, Y., Li, W. et al. Carbon coating of Li4Ti5O12-TiO2 anode by using cetyl trimethyl ammonium bromide as dispersant and phenolic resin as carbon precursor. Ionics 21, 1539–1544 (2015). https://doi.org/10.1007/s11581-014-1309-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1309-7