Abstract
Sporisorium scitamineum teliospores possess an organized cytoskeleton involved in important developmental and physiological processes. It has been described that microtubules appear to be fundamental for nucleus translocation during germination and hyphal growth, whereas actin polymerization is necessary for the formation of invaginations during teliospore displacement. Here, a global vision of the actin cytoskeleton organization throughout the life cycle of S. scitamineum cells is shown, providing evidence that a perfectly structured F-actin network is necessary to trigger smut pathogenicity. Moreover, although myosin presence in teliospores had been previously described, herein actin and myosin co-locations are demonstrated by confocal microscopy during both invaginations formation and germination. In turn, F-actin and microtubules (MTs) interact, jointly participating in the establishment of cell polarity. The resistant sugarcane cultivar Mayari 55-14 produces high molecular mass glycoproteins (HMMG) that differently affect F-actin organization at different stages of fungal development. HMMG first supported F-actin to induce the movement of teliospores towards the cytoagglutination points. At later stages of fungal development, HMMG disorganized F-actin which prevented the protrusion of germinative tube. A continuous exposure to HMMG provoked apoptosis in pathogenic, diploid cells and a delay in sporidia conjugation that could be crucial for plant resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Smut is one of the major pathologies of sugarcane plants. Sporisorium scitamineum, belonging to the Basidiomycetes, is the causal agent of the disease that produces significant losses in crops of Saccharum officinarum. Similar to the rest of Ustilaginales, diploid hyphae of S. scitamineum are infective, penetrate the host tissues, and damage their meristems (Waller 1970), although not all meristem cells are damaged as the plant continues to grow and to form healthy leaves until the formation of the whip. When fungal hyphae are responsible for carrying out penetration into host tissues, the growth of hyphae is directed towards depressions between the epidermal cells surrounding the stomata. It appears that the growth of hyphae depends on a reduced set of genes encoding plant cell wall hydrolyzing enzymes, which supports a biotrophic lifestyle in which host damage must be minimized (Que et al. 2014). However, the true nature of this mechanism is not yet clear. Finally, a network of hyphae invades the entire plant, producing teliospores. The mechanism is assumed to be a part of the machinery that is involved in cell growth and extension (Brand and Gow 2012).
When fungal spores are the invasive agent, the process is different. The smut spores, deposited on the wet surface of the leaves or stems, can infect other places further away from the deposition point by moving themselves towards new points of entry, wounds, or stomata. This movement is produced by successive contractions and relaxations of the actin cytoskeleton, as demonstrated by Sánchez-Elordi et al. (2016a). Since the pathogen can use the open stomata of host leaves to penetrate internal tissues, spores randomly deposited on the surface of a leaf, away from the stomata, should develop a mechanism of displacement towards the way of entry (Santiago et al. 2012). The two most frequently suggested mechanisms are the submicroscopic contractions of fibrils helically arranged within cell walls and the appearance of mobile appendages in zoospores (Brand and Gow 2012). Zoospores, on the other hand, are provided with one or two flagella that agitate within the surrounding water film, propelling the mobile cell. These flagella may be anterior, posterior, or lateral, attached to a slot in the cell body (Gleason and Lilje 2009).
Smut teliospores, on the other hand, accomplish chemotactic movement towards glycoproteins secreted by their host, S. officinarum. The migration of smut teliospores towards a source of these glycoproteins depends both on presenting time and the amount of glycoprotein used as a stimulant agent. The chemotactic movement of teliospores is inhibited by phalloidin and latrunculin A, acting on actin, as well as by blebbistatin, which inhibits the activity of a contractile protein similar to myosin II, responsible for the contraction-relaxation of the cytoskeleton (Sánchez-Elordi et al. 2016a). Since the organization of the actin cytoskeleton is required for cell polarity and cytokinesis, the binding of glycoproteins to their receptors inhibits the cell polarization and, consequently, the germination of teliospores. Therefore, these glycoproteins are considered as a defensive false quorum signal, since they are capable of agglutinating a large number of teliospores without germination ability (Sánchez-Elordi et al. 2015).
In addition, microtubules (MTs) also appear to be involved in the process since nocodazole (Noc) inhibits chemotactic displacement. Sugarcane glycoproteins also prevent the correct arrangement of MTs and cause nuclear fragmentation. As a result, nuclei cannot correctly migrate through the growing hyphae, causing germination failure. Arginase activity contained in defense glycoproteins is already described for preventing fungal germination (Millanes et al. 2005). Its enzymatically active form is considered as a link between the defensive capacity of glycoproteins and the MT disorganization in fungal cells (Sánchez-Elordi et al. 2016c). Active arginase is produced by healthy and resistant plants; conversely, it is not detected in the juice from susceptible varieties, which explains why MT depolarization, nuclear disorganization, as well as germination of teliospores are not significantly affected by glycoproteins from non-resistant plants (Sánchez-Elordi et al. 2016a).
After having demonstrated the role of F-actin in the movement of teliospores and suggested its implication in germination, this work tries (1) to provide a global view of the F-actin organization in teliospores throughout its germination, (2) to detect in cells the presence of both α and β actin, and (3) to demonstrate that cytoskeleton reorganization is induced by sugarcane glycoproteins “at its convenience” at different stages of S. scitamineum development, resulting a recurrent defense mechanism.
Material and methods
Plant material
Teliospores of the pathogen Sporisorium scitamineum (Syd.) and plants of Saccharum officinarum (L.), Mayarí (My) 55-14 cv. resistant to smut, and Barbados (B) 42231 cv., susceptible to smut, field grown in the Botanic Garden of Complutense University of Madrid (Madrid, Spain), were used throughout this work.
Teliospore germination and inoculation procedure
Teliospores of S. scitamineum were isolated from whips collected from diseased B 42231 plants in experimental crops of the National Institute for Sugarcane Investigation (INCA) in Matanzas, Cuba. Teliospores were sterilized in surface and incubated in sterile Lilly and Barnett medium (Lilly and Barnett, 1951) at 38 °C for 5 days, as previously described (Santiago et al. 2009). Single sporidial colonies were isolated and re-incubated in the same medium. In order to determine the mating type of each isolate, random mating experiments were performed. Mating reaction was evidenced by the appearance of aerial mycelium and isolates were arbitrarily designed as either plus or minus (Santiago et al. 2012). Ten-month-old plants of My 55-14 and B 42231 cvs. (five plants per cv.) were inoculated using a Hamilton syringe with 50 μL of a suspension containing 2 × 106 sporidia mL−1 of a 1:1 mixture (plus and minus) of the isolated mating cell types (inoculated plants). Five non-inoculated plants per variety were used as controls. Inoculation was carried out in the apical portion of the stem through the leaf sheath. Inoculum was injected into the stem 3 cm above the first leaf with a visible dewlap. Smut-inoculated and non-inoculated plants were sampled at 6 h post-inoculation. Stems were maintained at − 20 °C to be used for obtaining juices and to purify high molecular mass glycoproteins (HMMG).
Purification of HMMG sugarcane glycoproteins
HMMG sugarcane glycoproteins from inoculated and non-inoculated plants of My 55-14 and B 42231 cvs. were obtained as previously described (Sánchez-Elordi et al. 2015). Stalks from My 55-14 cv. were mechanically crushed immediately after cut. Juice was clarified by filtration and centrifugation (Legaz et al. 1995). To separate HMMG fraction, 5.0 mL of clarified juice was filtered through a Sephadex G-10 column (20 cm × 2.5 cm) embedded in 10 mM sodium phosphate buffer, pH 6.8. Elution was also carried out with the same buffer. The void volume (28 mL) was discarded, whereas the following 12 mL contained both HMMG and MMMG glycoproteins. This volume was concentrated in a SPD111 V Thermo concentrator. Concentrated fraction (4 mL) was then loaded onto a Sephadex G-50 column (35 cm × 2.5 cm) and eluted in the same way. The void volume (48 mL) was discarded. The next 20 mL contained HMMG (Legaz et al. 1995), and they were concentrated as above. Protein in HMMG fraction was determined according to Lowry et al. (1951).
α-Actin and nuclear location in germinating S. scitamineum teliospores
The simultaneous location of α-actin and nuclear material in smut teliospores was carried out by confocal microscopy. Pre-hydrated for 1.0 h in distilled water (1.0 mg mL−1) spores was incubated in the presence of (a) 5 μM jasplakinolide, Jas (Calbiochem, Bad Soden, Germany); (b) 10 μM Jas; (c) distilled water (control sample); (d) HMMG from uninoculated plants of My 55-14; (e) HMMG from inoculated plants of My 55-14; (f) HMMG from uninoculated plants of B 42231; or (g) HMMG from inoculated plants of B 42231, always at a concentration of 0.5 mg mL−1. After 3 h, the incubation media were removed and actin/nucleus labellings were carried out according to Baluska et al. (1992) with modifications described in Sánchez-Elordi et al. (2016b). For the binding of the primary antibody, teliospores were incubated at 37 °C for 2 h in the presence of a polyclonal anti-actin antibody from corn kindly provided by Christopher J. Staiger (Purdue University, IN, USA), produced in rabbit, diluted 1:200 in BSA, and was used for actin localization. The secondary antibody used was a rabbit anti-immunoglobulin G (IgG) antibody conjugated to Alexa Fluor®-488 (MP Biomedicals, Santa Ana, CA, USA) and diluted 1:200 in BSA. Nuclei were visualized after incubation with 5 μM DAPI (Sigma-Aldrich, Saint Louis, MO, USA). All volumes discarded during the process were removed by centrifugation in a Capsule HF-120 Microcentrifuge (Tomy Seiko, Tokyo, Japan) for short centrifugations or spins.
Fluorescent cells were observed using a confocal microscope Olympus FluoView FV-1000 (Olympus FluoView, Germany) equipped with program 4.0 Fluoview for image analysis. For the actin visualization, the excitation and emission lines of 488 nm and 520 nm, respectively, were used. The excitation and emission lines used for the visualization of the nuclear stain were 405 and 461 nm, respectively. The projections on the Z-axis were obtained by superimposition of 15–30 images obtained in series and corresponding to optical sections of 0.4–0.6 μm.
Detection by confocal optical microscopy: actin and myosin co-localization
Pre-hydrated for 1.0 h in distilled water (1.0 mg mL−1) spores were incubated in the presence of (a) distilled water (control sample), (b) 0.5 mg mL−1 HMMG from non inoculated My 55-14 plants, (c) 0.5 mg mL−1 HMMG + 5 μM Lat A (Lat A) (Sigma-Aldrich, Saint Louis, MO, USA), or (d) 0.5 μg mL−1 Noc (EMD Biosciences, Billerica, MA). After 3 h, the incubation media were removed and the samples of collected cells were immobilized on a slide that had been coated with poly-l-lysine (1.0 μM in MiliQ water, Merck KGaA Millipore, Darmstadt, Germany) for 2 h. Spores were fixed in formaldehyde for 10 min, washed in 10 mM PBS buffer, pH 6.7, and permeabilized by 7% (w/v) polyvinylpyrrolidone (PVP) for 1.0 h. Without removing the PVP, anti-phosphorylated MLC-Ser19 rabbit antibody (Rockland Immunochemicals, Gilbertsville, PA, USA), diluted 1:500 in PBS buffer and containing 0.1% saponin (v/v), was added to samples. Incubation was carried out for 2 h at 37 °C. After that, samples were washed in PBS buffer. Then, anti-IgG peroxidase conjugated antibody from rabbit (Pierce Thermo Fisher Scientific Inc., Rockford, IL, USA), diluted 1:500 in PBS buffer, contained 0.1% saponin (v/v), and was added to samples. At the same time, Alexa Fluor®-488–conjugated phalloidin (MP Biomedicals, Santa Ana, CA, USA) was added. After 1 h in dark, excess of secondary antibody and phalloidin was eliminated. Samples were washed in PBS buffer and mounted in Mowiol-DABCO (Calbiochem, La Jolla, CA, USA) to avoid fluorescence decay.
Confocal images were collected on a Leica DFC 350 FX camera (Leica lasertechnik GmbH, Mannheim, Germany) adapted to an inverto-microscope Leica DMI-6000, using a 66×/1,44NA (oil) Leitz (Stuttgart, Deutschland) PlanApo objective. The laser employed was an Ar/Kr laser, and the excitation and emission wavelengths were 468 nm and 520–540 nm respectively. Images were analyzed by using Leica Confocal Software.
Polarization of actin cytoskeleton in cells previously incubated wit Noc was evaluated by collecting data from confocal microscopy images. A total of 100 cells were analyzed. Percent of asymmetric fluorescing cells is shown.
Nocodazole and latrunculin A affect teliospore germination
Germination assay was carried out as described in Sánchez-Elordi et al. (2015). Teliospores were sterilized in surface and incubated in sterile Lilly and Barnett medium (Lilly and Barnett 1951) at 38 °C for 5 days, in the absence or in the presence of 8 μg mL−1 Noc (EMD Biosciences, Billerica, MA) or 0.08 μM Lat A. Percent of germinative tubes and sporidia in samples were then quantified over time (0–18 h). Error bars denote the standard error (SE).
Observation of β-actin labelled with ferritin in the cells of S. scitamineum by TEM
Samples of 1.0 mg of teliospores previously hydrated with distilled water were incubated for 1.0 h (1.0 mg mL−1) in the presence of 1.0 mL of (a) 0.41 mg mL−1 HMMG from uninoculated plants of My 55-14, (b) 0.41 mg mL−1 HMMG + 5 μM Lat A, or (c) distilled water (control). Incubations were carried out for 5 h with agitation (125 oscillations min−1). After that time, the incubation medium was removed by centrifugation for 1.0 min at 9000×g in a Sigma 2 K15 centrifuge (Sigma, St. Louis, MO, USA). To the teliospores precipitate, 1.5 mg of ferritin-anti-β-actin (AC-15, Tebu-Bio Santa Cruz Biotechnology, Inc.) prepared in 2 mL of 10 mM PBS buffer, pH 6.7, was added incubating the mixture at 26 °C for 30 min (Molina et al. 1998). Subsequently, the cells were recovered by centrifugation at 5000×g for 10 min and washed with PBS buffer for 10 min. The cells were fixed in 2.5% (v/v) glutaraldehyde prepared in sodium phosphate Milloning buffer, pH 7.3 (Milloning, 1961), post-fixed with osmium tetroxide, dried, and included in Epon-812 resin. Ultrafine (70–90 nm) cuts were then made with a diamond blade in an ultramicrotome (OmU2-Reichert Jung). They were deposited on copper grids covered by pioloform and stained with 2% (v/v) uracil acetate and 0.4% (w/v) lead citrate (Reynolds, 1963) on a Petri dish to prevent exposure to air and subsequently washed with distilled water to remove excess dye. Micrographs was made under a JEOL JEM transmission electron microscope 1010 (JEOL, Tokyo, Japan) coupled to a megaview II camera for the capture of images.
Analysis of the growth and the conjugation of sporidia colonies of S. scitamineum
Drops of 0.4 mL of 4.2% (w/v) potato dextrose agar medium (PDA) were used to analyze the effects of HMMG glycoproteins on the growth and the conjugation of sporidia of S. scitamineum. A suspension of 2.6 × 106 sporidia mL−1 (5 μL), isolated (+), or compatible with each other (+ and −) was deposited on the solidified PDA drops containing 0.41 mg mL−1 HMMG from uninoculated/inoculated My 55-14 and B 42231 plants. Per each assay, drops that contained sporidia whose growth would be compared were deposited in a same Petri dish, in order to minimize environmental differences. A control, in which any agent was replaced by distilled water, was always incorporated to the Petri dish. Three replicates were prepared for each trial. All the plates were sealed with Parafilm M® paper (American Can Co., Neenah, WI) and transferred to a JP Selecta culture stove (Incudigit SA, Barcelona, Spain), where they were incubated during 48 h. Images of the growth process and colony conjugation were taken using a Leica EZ4 HD stereoscopic microscope (Leica Microsystems, Heerbrugg, Switzerland) with an integrated digital camera for imaging, which were processed using the Leica Acquire software (Leica Microsystems).
Results
Confocal microscopy of nuclear and cytoplasmic α-actin distribution in the early stages of S. scitamineum life cycle
Figure 1 shows the location of cell nucleus and α-actin in untreated cells at different stages of development. Moreover, the large number of images obtained by confocal microscopy allowed us to elaborate a scheme about how actin distribution is modified throughout the cycle. Figure 1 (1) shows a teliospore before germination. A homogeneous distribution of actin throughout the cytoplasm and a compact nucleus inside the cell can be observed. Cortical actin, close to the plasma membrane, can be also visualized. Nuclear migration through growing hyphae is seen in Fig. 1 (5) and (6), whereas Fig. 1 (7) shows the organization of actin in sporidia released to the medium which is probably being divided, since decondensed nuclear material can be observed. The conjugation of two sexually compatible sporidia (Fig. 1 (9)) allows the appearance of infectious dikaryotic mycelium (Fig. 1 (10)). In all these phases, α-actin organization in the cytoplasm and sometimes inside the nucleus (Fig. 1 (1), (6), and (9)) can be visualized.
Scheme representing the organization of the F-actin during the first stages of germination of teliospores of S. scitamineum. Agglutination of teliospores at the point of infection where germination is triggered (1), thinning of the cell wall (2), loss of cortical F-actin and migration of the nucleus towards the germinative pole (3), emergence of hyphae (4) and translocation of the nucleus towards the interior of the germinative tube (5), liberation of sporidia (6), their division by germination (7), conjugation of compatible sporidia (plus and minus) (8), formation (9), and growth of dikaryotic mycelium (10). Representing:  , cortical actin;
, cortical actin;  , actin filaments;
, actin filaments;  cell wall;
cell wall;  , monokaryotic nucleus;
, monokaryotic nucleus;  , dikaryotic nucleus;
, dikaryotic nucleus;  , compatible sporidia;
, compatible sporidia;  , dikaryotic mycelium. Confocal images are the projection on the Z-axis of series of 8–10 micrographs. BF, brightfield; α-A, localization of actin by the use of antibodies anti-IgG conjugated to Alexa Fluor-488; D, nuclear staining with DAPI; M, merged or combination. The bars of scale indicate 1.0 μm in the confocal images corresponding to stages 1 and 5; 2 μm in the images corresponding to stages 6; and 5 μm in the images corresponding to stages 7, 9, and 10
, dikaryotic mycelium. Confocal images are the projection on the Z-axis of series of 8–10 micrographs. BF, brightfield; α-A, localization of actin by the use of antibodies anti-IgG conjugated to Alexa Fluor-488; D, nuclear staining with DAPI; M, merged or combination. The bars of scale indicate 1.0 μm in the confocal images corresponding to stages 1 and 5; 2 μm in the images corresponding to stages 6; and 5 μm in the images corresponding to stages 7, 9, and 10
Confocal microscopy of actin and myosin co-localization
The location of the actomyosin cytoskeleton in smut teliospores was performed by confocal microscopy. Variations in its distribution were also analyzed in the presence of HMMG and Lat A, already described as actin polymerizing/depolymerizing agents, respectively, in the S. scitamineum cells (Sánchez-Elordi et al. 2016a). Images displayed are a selection of the numerous micrographs analyzed.
First, fluorescent label of actin and myosin was detected inside untreated teliospores (Fig. 2a, b). Many of control cells showed a homogeneous distribution of actin and myosin. During germination of the untreated cells, actin and myosin were distributed throughout the nascent hyphae (Fig. 2c, d). Actin-myosin co-localization was observed in all cases. It indicated that they should participate jointly in the establishment of the cell polarity in the teliospores of S. scitamineum. This might be intimately related to the need for an active transport of macromolecules during teliospore germination and development (Deacon 2006). After incubation in the presence of HMMG obtained from un-inoculated plants, cv. My 55-14, many of the cells showed a polarized distribution of both cytoskeletal components (Fig. 3a–d). The distribution of actin and myosin was also observed in the presence of HMMG and Lat A, an inhibitor of actin polymerization. Large alterations were detected after incubation with that drug, where the label of F-actin was difficult to identify in all the cases, probably due to the absence of polymerized actin in presence of the inhibitor (Fig. 3e, f), since phalloidin Alexa Fluor®-488 cannot bind to depolymerized actin. Labelling of myosin was more intense than that of actin with in cells treated with Lat A. Even so, its distribution was homogeneous, confirming the absence of cell polarity in the presence of the inhibitor for the whole actin-myosin complex.
Series of images obtained by confocal microscopy corresponding to a control teliospore (untreated with sugarcane HMMG or inhibitors of cytoskeleton functionality) before (a, b) and after (c, d) germination. a and c correspond to the labelling with Alexa Fluor®-488 conjugated phalloidin; b and d correspond to the labelling with anti-phosphorylated MLC-Ser19 antibody. Scale bars indicate 5 μm
Series of images obtained by confocal microscopy corresponding to a teliospores treated with sugarcane HMMG obtained from non-inoculated plants of the cv. My 55-14 (a–d) and, in addition, treated with HMMG glycoproteins produced by non-inoculated plants of the cv. My 55-14 + Lat A (e, f). a, c, and e correspond to the labelling with Alexa Fluor®-488 conjugated phalloidin. b, d, and f correspond to the labelling with anti-phosphorylated MLC-Ser19 antibody. Scale bars indicate 5 μm
Germination of teliospores in the absence and in the presence of cytoskeleton inhibitors
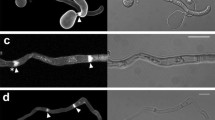
Knowing that both actin and MTs are necessary to teliospore germination and that the latter are indispensable for nuclear migration and to the liberation of formed sporidia, we wanted to confirm if MTs organization is necessary to trigger the same processes, since the absolute requirement of action organization has been previously found (Sánchez-Elordi et al., 2016a). Figure 4 a shows the percentage of germinative tubes and released sporidia in samples over a time period of 18 h. Germinative tubes and sporidia production increased in parallel during the whole experiment in un-treated samples. Noc slightly decreased the formation of germinative tubes and completely avoided sporidia releasing, as it had been previously reported by Sánchez-Elordi et al. (2016b) since polarized MTs are necessary to hyphal separation. Interestingly, neither tubes nor sporidia were found in the presence of Lat A, suggesting that actin and MTs participate in separated processes.
Percent of germinative tubes (straight line) and released sporidia (dash line) in the absence (asterisk) or in the presence of Lat A (filled triangle) or Noc (filled circle) over time (a). Percent of cells that showed an asymmetric actin distribution after incubation with 0.5 μg mL−1 Noc in relation to the control (b). Images of F-actin polarization in smut teliospores in the absence or in the presence of 0.5 μg mL−1 Noc. The scale bars represent 10 μm. Red arrows indicate polarized cells (c). The vertical bars indicate the standard error
Because of these results, Noc-treated teliospores that showed an asymmetric F-actin distribution were quantified during confocal analysis to check if F-actin and MT work independently (Fig. 4 b and c). Results show that the percentage of cells with polarized actin decreases when MTs are disorganized by Noc.
Effects of plant HMMG on teliospore α-actin and nuclear organization
The effect of sugarcane glycoproteins on α-actin and nuclear organization was evaluated by confocal microscopy of smut teliospores previously treated with HMMG obtained from inoculated and non-inoculated plants of both resistant and susceptible cvs. In parallel, some spores were incubated in the presence of jasplakinolide (Jas), an actin polymerizing agent, in order to include in the assay a positive control that revealed the disposition of the polymerized actin (Fig. 5). While the control cells revealed a homogeneous distribution of the actin filaments throughout the cytoplasm, the fungal cells, in the presence of 5 and 10 μM Jas, showed an asymmetrical organization of F-actin. The greatest effects of Jas were observed for a concentration of 10 μM of the drug, with a clear distinction between regions containing or not the polymerized actin filaments.
Z-axis projection of 8–10 micrographs obtained by confocal microscopy showing F-actin and nuclear organization in S. scitamineum teliospores after incubation with of 5.0 and 10 μM Jas, in the presence of HMMG produced by non-inoculated (a) or inoculated plants from cv. My 55-14 (b) and by uninoculated (c) and inoculated (d) plants from cv. B 42231. A control, incubated in the absence of external agents is also shown. BF, Brightfield; α-A, actin localization using anti-IgG antibodies conjugated to Alexa Fluor-488, D; nuclear staining with DAPI, M; merged images; DN, decondensed nucleus. The arrows indicate polarized marking. The scale bars represent 2 μm
Many of the teliospores, incubated with HMMG glycoproteins produced by resistant plants My 55-14 cv. (Fig. 5a), showed a polarized arrangement of the filaments, as it had already been proven in previous experiments (Fig. 3). Many of these teliospores showed a characteristic external label, in the form of ring, close to the cortical zone. In many occasions, nuclear label was diffuse (results not shown), which was associated with a possible nuclear decondensation caused by the glycoproteins that will make it difficult to visualize the label. In the presence of HMMG from inoculated plants from My 55-14 cv., the most external label disappeared (Fig. 5b), the nuclei appeared decondensed and fragmented and actin seemed to accumulate in the nuclear zone, tending to disappear from the cytoplasm. The glycoproteins produced by the susceptible cv. caused minor nuclear and actin alterations (Fig. 5 c and d). The distribution of cytoplasmic and nuclear actin was more like control, and the cores showed less damage.
Location of the fungal β-actin by electron microscopy
TEM technique was used to confirm the location of β-actin, whose presence in cells had been previously detected by Western blot (Sánchez-Elordi et al. 2016a), by using ferritin binding to specific antibodies. The deposition of ferritin inside the cell indicates the location of the antibodies and, therefore, the distribution of the protein that they recognize. The results are shown in Fig. 6. In Fig. 6a, corresponding to an untreated control cell, the presence of ferritin can be observed throughout the cytoplasm. However, it is only in the cortical region where it accumulates and organizes in a linear way, which should be related to the presence of filaments of actin in the outermost cytoplasmic zone. In Fig. 6 b and c, the stimulation of the cells with HMMG from uninoculated My 55-14 plants resulted in an increase in accumulation of ferritin in the cells, which showed darker regions (result of metal deposition) throughout the cytoplasm, and not only in its outermost region. The organization of β-actin into filaments seems to be necessary, together with α-actin, for the formation of the characteristic invaginations observed during chemotaxis in the presence of HMMG (Fig. 6 b and c). An organized deposition of ferritin in cells treated with Lat A was not detected (Fig. 6 d and e) except at some points in the cortical area (Fig. 6e). The drug affected the general morphology of the cells. Many of those showed impairment of the integrity of their membrane and cell wall. These damaged cells were able to prevent the release of the cytoplasmic components to the outside (Fig. 6d). Complete cell destruction was also detected, including the occurrence of apoptotic bodies in the cells that were previously treated with Lat A (Fig. 6e).
Micrographs obtained by TEM showing untreated (a) cells, stimulated with 0.41 mg mL−1 HMMG from non-inoculated plants from cv. My 55-14 (b, c) or incubated in the presence of 0.41 mg mL−1 HMMG + 5 μM Lat A (d, e). The detection of cellular F-actin was done in the presence of antibodies against β-actin linked to ferritin for the visualization of F-actin by metal deposition. The red arrows indicate regions with deposited ferritin, the white arrows indicate the presence of invaginations, and the black arrow the location of apoptotic bodies. b and d represent the enlargement of the corresponding micrographic region. The scale bars indicate 0.7 μm in the complete micrographs and 0.2 μm in the corresponding enlarged micrographs
Analysis of the growth and the conjugation of sporidia colonies of S. scitamineum
Finally, effect of glycoproteins in more advanced stages of pathogen development was studied. Growth and conjugation of sporidia in the presence of HMMG from un-inoculated (C) and inoculated (I) My 55-14 and B 42231 plants were analyzed. Results are shown in Fig. 7. Growth was not blocked by glycoproteins in any case. However, the change in the morphology of the colony, from rough to smooth, could be observed earlier in the presence of glycoproteins from inoculated My 55-14 plants.
Micrographs obtained by stereoscopic microscope corresponding to the different growth states of isolated (+) (a) or conjugated (plus and minus) (b) sporidia colonies in 4.2% (w/v) PDA medium containing 0.41 mg mL−1 HMMG from uninoculated (control, C) or inoculated with the pathogen (I, inoculated) plants from the resistant (My 55-14) or susceptible (B 42231) cvs. The assays were conducted for a sporidia concentration of 2.6 × 106 sporidia mL−1. The images were taken at 24, 29, and 48 h incubation. Red arrows indicate mycelium appearance. The scale bars indicate 1.0 mm
Most significant was the HMMG effect on conjugation. Glycoproteins from resistant variety caused a delay of sporidia conjugation. At 24 h, there had no evidences of conjugation in samples treated with My 55-14 HMMG while it was already observed in control and B 42231 samples.
Discussion
Implications of F-actin and MT assembly for teliospore germination
It has been demonstrated that germination of smut teliospores requires a previous organization of microtubules (MTs) in fungal cells (Sánchez-Elordi et al. 2016a). In the absence of this MT organization, nuclei cannot migrate correctly through the growing hyphae and germination fails. The inhibition of germination by phalloidin and Lat A suggests that also the dynamic alternation between polymerization and depolymerization of the F-actin filaments must collaborate in the emergence of the germinative tubes in the teliospores (Millanes et al. 2005; Sánchez-Elordi et al. 2016a). In particular, the actin polymerization seems essential, since the germination remained totally blocked in the presence of Lat A. Although actin role in germination has been previously suggested, investigations have focused on the role of actin organization in teliospores displacement, especially towards sugar cane glycoproteins.
The architecture of the ensemble of α-actin in the early stages of the life cycle of the pathogen is now revealed, and it is shown in Fig. 1. The information from images obtained by confocal microscopy allowed for the elaboration of a schema about the organization of the filaments during the early stages of development. These results confirmed that a distribution organized of F-actin must be the key condition for progress of the fungal growth and pathogenicity.
F-actin and myosin were jointly located in teliospores during the early stages of germination. It was found that actin and myosin are homogeneously distributed by the entire cytoplasm (including the cortical zone in the case of actin) in the teliospores (Fig. 2 a and b). Once the growth of the hyphae has begun, the actin label tends to disappear at the opposite pole to the germinative pore (mainly in the region of cortical) to condense in the cytoplasm of the germinative tube (Fig. 2 c and d), whose growth is then active. Thus, teliospores transport new material in the direction of the hyphal end as it is commonly the case for many other filamentous fungi (Banuett and Herskowitz 2002). However, at the same time that the cell content is translocated to the end of the emergent germinative tube, the teliospore body is losing its function and degenerates; the label F-actin is progressively diluted.
Both polymerized F-actin and MTs are necessary to germination, but do they work in parallel? Figure 4 a shows how actin organization is required to germinative tubes growth. Lat A avoided tube formation, and as a result, no sporidia were released to the medium. However, germination in the presence of Noc showed that the drug blocks the release of sporidia, probably through its interaction with MTs, but it does not avoid the emergence of germinative tubes, as it is shown in Fig. 4a and as it was described by Sánchez-Elordi et al. (2016b). These results propose that an organization of the actin prior to the germination seems to be necessary to the appearance of the germinative tube, while MTs seem to be indispensable, later, to liberate them to the medium but not for its emergency from the teliospore, as has been observed in other fungal cell types (Snyder et al. 1991; Jacobs et al. 1988; Page and Snyder 1993; Åström et al. 1995; Pillai et al. 1992). Thus, elongation of the germinative tubes and nuclear migration could be processes temporally and mechanically separated: the first was derived from the organization of the F-actin, and the second from the polymerization of the MT. HMMGs and Noc produce the same effect, indistinguishable by fluorescence or confocal microscopy, although they act at two different levels. While HMMGs seem to act preferably at the level of actin microfibrils, although a secondary action of tubulin cannot be excluded, Noc acts at the level of tubulin MTs. The final effect of both compounds is the inhibition of polarization (absence of capping), without observing summatory or synergistic effects.
However, localization of F-actin in the presence of Noc (Fig. 4b, c) showed that percentage of cells with an asymmetric distribution of F-actin decreased in the absence of intact MTs. Conserved MT–actin interactions have been described to favor cell movement and morphogenesis in a lot of cellular types in where asymmetry must be established (Rodriguez et al., 2003). Similarly, our results indicate that both MTs and F-actin in S. scitamineum are inter-connected, despite playing leading roles in different processes.
Actions of HMMG on the cytoskeleton
Knowing that both actin and myosin are necessary to S. scitamineum cells displacement after contact with HMMG chemoattractant (Sánchez-Elordi et al. 2016a), variations in organization of actin-myosin complex were visualized in the presence of the glycoproteins HMMG from resistant healthy My 55-14 cv. (Sánchez-Elordi et al. 2016a). The existence of myosin in smut cells had been demonstrated by Western blot, but the protein had not been still detected by microscopy. Actomyosin complex was polarized by HMMG glycoproteins, as expected, since the entire complex is necessary to induce movement. The fluorescence corresponding to F-actin almost completely disappeared during incubation with Lat A (Fig. 4 e and f), demonstrating again that the cytoskeleton of S. scitamineum is reorganized in response to external signals.
On the other hand, the effect of the different HMMG glycoproteins on germination had been previously demonstrated (Millanes et al. 2005; Sánchez-Elordi et al. 2016a, c). HMMG from a resistant cv. provoked an inhibitory effect on the germination of teliospores higher than that produced by the same one obtained from susceptible cvs. Most of the teliospores are not able to develop the germinative tube if they have been in contact with HMMG (Fig. 3) which indicates that glycoproteins block actin polarization, totally necessary to tube emergence (Sánchez-Elordi et al. 2016a). However, if they are formed, glycoproteins can then block MT polymerization. Both polymerization and polarization of the MTs are events necessary to achieve a correct positioning of the nucleus before the germination, according to the model of nuclear migration (Xiang and Plamann 2003; Morris 2000; Steinberg et al. 2001; Baluška and Barlow 1993; Jacobs et al. al. 1988). That is why some of the germinative tubes and sporidia formed during germination appeared devoid of nuclear material after the contact with the HMMG from non-inoculated plants of My 55-14 cv. (Sánchez-Elordi et al. 2016b).
It is interesting to note that HMMG support assembly of F-actin to induce movement but disorganize them to avoid germination. It implies that at least two F-actin networks could work independently: one of them involved in germination and one of them involved in movement, as it was suggested by Sánchez-Elordi et al. (2016a). This report described that glycoproteins increase the percentage of teliospores that show an asymmetric distribution of F-actin, despite the fact that the same contained a smaller amount of polymerized actin by cell.
TEM assays demonstrated that β-actin exists in S. scitamineum and it polymerizes after HMMG contact, in the similar way that it is observed also for α-actin (Fig. 6). Electron microscopy confirmed how Lat A is capable to disorganize the β-actin filaments in the teliospores of S. scitamineum. β-Actin filaments, in the absence of drugs, were organized in the cortical zone, even more in the presence of HMMG. Cortical F-actin could be the main responsible for the first stages of displacement, as has been described in other cellular types (Takenawa & Miki 2001).
A simultaneous labelling of the actin (by immunolabeling) and the nucleus (by staining with DAPI) of teliospores after contact with the different HMMG (Fig. 5a–d) was observed. Many of the cells incubated in the presence of glycoproteins produced by non-inoculated My 55-14 presented a polarized distribution of actin, in agreement with the effects of the HMMGs described above. However, some cells show a ring-shaped polarization in the more cortical zone, not related to invaginations formation. This one, accompanied by a loss of nuclear material, suggested that apoptosis could have been triggered in the cells after continuous exposure to the glycoproteins. Moreover, apoptotic bodies were also observed in TEM assays (Fig. 6e). The presence of a peripheral actin ring (apoptotic ring) has been defined in numerous cell types in the stages the early stages of apoptosis (Mills et al. 1999; Rosenblatt et al. 2001). This process is highly stimulated by the presence of ROS, accompanied by a disruption of MTs and nuclei, and immediately followed by the loss of polymerized actin throughout the cytoplasm (Marx et al. 2008; Mills et al. 1999). In some cases, the nucleus decondensation observed in cells after their exposure to HMMG from My 55-14 plants was so intense that nucleus could not be visualized (Fig. 5a, b). We propose that the glycoproteins produced My 55-14 plants, after exercising their chemoattractant-cytoagglutinant actions and after have blocked the germination of the teliospores, would trigger the death of the fungal cells.
But what would happen if some teliospores will escape the effect of the HMMG glycoproteins and achieve germination and to produce infective sporidia? In relation to the effect that the glycoproteins provoked on the growth of spore colonies isolated (+) and compatible (+ and −), it was found that the glycoproteins produced by inoculated and non-inoculated plants from the resistant cv. produce a delayed conjugation of compatible sporidia, which could be the key to stop the establishment of infection (Fig. 7). It has been shown that the merger of the cells during the process of conjugation depends on a distribution organized by F-actin in U. maydis. In addition, MTs, which do not appear to directly affect that process, could be involved in the conjugation process by means of their interaction with actin cytoskeleton (Fuchs et al. 2005). Similarly, the disorganization of F-actin by defense glycoproteins could prevent the conjugation of the sporidia of S. scitamineum, as it has been proved to polymerized actin appear to be necessary in conjugant sporidia (Fig. 1).
Global vision: HMMG from resistant cv. affects cytoskeletal reorganization through S. scitamineum life cycle
Resistance of My 55-14 plants is the result of major disruptions of the organization of the cytoskeleton via the HMMG in the early stages of pathogen development. These disruptions occur at different points of S. scitamineum life cycle. All of them are schematized in Fig. 8. Firstly, HMMG glycoproteins induce actin polymerization in order to move fungal cells to a defensive agglutination point (Sánchez-Elordi et al. 2016a). Secondly, HMMG glycoproteins disorganize the integrated cytoskeleton, composed of F-actin and microtubular networks, which avoids tube emergence and nuclear migration. After continuous exposure to the HMMG glycoproteins, F-actin participates in apoptotic response of cells, in collaboration with MT, which results in nucleus fragmentation. Finally, sporidia that “escape” the first effects of My HMMG have more difficulties to conjugate. This delay in conjugation (that may not seem important) could provide the necessary time to activate an effective systemic response in plants.
Scheme that represents the effects of defense glycoproteins at different stages of development of the pathogen. On the left, organization of cytoskeleton throughout the life cycle of S. scitamineum in absence of HMMG is shown. Glycoproteins are able to stimulate a intense chemoattraction of teliospores by means of polarization of F-actin involved in displacement. It results in a defensive agglutination since degradative enzymes contained in HMMG break down agglutinated cell walls (A). Glycoproteins from resistant variety can induce F-actin (B) and MT (C) depolymerization to avoid germination in still-living teliospores. Finally, HMMG could trigger F-actin disorganization in sporidia if they had achieved be released to the medium. This disorganization could be responsible for a delay in conjugation (D)
Abbreviations
- B:
-
Barbados
- BSA:
-
Bovine serum albumin
- DAPI:
-
4′,6-Diamidino-2-phenylindole dihydrochloride
- HMMG:
-
High molecular mass glycoproteins
- IgG:
-
Immunoglobulin G
- Jas:
-
Jasplakinolide
- Lat A:
-
Latrunculin A
- MT:
-
Microtubules
- My:
-
Mayarí
- Noc:
-
Nocodazole
- PBS:
-
Phosphate saline buffer
- PVP:
-
Polyvinylpyrrolidone
- TEM:
-
Transmission electron microscopy
References
Åström H, Sorri O, Raudaskoski M (1995) Role of microtubules in the movement of the vegetative nucleus and generative cell in tobacco pollen tubes. Sex Plant Reprod 8:61–69
Baluška F, Barlow PW (1993) The role of the microtubular cytoskeleton in determining nuclear chromatin structure and passage of maize root cells through the cell cycle. Eur J Cell Biol 61:160–167
Baluška F, Parker JS, Barlow PW (1992) Specific patterns of cortical and endoplasmic microtubules associated with cell growth and tissue differentiation in roots of maize (Zea mays L.). J Cell Sci 103:191–200
Banuett F, Herskowitz I (2002) Bud morphogenesis and the actin and microtubule cytoskeletons during budding in the corn smut fungus, Ustilago maydis. Fungal Gen Biol 37:149–170
Brand A, Gow NAR (2012) Tropic orientation responses of pathogenic fungi. In: Pérez-Martin J, Di Pietro A (eds) Morphogenesis and pathogenicity in Fungi. Springer Verlag, Berlin, pp 21–41
Deacon J (2006). Fungal structure and ultrastructure. In: Fungal biology (4° ed). Malden: Blackwell Publishing Ltd, pp.48–66
Gleason FH, Lilje O (2009) Structure and function of fungal zoospores: ecological implications. Fungal Ecol 2:53–59
Fuchs U, Manns I, Steinberg G (2005) Microtubules are dispensable for the initial pathogenic development but required for long-distance hyphal growth in the corn smut fungus Ustilago maydis. Mol Biol Cell 16:2746–2758
Jacobs CW, Adams AEM, Szaniszlo PJ, Pringle JR (1988) Functions of microtubules in the Saccharomyces cerevisiae cell cycle. J Cell Biol 107:1409–1426
Legaz ME, Pedrosa MM, Martínez M, Vicente C (1995) Soluble glycoproteins from sugar cane juice analyzed by SE-HPLC and fluorescence emission. J Chromatogr 697:329–335
Lilly VG, Barnett HL (1951) Physiology of the Fungi. McGraw-Hill, New York
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marx F, Binder U, Leiter E, Pocsi I (2008) The Penicillium chrysogenum antifungal protein PAF, a promising tool for the development of new antifungal therapies and fungal cell biology studies. Cell Mol Life Sci 65:445–454
Millanes AM, Fontaniella B, Legaz ME, Vicente C (2005) Glycoproteins from sugarcane plants regulate cell polarity of Ustilago scitaminea teliospores. J Plant Physiol 162:253–265
Milloning G (1961) Advantages of a phosphate buffer for OsO4 solutions in fixation. J Appl Phys 32:1637–1650
Mills JC, Stone NL, Pittman RN (1999) Extranuclear apoptosis. J Cell Biol 146:703–708
Molina MC, Stocker-Wörgötter E, Turk R, Bajon C, Vicente C (1998) Secreted, glycosylated arginase from Xanthoria parietina thallus induces loss of cytoplasmic material from Xanthoria photobionts. Cell Adh Commun 6:481–490
Morris NR (2000) Nuclear migration from fungi to the mammalian brain. J Cell Biol 148:1097–1101
Page B, Snyder M (1993) Chromosome segregation in yeast. Ann Rev Microbiol 47:201–231
Pillai MC, Baldwin JD, Cherr GN (1992) Early development in an algal gametophyte: role of the cytoskeleton in germination and nuclear translocation. Protoplasma 170:34–45
Que Y, Xu L, Wu Q, Liu Y, Ling H, Liu Y, Zhang Y, Guo J, Su Y, Chen J, Wang S, Zhang C (2014) Genome sequencing of Sporisorium scitamineum provides insights into the pathogenic mechanisms of sugarcane smut. BMC Genomics 15:996–1014
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain for electron microscopy. J Cell Biol 17:208–212
Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM (2003) Conserved microtubule–actin interactions in cell movement and morphogenesis. Nat Cell Biol 5(7):599–609
Rosenblatt J, Raff MC, Cramer LP (2001) An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol 11(23):1847–1857
Sánchez-Elordi E, Morales de los Ríos L, Vicente C, Legaz ME (2015) Sugar cane arginase competes with the same fungal enzyme as a false quorum signal against smut teliospores. Phytochem Lett 14:115–122
Sánchez-Elordi E, Vicente-Manzanares M, Díaz E, Legaz ME, Vicente C (2016a) Plant–pathogen interactions: sugarcane glycoproteins induce chemotaxis of smut teliospores by cyclic contraction and relaxation of the cytoskeleton. South Afr J Bot 105:66–78
Sánchez-Elordi E, Baluška F, Echevarría C, Vicente C, Legaz ME (2016b) Defence sugarcane glycoproteins disorganize microtubules and prevent nuclear polarization and germination of Sporisorium scitamineum teliospores. J Plant Physiol 200:111–123
Sánchez-Elordi E, Morales-de los Ríos L, Díaz EM, Ávila A, Legaz ME, Vicente C (2016c) Defensive glycoproteins from sugarcane plants induce chemotaxis, cytoagglutination and death of smut teliospores. J Plant Pathol 98:493–501
Santiago R, de Armas R, Fontaniella B, Vicente C, Legaz ME (2009) Changes insoluble and cell-wall-bound hydroxycinnamic and hydroxybenzoic acids in sugarcane cultivars inoculated with Sporisorium scitamineum sporidia. Eur J Plant Pathol 124:439–450
Santiago R, Alarcón B, de Armas R, Vicente C, Legaz ME (2012) Changes in cinnamyl alcohol dehydrogenases from sugarcane cultivars inoculated with Sporisorium scitamineum sporidia. Physiol Plant 145:245–259
Snyder M, Gehrung S, Page BD (1991) Studies concerning the temporal and genetic control of cell polarity in Saccharomyces cerevisiae. J Cell Biol 114:515–532
Steinberg G, Wedlich-Söldner R, Brill M, Schulz I (2001) Microtubules in the fungal pathogen Ustilago maydis are highly dynamic and determine cell polarity. J Cell Sci 114:609–622
Takenawa T, Miki H (2001) WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci 114:1801–1809
Waller JM (1970) Sugarcane smut (Ustilago scitaminea) in Kenya: II infection and resistance. Trans Br Mycol Soc 54:405–414
Xiang X, Plamann M (2003) Cytoskeleton and motor proteins in filamentous fungi. Curr Op Microbiol 6:628–633
Funding
This work has been supported by a grant from the Complutense University (Spain) UCM, GR3/14.910081.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Marc Stadler
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sánchez-Elordi, E., Baluška, F., Vicente, C. et al. Sugarcane glycoproteins control dynamics of cytoskeleton during teliospore germination of Sporisorium scitamineum. Mycol Progress 18, 1121–1134 (2019). https://doi.org/10.1007/s11557-019-01510-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-019-01510-5