Abstract
Despite the recent mycologists’ interest in relationships between gasteroid taxa and agaricoid Basidiomycetes lineages and an intensive debate on the evolution of enigmatic secotioid fungi, systematic studies of the genus Galeropsis have received little attention. Here, the taxonomy of this genus is revised based on morphological and nuclear ribosomal DNA (ITS and LSU) data. The genus is shown to be polyphyletic, with at least five phylogenetic lineages corresponding to known genera of Agaricomycetes (Panaeolus, Agrocybe, Parasola, Conocybe, and Leratiomyces). The type species of Galeropsis, G. desertorum, is combined to Panaeolus, and other six new combinations are proposed. In total, eight type collections are studied. The lectotype for Psammomyces plantaginiformis is designated here. Gastrocybe iberica is placed as synonym of Panaeolus desertorum, and the names Galeropsis andina and Galeropsis bispora are synonymized under Panaeolus plantaginiformis. The detailed morphological descriptions and illustrations of microscopic structures for all studied species are given. The phylogenetic inference and our current understanding of the phylogenetic structure and ecology of Galeropsis are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the time of its description nearly 100 years ago (Velenovský 1930), the genus Galeropsis has long been regarded by the unanimous opinion of mycologists as a rather unique group of secotioid fungi (Zeller 1943; Pilát 1948; Singer 1963; Thiers and Watling 1971; Gorovoi and Batyrova 1986). The type species of the genus Galeropsis desertorum Velen. & Dvořák was collected by Dvořák in Czechoslovakia (currently Czech Republic) (Velenovský 1930). At least 15 species have been considered members of the genus at different times. The first collection was found by Harley in South Africa in 1841 and published by Berkeley (1844) as Bolbitius mitraeformis but subsequently attributed to Galeropsis by Heim (1950). The last species described in the genus was Galeropsis aporos collected by Divet from France in 1992 and described by Courtecuisse (1993). It is quite typical that species ultimately attributed to Galeropsis were often originally described under other generic names or were ever transferred from one genus to another (including Galera, Bolbitius, Conocybe, Agrocybe, Gastrocybe, Secotium, Weraroa, Cyttarophyllum, Psammomyces) which further stressed the unobvious taxonomic status of Galeropsis.
These fungi are extremely rare and have a distribution closely linked to xerophytic or subxerophytic environmental conditions in different regions (from deserts in South America and mountains of North America, dry sandy places of South Africa and Madagascar to the deserts of Central and East Asia and steppe regions of Central Europe). Being treated for a long time as a member of Secotiaceae (or family Galeropsidaceae close to Secotiaceae, according to Singer 1963) essentially due to its morphology, the genus Galeropsis do in fact combine the characters inherent in gasteroid and agaricoid taxa. This fact was emphasized by many authors (Heim 1950, 1971; Kotlaba and Pouzar 1959; Singer 1963; Watling and Martín 2003), who were inclined to consider Galeropsis as a transitional taxon between the above-mentioned groups of fungi owing to the combination of following characters: partly enclosed and ovoid pileus; lacunar hymenophore or in some cases regularly developed lamellae often connected by anastomoses; differentiated stipe; passive spore discharge; ochre-brown color of spores, their germ pore; the structure of pileipellis; the presence in some species of hymenial cystidia; clamped hyphae.
Some authors (Singer 1963; Watling et al. 1986) believed the family Galeropsidaceae to be a rather artificial assemblage of bolbitiaceous and strophariaceous elements; hence, it was often emphasized the particular affinities of Galeropsis to Conocybe, Bolbitius, and Agrocybe. However, in spite of recent attempts to find relationships between gasteroid and agaricoid morphs based on modern taxonomic methods associated with DNA sequencing (Krüger et al. 2001; Moncalvo et al. 2002; Matheny et al. 2006; Baroni and Matheny 2011; Braaten et al. 2014; and others), any complete revision of the genus Galeropsis has not been implemented so far. The molecular phylogenetic analyses conducted by Tóth et al. (2013) merely questioned a monophyly of Galeropsis, i. e., the fact that it represents a true evolutionary group within the Basidiomycota, but only one collection was involved in the analysis. Nevertheless, an interesting and new result of the mentioned work was the placement of the type species of Galeropsis (G. desertorum) in the Panaeolus-Panaeolina clade with a pretty morphologically diverse set of species as closest relatives based on the sequences of three loci (ITS, nrLSU, and tef1-alpha).
Since no molecular systematic work has been published investigating the taxonomic status of Galeropsis and species boundaries in the genus, the goal of our study was to shed light on the molecular phylogeny of Galeropsis by examining as many species as possible, including all available type collections. For this purpose, we generated nucleotide sequences of two nuclear ribosomal DNA regions: internal transcribed spacer (ITS) and large ribosomal RNA subunit (LSU).
In the present study, we sequenced, re-examined, and thoroughly illustrated 29 collections and among them eight type specimens. Besides, the value of morphological features and geographic distribution of species was reconsidered. The phylogenetic analysis led to splitting Galeropsis into three main lineages within known agaricoid genera, and, as a result, eight new combinations in Panaeolus, Agrocybe, and Parasola were proposed.
Materials and methods
Studied collections, morphological examination
Type collections from LE, LIP, S, NYS, BRNM, BAFC, and AH herbaria were studied. Herbarium acronyms are given according to Thiers (2016). Macroscopic descriptions were based on fresh basidiocarps as well as on photos taken on site. Microscopic features were described from dried material. The collections were examined and drawn using standard microscopic techniques (Clémençon 2009) with Axio Imager.A1 (Carl Zeiss, Germany) microscope. For statistical evaluation of spore dimensions, at least 30 spores were measured from each basidiocarp; [100, 5, 5] indicates measurements based on 100 spores from 5 basidiocarps in 5 collections. Spore dimensions are given following the form (a–)b–c(–d), with b–c containing at least 90% of all values and the extremes (a, d) enclosed in parentheses, and avl × avw are the mean value of length and width of the total spores measured. Q indicates the basidiospore length/width ratio, avQ represents the mean length/width quotient of the total spores measured.
Molecular techniques
In total, 39 specimens were selected for molecular sampling (Table 1). DNA was extracted from small fragments of dried basidiocarps using the NucleoSpin Plant II Kit (Macherey-Nagel GmbH & Co. KG) according to the manufacturer’s protocols, without modification. The following primers were used for amplification and sequencing: ITS1F-ITS4B (White et al. 1990; Gardes and Bruns 1993) for the ITS1-5.8S-ITS2 fragment; LROR-LR5 (White et al. 1990; Vilgalys and Hester 1990) for part of nrLSU region. Whenever unique ITS fragments were amplified, they were directly sequenced. For old type or poorly preserved specimens that were often contaminated with ascomycetous fungi, a cloning of the PCR products was performed using Quick-TA Cloning Kit (Evrogen, Russia), according to the manufacturer’s instructions. At least 3–4 positive clones from each sample were then re-amplified and sequenced with the same primers.
PCR products were purified applying the GeneJET Gel Extraction Kit (Thermo Scientific, Thermo Fisher Scientific Inc., MA, USA). Sequencing was performed with an ABI model 3130 Genetic Analyzer (Applied Biosystems, CA, USA). Raw data were edited and assembled in MEGA X (Kumar et al. 2018).
Microscopic and molecular studies of specimens from LE, LIP, S, NYS, BRNM, and BAFC herbaria were carried out at the Center for collective use of scientific equipment “Cellular and molecular technology of studying plants and fungi” (Komarov Botanical Institute, Russian Academy of Sciences, St. Petersburg).
Phylogenetic analyses
For this study, 31 nrITS and 34 nrLSU sequences were generated (Table 1). In addition to the newly generated sequences, 36 nrITS and 37 nrLSU were retrieved from the GenBank database (www.ncbi.nlm.nih.gov/genbank/) using the BLAST application and taxonomic considerations. The taxonomic identities of these sequences are given (Table 1) as they appear in GenBank.
Sequences were aligned with the MAFFT version 7 web tool (http://mafft.cbrc.jp/alignment/server/) using the Q-INS-i option for both datasets.
Phylogenetic reconstructions were performed with maximum likelihood (ML) and Bayesian inference (BI) analyses. Before the analyses, the best-fit substitution model was estimated separately for the nrITS and nrLSU alignments based on the Akaike information criterion (AIC) using FindModel web server (http://www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html). GTR model was chosen for all datasets and therefore was used for concatenated dataset (ITS+LSU). Maximum likelihood analysis was run on RAxML servers, v.0.6.0 (http://raxml-ng.vital-it.ch/#/) with 100 rapid bootstrap replicates. BI analysis was performed with MrBayes 3.2.5 software (Ronquist et al. 2012), for two independent runs, each with 5 million generations under described model and four chains with sampling every 100 generations. To check for convergence of MCMC analyses and to get estimates of the posterior distribution of parameter values, Tracer v1.7.1 was used (Rambaut et al. 2018). We accepted the result where the ESS (effective sample size) was above 200 and the PSRF (potential scale reduction factor) was close to 1.
Newly generated sequences have been deposited in GenBank with corresponding accession numbers (Table 1). The alignment has been deposited in TreeBASE (S23820).
Results
Phylogenetic analyses
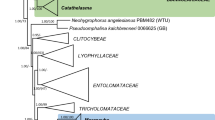
The final nrITS+nrLSU combined dataset consisted of 1721 characters, including gaps. The overall topologies of the ML and BI trees are almost congruent. In all analyses, the studied Galeropsis collections are distributed among separate divergent lineages corresponding to different genera within Agaricomycetes (Fig. 1):
Combined nrITS + nrLSU topology from Bayesian inference analysis showing the placement of Galeropsis species within genera of the Agaricales. Collection numbers are given for all specimens. Newly generated sequences are indicated in bold and the type collections are marked with black circles. Support values (Bayesian posterior probability/maximum likelihood bootstrap values) are given above the branches. Scale bar shows expected changes per site
-
1.
One of the clades including the Galeropsis generic type (G. desertorum) and receiving very high support (bs = 93%, pp = 0.99) is placed within the genus Panaeolus, in agreement with previous findings (Tóth et al. 2013). The clade was found to contain the authentic (leg. by R. Dvořák) and modern collections of G. desertorum from the Czech Republic, as well as type specimens of Gastrocybe iberica, which confirms the synonymy of both taxa in accordance with Moreno et al. (1989).
As indicated in our phylogenetic reconstruction, at least one more group at species level comprising sequences of Galeropsis can be distinguished aside from G. desertorum within Panaeolus. This clade receiving medium support (bs = 70%, pp = 0.63) encompasses the lectotype of Psammomyces plantaginiformis as well as type specimens of Galeropsis bispora and G. andina, and is not even sister to G. desertorum. It also includes some other collections previously identified as G. desertorum but with Asian distribution, whereas all European collections grouped in the first clade.
The recognition of these two taxa and their placing in the genus Panaeolus cause a reassessment of morphological criteria relevant to the species delimitation. The discussion concerning this issue is given in the taxonomic part of the paper.
-
2.
The type collection of Bolbitius liberatus, together with several specimens originally identified as G. besseyi, is placed in the Agrocybe clade and grouped together with Agrocybe pediades lineage with medium statistical support (bs = 73%, pp = 0.74). However, all studied collections are clearly different in their DNA sequences, morphology, and geographic distribution, both among themselves and from A. pediades. Hence, we consider Galeropsis liberatus as separate species and combine it to Agrocybe based on molecular evidences. The revision of morphology in the light of new data also allowed us to treat some other species formerly known as members of the genus Galeropsis but not included in molecular study (G. angusticeps and G. deceptiva) as now belonging to Agrocybe (see taxonomic part).
-
3.
The type collection of Galera besseyi occupies a sister position to the “B. liberatus-A. pediades” clade. The similarity of G. besseyi and B. liberatus is also confirmed morphologically. However, the differences in nucleotide structure of nrITS sequences (ten positions) of type specimens of B. liberatus and G. besseyi are sufficient to treat them as separate species.
-
4.
The type collection of Galeropsis aporos is nested within the Parasola clade. Based on morphological and ecological characteristics, G. aporos resembles some members of Conocybe but phylogenetically the most closely related taxon is Parasola plicatilis-similis. Hereby, we propose a new combination Parasola aporos.
Type studies
Galeropsis aporos Courtec., Documents Mycologiques 22(88): 4 (1993) (Fig. 2).
Original descriptionGaleropsis (Gastrocybe) lateritia (Watling 1968) Moreno et al. Moreno et al. 1989 valde similis sed robustior. Sporae 11–18 × 7–9.5 μm, ovo-ellipticae vel subpiriformes, poro germinativo orbae. Basidia tetrasporica. Cheilocystidia 40–65 × 5–45 μm, utrilageniformia. Pileipellis ixo-hymeniformis ex cellulis clavatis vel piriformibus 30–50 × 10–18 μm.
Type: FRANCE, lawns of Chartres Hospital (Eure-et-Loir), gregarious, Nov. 7 and Nov. 9, 1992, leg. C. Divet (No. RC/F92.191, LIP).
Morphological examination Macroscopic (and partly microscopic) characters are given taking into account the original source (Courtecuisse 1993):
Pileus 10–12(16) mm high and 3–4 mm broad, chocolate brown, finely striate up to disc like a Coprinus, remaining constantly cylindrical, not spreading with age, glabrous, shiny. Lamellae not described, but clearly visible on dry samples, apparently without anastomoses. Stipe 10–12(16) cm high, very thin, uniformly white, cartilaginous, smooth, easily separable from pileus.
Basidiospores 11–18 × 7–10 μm, broadly ellipsoid, oblong ellipsoid or ovoid, some pyriform or almost triangular (with wide base and narrowed apex, 14.5–17.5 × 7.5–9.5 × 5.5–7 μm), bean-shaped or irregularly shaped, avl × avw = 15.2 × 8.5 μm, Q = 1.55–2.07, avQ = 1.79, slightly thick-walled, grayish brown in KOH, vacuolated, with broad apiculus and without germ pore. Basidia 4-spored (rarely 2-spored), 27–33(40) × 10–12 μm, broadly clavate, thin-walled. Pseudoparaphyses present, broadly clavate, up to 20–25 μm in length. Trama made up of branched hyphae 4–7(10) μm wide, with numerous septa. Cheilocystidia quite numerous, 40–65 × (5)18–25(45) μm, utriform or lageniform, thin-walled, colorless. Pleurocystidia absent. Pileipellis an ixo-hymenoderm, with upper layer of narrowly clavate, cylindrical or piriform elements, often capitated at apex, 30–60(80) × (5)10–20 μm. Stipitipellis a cutis, made up of thin-walled hyaline hyphae 4.5–12(25) μm broad. Caulocystidia absent. Clamp connections present, abundant.
Studied collection Holotype.
Bolbitius liberatus Kalchbr., Bulletin de la Société Impériale des Naturalistes de Moscou: no 1302 (1879) (Fig. 3).
Original description Pileo elongato, acuminato, deorsum contracto et sic sublanceolato (ad formam calyptrae Polytrichi juniperini), laevi, glabro, colore recentis corii; stipites fistuloso, gracili, basi bulbilloso, subconcolori; lamellis linearibus, ochraceis.
Type: Hab. ad Somerset-East Promont. Bonae Spei, 1877, leg. P. Mac Owan. No 1302 in: Thümen, F. von. 1879. Mycotheca Universalis, Centurie 14 (1301–1400). Bulletin de la Société Impériale des Naturalistes de Moscou.
Morphological examinationPileus cylindrical to ovoid, 8–15 mm high and 7–10 mm broad, slender, membranous, with acute apex, margin incurved or adhered to stipe, glabrous, dried fragile, grayish brown or straw-colored, striate to disc. Lamellae narrow, crowded, rust-brown, with concolorous margin. Stipe slender, 30–60 × 1.5–3 mm, slightly broadened towards base, glabrous, yellowish.
Basidiospores [30, 1, 1] 11–13(14.5) × (6)7–9(11) μm, avl × avw = 12.3 × 7.9 μm, Q = 1.23–2.15, avQ = 1.58, broadly ellipsoid, pip-shaped, some ovoid or lentiform, more rarely somewhat amygdaliform, slightly thick-walled, honey brown or yellow-brown in KOH, with very prominent large (up to 2 μm), central or slightly excentric germ pore and broad apiculus. Basidia 1-, 2-, and 4-spored (2-spored predominate), 14–27 × 7–8 μm, narrowly to broadly clavate, thin-walled, sterigmata prominent and wide. Pseudoparaphyses present, broadly clavate or utriform, similar in size to basidia. Cheilocystidia quite numerous, 19–32 × 8–12 μm, lageniform with subcapitate apex, more rarely broadly lanceolate or fusiform, thin-walled, colorless or some with yellowish content. Pleurocystidia absent. Pileipellis a trichohymeniderm consisting of palisade of cylindrical or narrowly clavate terminal elements, 10–18 × 4–7 μm, with yellow-brown intracellular pigment. Stipitipellis a cutis, made up of thin-walled, yellow-brown hyphae 3–4 μm broad. Caulocystidia absent. Clamp connections present.
Studied collection Isotype (F14242, S).
Galera besseyi Peck, Bulletin of the New York State Museum 131: 35, Pl. V, Figs. 15–20 (1909) (Fig. 4).
Original description Pileus tenuis, ovatus ovalisve, rare subglobosus, obtusus, glaber, nunquam expansus, isabellinus vel subochraceus, in margine abrupte contractus et stipitem amplexans; lamellae tenues, confertae, ascendentes, adnatae, ferrugineol-brunneae; stipes gracilis, subflexuosus, glaber, levis vel substriatus, pileo in colore similis; sporae ellipsoideae, 14–15 × 10–12 μ. Pileus 5–12 mm longus, 4–10 mm latus; stipes 2.5–5 cm longus, 1–2 mm crassus.
Type: USA, Garden of the Gods, Manitou, El Paso Co., Colorado, 8 August 1908, sandy soil, leg. C.E. Bessey and E.A. Bessey (NYSf 453.1).
Morphological examination Description of macroscopic characters derived from a protologue: “Pileus thin, ovate or oval, rarely subglobose, obtuse, glabrous, never expanding, isabelline or pale dingy ochraceous, the margin abruptly contracted and closely embracing the stem; lamellae thin, connected by transverse anastomoses, ascending, adnate, ferruginous brown; stem slender, slightly flexuous, hollow, glabrous, even or slightly striate, colored like the pileus; spores broadly ellipsoid, .00055–.00065 of an inch long, .0004–.0005 broad. Pileus 2.5–6 lines long, 2–5 lines broad; stem 1–2 inches long, .5–1 line thick.”
Basidiospores [30, 1, 1] 12.5–16.5(17) × (7)8–10.5(12) μm, avl × avw = 14.7 × 9.4 μm, Q = 1.41–1.73, avQ = 1.57, broadly ellipsoid, pip-shaped, some lentiform, distinctly thick-walled, sepia or yellow-brown in KOH, with very prominent large (up to 2 μm), central or sometimes slightly truncated germ pore and broad apiculus. Basidia 1- or 2-spored (2-spored predominate), 23–30 × 7–10 μm, narrowly to broadly clavate, almost cylindrical, with short pedicel and undulating walls, thin-walled, sterigmata prominent and inflated. Pseudoparaphyses absent. Cheilocystidia quite numerous, 28–37 × 6–12 μm, lageniform with subcapitate or capitate apex, thin-walled, colorless. Pleurocystidia not seen. Pileipellis a hymeniderm, with outer layer consisting of spheropedunculate, pyriform or broadly clavate elements, 15–25 × 6–12 μm, slightly thick-walled and colorless. Pileocystidia scattered, 15–25 × 4–8 μm, cylindrical or narrowly clavate, with golden brown intracellular pigment. Stipitipellis a cutis, made up of thin-walled, yellowish hyphae 3–5 μm broad. Caulocystidia present, numerous, 12–30 × 4–8 μm, cylindrical with capitate apex or narrowly clavate, slightly thick-walled, yellow-brown. Clamp connections present.
Studied collection Holotype.
Gastrocybe iberica G. Moreno, Illana & Heykoop, Cryptogamie Mycologie 8(4): 323 (1987) (Fig. 5).
Original description Pileus (0.6) 0.8–2.2 (2.5) cm longus, 0.2–0.4 cm latus, cylindricus, acuto apice, colori paleae simili, at cinereo in herbario, sine striis glaber, cuius inferior pars clausam atque pedi adhaerentem se praebet. Stipes, qui fit in basi paulo amplior (1) 2–4 (5.5) cm longus, fere 0.1 cm est latus. Laminae sunt ascendentes, pressae, proba forma, haud anastomosantes, interdum bifurcatae sunt ascendentes, pressae, proba forma, haud anastomosantes interdum bifurcatae in basi, ochraceo-ferrugineo colore. Lamellulae non observantur. Velum abest. Basidiosporae 15–20 (23) μm longae, 9–13 (18) μm sunt latae, quarum forma inter ellipsoidea et amygdaliformis, ochraceo colore, germinativo poro praeditae. Basidia bisporica sunt, 18–22 μm longa, 9–11 μm lata. Cystidia absunt. Pileipellis hymeniformis, cellulis quarum diameter est 9–17 μm longus constituta. Pileocystidia frequentissima, hyalina, lageniformia, quorum est longitudo maxima 60 μm, latitudo e 15 μm vergit in 6 μm rursusque in 8 μm. Fibulae adsunt.
Habitat: in pratis hygrophytis (Poaceae sps., Trifolio fragiferi-Cynodontetum), basico in solo (pseudogrey) populeti (Populus alba). Item in pratis (Hordeion leporini), basico in solo (rendsinas).
Type: In praedio cui nomen “La Oruga,” Compluti (Matriti) 30TVK7282, leg. G. Moreno, C. Illana & M. Heykoop, 9–X–1986 (AH 9990).
Morphological examination Description of macroscopic (and partly microscopic) characters is given based on original source (Moreno et al. 1987):
Pileus (6)8–22 × 2–4 mm, cylindrical, acute apex, cream-straw colored, ash-colored when dried, non-striated and glabrous, touching the stipe with lower narrow part. Lamellae ascendant, narrow, well formed, not anastomosing but sometimes forked as the bases, ochraceous-ferruginous. Lamellulae not observed. Stipe becoming slightly broader at the base (10)20–40(50) mm high an approximately 1 mm broad. Veil absent.
Basidiospores [30, 1, 1] 12.5–19.7(21.7) × 6.3–14.3 μm, avl × avw = 16.0 × 9.8 μm, Q = 1.25–2.25, avQ = 1.64, variable in shape, broadly ellipsoid, ellipsoid-amygdaliform, some lentiform or pyriform, ochraceous, with pronounced hilar appendage, and with germ pore not clearly visible under the optical transmitted-light microscope but very clear with “Nomarski” optics (up to 2–2.5 μm, sometimes papillate), thin- or slightly thick-walled. Basidia 1- and 2-spored in equal proportion (3-spored form was observed once), 15–23 × 8–11 μm, sterigmata long, up to 7 μm in length, sometimes pigmented. Hymenial cystidia not observed. Pileipellis hymeniform, formed by clavate to pyriform or subglobose cells, 9–17 μm in diameter. Pileocystidia very abundant, lageniform with inflated base and long cylindrical neck and subcapitate apex, (16)25–60 × 10–15 × 4–6(8) μm, hyaline. Stipitipellis a cutis, made up of thin-walled, yellowish hyphae 5–10 μm broad. Caulocystidia abundant, in clusters, 20–60 × 5–7 μm, narrowly clavate or cylindrical, often sinuous, with subcapitate or rounded apex, thin-walled, hyaline. Clamp connections present.
Studied collection Holotype.
Galeropsis andina Singer, Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen Section C 66: 107 (1963) (Fig. 6).
Original description Gastrocarpio conico-obtusato, cylindraceo vel ovoideo-elongato, haud acutissimo nec acute-appendiculato, 6–33 mm alto, 4–12 mm lato; peridio alutaceao-stramineo vel corricolori-ochraceo, subbrunnescente, haud nitente, haud viscido, levi, demum frequenter rivuloso, vel rimoso, tenui; columella stipitem elongante et cum hoc continua; gleba lamelliformi, lamellis subverticaliter ascendentibus, angustis, venis transversalibus, frequenter, corrugates sed haud anastomosantibus nec furcates; velo nullo; stipites pileo longoire vel rarius paullo breviore, pallide albo. – Sporis 11.3–18 × 7.2–11 μ (illis quae e basidiis tetrasporis natae sunt, 11.3–16.5 × 7.2–10 μ metientibus), poro germinativo paulum evoluto, haud vel vix truncates, apice membrana haud vel vix modificate sed in zona angusta pigmento destitute praeditis, pallide melleis, rarius melleis (KOH); basidiis aut omnibus tetrasporis aut bi- et tetrasporis mixtis; cheilocystidiis nullis; epicute haud gelatinosa, fasciculis densis sed sparse dispositis obsita, e dermatocystidiis pseudoparaphysoideis efformatis.
Type: ARGENTINA, Mendoza, Dpto. San Carlos, Arroyo de Cruz de Piedras, leg. A. Ruiz Leal, 20 I 1941 (no. 7287).
Morphological examination Description of macroscopic (and partly microscopic) characters is given based on original source (Singer 1963):
Pileus conic-elongated or campanulate-elongated, often also cylindric or obovoid-elongated, always much longer than broad, 6–33 × 4–12 mm, obtuse at apex or rarely subacute or mucronate, leather-straw-colored to dull ochraceous, in herbarium more brownish, not shining, not viscid, smooth, in age becoming rivulose or rimose but not plicate, thin. Lamellae narrow, corrugated by transversal veins, but not anastomosing, not forked, adnate, uniformly ochraceous brown. Stipe 13–70 × 1–2.5 mm, usually longer than pileus, slender, equal or subequal, rigid-brittle, white, later slightly buffish pallid, fistulose-hollow, often slightly curved, spirally contorted and striate in dried specimens, glabrous or subglabrous, dry.
Basidiospores [30, 1, 1] 10.5–14.5(16) × (6.5)7–9 μm, avl × avw = 13.0 × 7.9 μm, Q = 1.38–2.05, avQ = 1.65 (the spore size slightly differs from Singer’s description—11.3–16.5 × 7.2–10 μm; also, he noted the presence of giant spores 18.2–15.8 × 11–13.3 μm that we did not observed), variable in shape, mostly broadly ellipsoid, pip-shaped, lentiform or ovoid, some cylindrical or irregularly shaped, slightly thick-walled, sepia or yellow-brown, with indistinct (often visible only as a narrow hyaline zone of the wall) central germ pore and broad apiculus. Basidia 1–4-spored (4-spored predominate), (17.5)20–28(33) × 9–13 μm, broadly clavate, with short pedicel, thin-walled, sterigmata prominent and inflated, in some cases very long (up to 23 μm). Pseudoparaphyses difficult to distinguish from the basidioles. Cheilocystidia and pleurocystidia absent. Pileipellis a hymeniderm, not gelatinized, with outer layer consisting of spheropedunculate, pyriform or broadly clavate elements, 17–30 × 8–15 μm, slightly thick-walled, and colorless. Stipitipellis a cutis, made up of thin-walled, brownish hyphae 4–10 μm broad. Caulocystidia not seen. Clamp connections present.
Studied collection Isotype (BAFC No 31514).
Galeropsis bispora Vassilkov, Acta Inst. bot. Komarov. Acad. Sci. URSS, Pl. Crypt., Ser. II, 9: 463 (1954) (Fig. 7).
Original description Receptaculum 1–2.5 cm elatum. Peridium pileiforme, 1–1.3 × 0.3–0.5 cm, cylindrico-conicum, vel fusiforme, tenuiter-carnosum, longitudinaliter-sulcatum, rugosum, innato-fibrosum, subgriseo-fuscum, margine subdirumpo. Stipes (columella exclusa) 0.3–1.5 × 0.1 cm cylindricus, longitudinaliterfibrosus, sordide-albidus. Gleba foveolato-lamellata, obscurofusca. Basidia 18–24 × 8–10 μ, late-clavata, ovata, saepius sacculiformia, interdum curvata, 2 (1, 3) sterigmatis rectis, conicis vel leniter curvatis, firmis, ad 4.5 μ longis, vulgo subfusci colorati. Sporae 10–16–(18) × 6–10–(11) μ, ovatae, late-ellipsoideae, amygdalaeformes, interdum monstrosae, crassetunicatae, brunescente-cinnamomeae, pora germinativa.
Type: Uzbekskaja RSS (presently the REPUBLIC OF UZBEKISTAN), vicinity of Fergana, desert, on moist grass-covered clay soil, leg. N.G. Achafeev, 16 III 1950 (LE 2863).
Morphological examination Description of macroscopic characters is given based on original source (Vassilkov 1954):
Basidiocarp 1–2.5 cm high. Pileus 1–1.3 × 0.3–0.5 cm, cylindrical-conical or fusiform, thin, longitudinally sulcate, wrinkled, fibrillose, with disrupted margin, dull grayish brown. Lamellae narrow, crowded, rust-brown, with concolorous margin. Stipe 0.3–1.5 × 0.1 cm, cylindrical, longitudinally fibrillose, whitish.
Basidiospores [30, 1, 1] 10.5–15 × 7–9.5 μm, avl × avw = 12.8 × 8.4 μm, Q = 1.18–1.78, avQ = 1.52 (the spores are slightly smaller than Vassilkov observed—10–16–(18) × 6–10–(11) μm), variable in shape, predominantly broadly ellipsoid, lentiform or ovoid, some bean-shaped or irregularly shaped, strongly thick-walled, yellow- or rust-brown, with distinct wide central or slightly excentrical germ pore, and broad apiculus. Basidia 1–2-spored (sparse 3-spored also present), 13–25 × 8–11 μm, broadly clavate or saccate, with short pedicel, thin- or thick-walled, with prominent and thick (up to 6–7 μm long and 3 μm wide), often golden brown peculiar sterigmata. Pseudoparaphyses and hymenial cystidia not seen. Pileipellis a hymeniderm, with outer layer consisting of spheropedunculate, pyriform or broadly clavate elements, 20–40 × 10–20 μm, slightly thick-walled, and colored at base (with intracellular and encrusting yellow-brown pigment). Stipitipellis a cutis, made up of thin-walled, hyaline hyphae 6–7 μm broad with clamps. Caulocystidia-like elements present, cylindrical or clavate, 12–35 × 6–10 μm, hyaline, thin-walled. Clamp connections numerous in all tissues.
Studied collection Holotype.
Psammomyces plantaginiformis Lebedeva, Bull. Pl. Prot. 5(1): 117 (1932) (Fig. 8).
Original description Peridium pileiforme, cylindraceo-conicum vel anguste fusiforme, siccus, tenui-carnosum, gelatinosum, cum plicis spiraliter tortis, rugulosum, ad superficiem cum fibris tenuibus, conglutinates, flocciformibus, cinnamomeo-fuscum, ad marginem inferiorem inaequale, tenui-plicatile, 1.5–3 cm long., 0.6–0.8 cm diam. Contextus interior exoperidii e prosenchymate, raro septato, tenui tunicato. Inter exoperidium et endoperidium prosenchyma laxum, glutinosum, tenui-tunicatum. Gleba e lamellis verticalibus, anastomosantibus, interdum in loculos abeuntibus. Basidiae clavatae vel cylindraceae, vel ovoideae, hyalinae vel fuscescentes, 20–30 μm long., 10–15 μm lat., sterigmata quatuor, filiformia, elongate, flexuosa, 10–15 μm lg., 0.5–1.5 μm lat. Basidiosporae ovato-ellipsoideae, saepe inaequilaterales, leves, fuscae, crassiuscule-tunicatae, 8–10(−15) μm long., 5–6 μm lat. Stipes centralis, lignosus, tortus, fuscescens, superne in columellam endoperidium attingentem, cavam abeuns, extus e cellulis tenuibus, intus e cellulis crasse-tunicatis, valde elongates, raro septalis, fuscescentibus, prosenchymaticis contextus. Cavus cylindri axillaris stipites et columellae hyphis fusco-cinnamomeis, septalis, rectis vel flexuosis vestitus.
Type: Habitat in arena prope pagum Ischey-Metschet, districti Prikumensis, Regionis Terek, Caucasi borealis, 12 junio 1925 legit A. Lobik.
Morphological examination The macroscopic description is based on dried material: Pileus 12–40 mm high and 4–10 mm broad, cylindrical, narrowly conical-elongated or fusiform, sometimes spirally contorted, always longer than broad, mucronate at apex, margin incurved and often torn, longitudinally striate, fulvous or dull gray-brown, smooth. Lamellae narrow, crowded, often agglutinate, adnate, henna, or rusty brown. Stipe 30–100 × 2–4 mm, slender, cylindrical, longitudinally fibrillose, often helically twisted, ochraceous, shining, glabrous.
Basidiospores [30, 1, 1] (9.5)11–13 × 6–7.5(8.5) μm, avl × avw = 11.7 × 7.0 μm, Q = 1.49–1.88, avQ = 1.68, narrowly to broadly ellipsoid, narrowly to broadly amygdaliform, some nonstandard and irregularly shaped, strongly thick-walled, lightly colored, ochraceous or yellow-brown, with wide (to 2 μm), central, sometimes slightly papillate, germ pore, and broad apiculus. Basidia 3- and 4-spored, 21–32 × 7–10 μm, cylindrical or narrowly clavate, hyaline or yellowish, often with medial constriction, with long thin and curved sterigmata (up to 10 μm long). Pseudoparaphyses not seen. Hymenial cystidia absent. Pileipellis a hymeniderm, consisting of chains of spheropedunculate, pyriform or broadly clavate, slightly thick-walled, yellow-brown elements, 27–45 × 10–25 μm. Stipitipellis a cutis, made up of thin-walled, hyaline hyphae 5.5–7 μm broad. Caulocystidia absent. Clamp connections present.
Studied collection The holotype appears to be lost and is not found in either LE or other herbaria. However, there is an authentic collection kept in LE and definitely taken into account by Lebedeva for protologue, for which the description is given above. We designate it as lectotype here:
RUSSIA, Caucasus, Terek Region, Prikumen district, steppe solonchak, on sandy soil, 16 VI 1925, leg. A. Lobik (LE 2862, lectotype, here designated, MBT 386209).
Taxonomic conclusions
Below, we consider all taxa ever placed in the genus Galeropsis and give a discussion on their current taxonomic concepts.
Parasola aporos (Courtec.) E.F. Malysheva, comb. nov.
Basionym: Galeropsis aporos Courtec., Documents Mycologiques 22(88): 4 (1993).
MycoBank No. MB 829492
Based on some macroscopic characters (such as cylindrical, striated, not expanding at maturity pileus), Galeropsis aporos agrees with the general concept of Galeropsis. However, the species is also highly similar to Conocybe deliquescens Hauskn. & Krisai (= Gastrocybe lateritia Watling) in terms of shape and color of pileus, very long and thin stipe, spore size, and structure of pileipellis. Accordingly, there are several morphological characters of G. aporos matching different genera concepts and the species delimitation mainly relies on the peculiar, mostly pyriform, grayish-colored spores without germ pores, presence of pseudoparaphyses and numerous utriform cheilocystidia.
The results of our study revealed G. aporos is phylogenetically closest to the recently described European species Parasola plicatilis-similis L. Nagy, Szarkándi & Dima (Szarkándi et al. 2017) and both taxa are placed in one highly supported clade within the Parasola monophyletic group (Fig. 1). The nrITS sequences of type collections of both species (MH196348 and KY928620) have 100% similarity. Nevertheless, G. aporos morphologically differs from P. plicatilis-similis in significantly lighter and larger spores (11–18 × 7–10 μm vs 10.5–13.5 × 8–11.5 μm) with lack of germ pore, the absence of pleurocystidia and habit of basidiocarps (P. plicatilis-similis is characterized by typical coprinoid appearance).
In the present study, we found that the taxonomic value of some morphological characters of the studied species needs to be amended in concordance with phylogenetic inference. Nevertheless, based on our observation and at this stage of knowledge, it would be reasonable to consider G. aporos as a separate species but not as a secotioid morph of P. plicatilis-similis because of the strong morphological differences found between them until we get additional data to prove otherwise. Hence, we propose here a new combination Parasola aporos.
Ecology In large groups (of 10 to 20 basidiocarps), occupying a vast area of about 200 m2, among grass, on lawns, or open places.
Distribution Known from Europe (France).
Panaeolus allosporus (Singer) E.F. Malysheva, comb. nov.
Basionym: Galeropsis allospora Singer, Lilloa 23: 239 (1952).
= Galeropsis allosperma Singer, Lilloa 22: 733 (1951).
MycoBank No. MB 829486
The species was originally described by Spegazzini as Galera paradoxa Speg. in 1900 (Spegazzini 1913, republication) based on a collection from sandy grasslands along the Rio Chubut in Patagonia (Argentina). After studying Spegazzini’s original collection, Singer (1949, 1950) reached a conclusion that this species, having many similar features with Galeropsis desertorum, should be treated as a member of the genus Galeropsis. He distinguished it from another species, Galeropsis paradoxa (Mattir.) Pilát, which was based on an African collection of Galera paradoxa Mattir., by proposing a new replacement name Galeropsis allosperma Sing. ad interim (Singer 1949) for Spegazzini’s species, and then, after more careful consideration of the issue, Galeropsis allospora Singer (1950).
The main morphological characters (following the original description of Spegazzini (1913) as well as the type revision of Singer (1950)) are cylindrical pileus acuminated at apex, chestnut or rusty brown, 20–40 mm high and 4–7 mm broad; developed narrow lamellae scarcely forked; long stipe 60–100 × 1.5–3 mm; the absence of cheilocystidia; thick-walled, ferruginous, asymmetric spores, 16.4–21.3 × 9.8–14.7 μm, with poorly determined germ pore (often like hyaline zone).
Although the sequencing of the type specimen has not yet been implemented, we incline to consider this taxon to belong to the genus Panaeolus, like the Galeropsis generic type G. desertorum, because of their morphological similarity until it is eventually confirmed or confuted by molecular data.
Ecology On sandy soil, in grassy place.
Distribution South America (Argentina). It seems to be known so far only from the type locality.
Panaeolus desertorum (Velen. & Dvořák) E.F. Malysheva, G. Moreno, Svetash. & M. Villarreal, comb. nov. (Figs. 9, 10 and 11).
Basionym: Galeropsis desertorum Velen. & Dvořák, Mykologia 7(9–10): 106 (1930).
= Gastrocybe iberica G. Moreno, Illana & Heykoop, Cryptogamie Mycologie 8(4): 323 (1987).
MycoBank No. MB 829487
Morphological descriptionPileus (6)10–37 mm high and (2)3–8 mm broad, cylindrical-conical, elongated or fusiform, often spirally contorted, always longer than broad, mucronate at apex, margin incurved or adhered to stipe, usually longitudinally striate but not sulcate, fibrillose, hygrophanous, leather-straw-colored to dull ochraceous, in herbarium dull grayish brown or ash-colored, not shining, not viscid, smooth. Lamellae narrow, crowded, not anastomosing but sometimes forked as the bases, often agglutinate, adnate, rufous or ochraceous brown. Stipe (10)15–55(80) × 1–2.5 mm, slender, cylindrical or slightly broadened towards base, longitudinally fibrillose, uniformly pruinose, ochraceous.
Basidiospores [160, 6, 6] (12.5)13.5–18(21.5) × 7–12(17.7) μm, avl × avw = 15.0 × 8.8 μm, Q = 1.22–2.25, avQ = 1.70, extremely variable in shape and size, ellipsoid or broadly ellipsoid, amygdaliform or limoniform, some lentiform, pyriform or bean-shaped, slightly to strongly thick-walled, lightly colored, ochraceous, honey-colored or grayish brown, with wide (to 2 μm), central, often papillate, germ pore or broad hyaline zone instead, and with broad apiculus. Basidia 1–4-spored (in different basidiocarps different type predominates), 18–25(35) × 8–12(16) μm, broadly clavate or saccate, with short pedicel and long thick sterigmata, sometimes pigmented (up to 10–20 μm long and 3 μm wide). Pseudoparaphyses often present, broadly clavate or utriform, similar in size to basidia. Hymenial cystidia not observed. Pileipellis a hymeniderm, consisting of chains of spheropedunculate, pyriform or broadly clavate, thin-walled or slightly thick-walled, hyaline or yellowish elements, 10–35 × 8–22 μm, (but in one collection the outer layer of pileipellis made up of long, cylindrical or narrowly clavate elements, 10–35 × 5–9.5 μm). Pileocystidia usually absent or present, if present then very abundant, hyalines, lageniform at the base, with long cylindrical neck and subcapitate apex, up to 60 × 15 × 6(8) μm. Stipitipellis a cutis, made up of thin-walled, hyaline hyphae 4–10 μm broad. Caulocystidia rather numerous (as an exception absent), 10–45(60) × 5.5–12 μm, often in bundles, variable in shape, cylindrical with subcapitate apex, clavate, utriform or lageniform, hyaline, thin- or slightly thick-walled. Clamp connections present.
Ecology Solitary or in small groups; on different soil in wet grassy areas (meadows, or ruderal places) or in steppe areas.
Distribution Western and Eastern Europe.
Studied collections SPAIN, Madrid, Alcalá de Henares, Finca La Oruga, 30TVK7282, in hygrophytic meadow (Poaceae sp., Trifolio fragiferi-Cynodontetum), on basic soil (pseudogley) from poplar grove (Populus alba); and in meadows of Hordeion Leporini, basic soil (rendsinas), leg. C. Illana, 11–X–1986, AH 9991 (Paratypus of Gastrocybe iberica); Ibidem, Madrid, Alcalá de Henares, climbing from the Finca La Oruga to the hill Ecce-Homo, 30TVK7281, leg. C. Illana, E. Illana & J. Chico, 2–XI–1986, AH 9993 (Paratypus of Gastrocybe iberica); Ibidem, Madrid, Alcalá de Henares, Los Catalanes, 30TVK7081, leg. C. Illana, I. López & P. Sánchez, 2–XI–1986, AH 9992 (Paratypus of Gastrocybe iberica). Ibidem, Madrid, Alcalá de Henares, Tabla Pintora, 30TVK6979, 620 m, leg. C. Illana & M. Heykoop, 9–XI–1986, AH 9994 (Paratypus of Gastrocybe iberica); Ibidem, Alcalá de Henares, Subida hacia el Gurugú, 30 T0469844789, meadows with Pinus halepensis, nitrophilous thickets and predominant graminoid grasslands, basic soil (calcareous marls and gypsum) eutrophized, leg. G. Moreno, G. Rodríguez & F.J. Rejos, AH 42860; Ibidem, Alcalá de Henares, Campus Universitario, pasture, leg. J.C. Pastor, C. López del Rincón & C. Illana, 12–XI–1987, AH 10396 y AH 10397; Madrid, Hoyo de Manzanares, Urbanización Zodiaco, grassland fertilized with cow dung, XI–1987, leg. Remmert Daams, AH 10791; Jaen, Sierra Morena, El Centenillo, Cerro del Hospital, pasture, leg. R. Galán & F.E. Martínez, 24–X–1987, AH 10493; Isotypus of Gastrocybe iberica in the herbarium of Dr. R. Watling in Edimburg (E) an in the herbarium of the Royal Botanic garden of Madrid (MA-Fungi). CZECH REPUBLIC, Moravia, near Mohelno, 14 X 1930, leg. and leg. R. Dvořák (BRNM 126071); Havraníky (okr. Znojmo), northwest outskirts of the village, ca 70 m from the chapel on the edge of the Havranického, on a regularly cut grass in front of a family house and in lawn, 4 X 2014, leg. J. Běťák, M. Čapoun (BRNM 772116). GREECE, bank of Megali Prespa Lake, meadow, on soil, 14 X 2014, leg. T. Svetasheva (LE 313091). RUSSIA, vicinity of Stavropol, cattle breeding farm, lawn, on soil, 23 IX 2009, leg. T. Svetasheva (LE 313090, 313250).
NotesGaleropsis desertorum was described in 1930 by Prof. Josef Velenovský based on specimens collected from the Mohelenská hadcová steppe (Moravia, Czech Republic) by amateur mycologist Rudolf Dvořák (Velenovský 1930). The very special habit of their basidiocarps as well as other morphological features that have not yet been mentioned for any known genera led Velenovský to propose a new genus along with a new species name for this fungus. In 1948, Pilát updated the original description of G. desertorum based on re-examination of Dvořák’s authentic collections and pointed the following characters as the most taxonomically important for the species: pileus 15–20 mm high and 2.5–3.5 mm wide, cylindrical or conical with pointed top, striated in its full length; developed lamellae; stipe 30–80 mm long, very thin, tubular hollow; spores vary considerably in size, in average 11–12.5 × 5.5–8.5 μm, ellipsoid-fusiform, with small germ pore, yellow-rusty. In addition, comparing morphological features of G. desertorum with another species G. plantaginiformis (Leb.) Singer, Pilát extended the concept of G. desertorum, having considered the two species as synonymous. This point of view allowed him to assume not only a European, but also Asian distribution of the species. Following this opinion, the authors of a recent paper (Li et al. 2015) identified the collection found in North China as G. desertorum.
Kotlaba and Pouzar (1959) reported a second find of G. desertorum in Slovakia, defining 2- and 4-spored basidia with slightly longer spores in comparison with the holotype, 11–14 × 7–8 μm. Bět’ák and Čapoun (2015) documented a modern find of the species in the Czech Republic, and the description of their collection fits the original and Slovak specimens.
In 1989, Moreno with co-authors (Moreno et al. 1989) studied again the holotype of G. desertorum and supplemented its description with the information on the presence of lageniform pileocystidia and hymeniform structure of pileipellis, which were not previously mentioned. They also accentuated, following Pilát (1948), a considerable variability in basidiocarp and spore size. One more conclusion made in the paper of Moreno et al. and presently confirmed by our new molecular data was the synonymy of Gastrocybe iberica and Galeropsis desertorum.
Our study of Dvořák’s authentic collection (BRNM 126071) revealed a greater range of variation in spore sizes versus Pilat’s conclusion (viz. 10.5–13.5(16) × 6.7–9(10.8) μm) as well as the presence of 2- and 4-spored basidia. The re-examination of a modern Czech collection (BRNM 772116) resulted in discovery of numerous pileo- and caulocystidia, which were not previously mentioned by the authors of the find (Běťák and Čapoun 2015).
As a result of our research, the taxonomic concept of G. desertorum was significantly amended. Furthermore, the morphological limits of the species were slightly extended based on new data obtained. Taken together, our findings are consistent with the opinion that G. desertorum is a European species that can be characterized by: basidiocarps with conical, elongated, or fusiform pileus up to 40 mm high and 8 mm broad; thin and long (up to 80 mm) stipe; 2–4-spored basidia; predominantly ellipsoid, amygdaliform, or limoniform basidiospore variable in size, 11–18(21.5) × 5.5–12(14.5) μm (though monstrous or malformed ones are normally absent), with prominent germ pore; the absence of cheilocystidia; and the presence of pileo- and caulocystidia.
The molecular data showed that the nrITS and nrLSU sequences of the species are nested within the Panaeolus clade on the phylogenetic tree (Fig. 1). Accordingly, a new combination, Panaeolus desertorum, is proposed.
Panaeolus plantaginiformis (Lebedeva) E.F. Malysheva, comb. nov. (Fig. 12).
Basionym: Psammomyces plantaginiformis Lebedeva, Sb. Vsesojuzn. Inst. Zašć. Rast. 5(1): 117 (1932).
≡ Galeropsis plantaginiformis (Leb.) Singer, Beih. Botan. Centralbl., Abt. B 56: 148 (1937).
= Galeropsis andina Singer, Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen Section C 66: 107 (1963). ≡ Galeropsis andina Singer var. andina, Proc. Acad. Sci. Amst., Ser. C 66: 107 (1963). ≡ Galeropsis andina var. gracilior Singer, Proc. Acad. Sci. Amst., Ser. C 66: 107 (1963).
= Galeropsis bispora Vassilkov, Acta Inst. bot. Komarov. Acad. Sci. URSS, Pl. Crypt., Ser. II, 9: 463 (1954). ≡ Galeropsis desertorum var. bispora (Vassilkov) G. Moreno, Heykoop & Illana, Mycotaxon 36(1): 66 (1989).
MycoBank No. MB 829488
Morphological descriptionPileus 10–40 mm high and 3–12 mm broad, cylindrical-conical, ovoid, elongated or fusiform, always longer than broad, mucronate at apex, margin usually incurved or adhered to stipe, longitudinally striate (rarely sulcate or wrinkled), fibrillose, hygrophanous, straw-colored, ochraceous or yellow-brownish, in herbarium dull grayish brown, sometimes shining, not viscid, smooth. Lamellae narrow, crowded, often agglutinate, not anastomosing, adnate, ochraceous brown or orange-brown. Stipe (10)30–80(100) × (1)2–4 mm, cylindrical, often slightly curved, spirally contorted, longitudinally fibrillose and often striate, glabrous or pruinose, whitish to ochraceous.
Basidiospores [130, 5, 5] originated from 4-spored basidia 9.5–15.5 × 5.5–8(9.2) μm, avl × avw = 12.0 × 7.3 μm, Q = 1.32–2.05, avQ = 1.65, [100, 4, 4] originated from 2-spored basidia (9.5)12–16.5 × 5.5–13 μm, avl × avw = 13.5 × 8.7 μm, Q = 1.18–2.14, avQ = 1.58, extremely variable in shape and size, ellipsoid to broadly ellipsoid, ovoid, broadly fusiform, pip-shaped, amygdaliform or limoniform, some reniform or even cylindrical, large proportion irregularly shaped or monstrous, slightly to strongly thick-walled, most lightly colored—ochraceous, golden brown or rust-brown (in only one collection LE 2867 dark-colored, cacao-brown, or topaz brown), with wide (to 2–2.5 μm) but often indistinct (visible only as a narrow hyaline zone of the wall), central, often papillate or truncated, germ pore and broad apiculus. Basidia 1–4-spored (in different collections different type predominates), (13)15–30(33) × 8–13 μm, cylindrical, narrowly to broadly clavate or saccate, often with medial constriction, pedicellate or not, with long thick, sometimes yellow-brown and opalescent, sterigmata (up to 10(23) μm long and 3 μm wide). Pseudoparaphyses absent or similar to basidioles. Hymenial cystidia usually absent but present in one collection (LE 2867)—lageniform with thick neck and subcapitated apex or utriform, 22–40 × 9–12 μm, colorless, thin-walled. Pileipellis a hymeniderm, consisting of chains of spheropedunculate, pyriform or broadly clavate, thin-walled or slightly thick-walled, hyaline or yellow-brown elements, 15–45 × 8–25 μm. Pileocystidia absent. Stipitipellis a cutis, made up of thin- or thick-walled, hyaline hyphae 5–12 μm broad. Caulocystidia absent, in some collections present, when present then numerous, (12)20–50 × 6–12 μm, variable in shape, cylindrical, clavate or lageniform, hyaline, thin-walled or slightly thick-walled. Clamp connections present or absent.
Ecology Solitary or in small to large groups; on soil, dead stems or roots of herbaceous plants; in moist or wet (often grass-covered) places in dry regions—steppe area, the alpine zone, or deserts.
Distribution The taxon seems to be quite widespread. It has been found so far in: Argentina, Peru, Caucasus, Central and East Asia, South Siberia.
Additional studied collections REPUBLIC OF UZBEKISTAN, Kuldzhuktau Mountains, Ayakguzhumdy, the Kyzyl-Kum Desert Station of the Institute of Botany of the Academy of Sciences Uzbek SSR, in sedge, 24 IV 1964, leg. Gaponenko, Ubajdullaev, det. B. P. Vassilkov (originally labeled as Galeropsis mitraeformis) (LE 2867); Syrdarya Region, Khavast District, Golodnaya Steppe, wormwood steppe (Artemisia diffusa, Poa bulbosa, Carex pachystylis), 23 IV 1957, leg. Bajmuratova (LE 2868); Samarkand Region, foothill of Nuratau Mountains, 30 III 1958, leg. Zaprometova, det. T. S. Panfilova (originally labeled as Galeropsis desertorum) (LE 2870); the same place, at the foot of the south slope, 17 III 1957, leg. Zaprometova, det. T. S. Panfilova (originally labeled as Galeropsis desertorum) (LE 2864); the same place, 15 III 1958, leg. Zaprometova, det. T. S. Panfilova (originally labeled as Galeropsis desertorum) (LE 2865). RUSSIA, Krasnoyarsk Territory, Field Station of Pedagogical Institute, steppe slope, 5 VIII 1960, leg. L. I. Kashina, det. B. P. Vassilkov (originally labeled as Galeropsis desertorum) (LE 2869); Republic of Altai, Kosh-Agach District, vicinity of Chagan-Uzun, wormwood-grass steppe, on soil, 21 VI 2015, leg. I. A. Gorbunova (LE 313092).
Notes In 1932, L.A. Lebedeva studied several specimens collected from the North Caucasus by Lobik and from the Central Asia by Fetissow for which she introduced a new fungal genus and species Psammomyces plantaginiformis in her paper “On the new fungus of the family Secotiaceae” (Lebedeva 1932). According to the author, the main features of the fungus are pileus 1.5–3 × 0.6–0.8 cm; thin and long stipe, 5–8 × 0.2–0.3 cm; ovoid-ellipsoid basidia, 20–30 × 10–15 μm, with 4 sinuous sterigmata up to 10–15 μm long; basidiospores ovoid-ellipsoid, with non-equal sides, 8–10(15) × 5–6 μm. In her almost forgotten article, Lebedeva also discussed possible generic placement of Karsten’s species Raddetes turkestanicus P. Karst. and its relation to the studied collections of P. plantaginiformis from Turkestan and Caucasus to solve the question of priority. As Lebedeva indicated in the description, both species possessed some similar features but their conspecificity was not ultimately accepted by the author. Our recent work (Liu et al. 2018) based on molecular study of type material demonstrated that the enigmatic fungus known as R. turkestanicus is phylogenetically far from both P. plantaginiformis and G. desertorum. It belongs to the genus Conocybe and should be further called Conocybe turkestanica (P. Karst.) E.F. Malysheva, in accordance with an earlier opinion of Donk (1941).
Singer (1936) re-examined type material of P. plantaginiformis together with additional Asiatic collections. He found that the two genera (Galeropsis and Psammomyces) were identical, which was the reason for proposing a combination Galeropsis plantaginiformis (Leb.) Singer because of the priority of Velenovský’s generic name. However, he revealed that the morphological characters of both species were not completely congruent, which prevented him from synonymizing them too. The differences were found to consist mainly of the basidia, sterigmata, and spore size (following Singer, they were significantly smaller in G. plantaginiformis).
This point of view on the independence of the two species was also shared by Heim (1950) and Kotlaba and Pouzar (1959), сontrary to the opinion of Pilát (1948), Vassilkov (1954), and Moreno et al. (1989) who believed in these species identity and great variability of morphological features.
Wasser (1974) accepted the species Galeropsis plantaginiformis and indicated its distribution in the Ukraine, Kyrgyzstan, Kazakhstan, Dagestan, and Afghanistan. Unfortunately, in his work, there were no references to the studied collections; hence, it is not possible to verify this information.
During the present work, we studied an authentic specimen from the Caucasus (designated in the present paper as lectotype), previously examined by Lebedeva and Vassilkov, as well as additional materials collected from Uzbekistan and Siberia. Our morphological observations are generally consistent with the original description of Lebedeva, with the exception of broader basidiospores that were also mentioned by Singer (1936) and Vassilkov (1954). All studied collections are placed along with type specimens of Galeropsis bispora and G. andina within the same clade in the genus Panaeolus on the phylogenetic tree (Fig. 1). The nrITS sequences of type specimens of the three species (P. plantaginiformis, G. bispora, G. andina) are almost identical, and the genetic distance between them is less than 1% (0.7%).
Galeropsis bispora was described by Vassilkov based on one collection from Uzbekistan. In his original description (Vassilkov 1954), Vassilkov pointed out the small size of basidiocarps, 2-spored basidia with peculiar thick and colored sterigmata, and large basidiospores as the main distinguishing characters of his newly described taxon from Galeropsis desertorum. It should be said that, after the examination of isotype collection of G. bispora, Moreno and co-authors (Moreno et al. 1989) also indicated the presence of pileocystidia similar to those of G. desertorum, which were never detected in holotype material. The isotype revision prompted the authors to reduce the taxonomic level of Galeropsis bispora to a mere variety of Galeropsis desertorum (Moreno et al. 1989), interpreting larger spores as a logical product that can be generated on bisporic basidia. The results of the current study of all additional 2-spored collections are quite similar to the holotype description in terms of spore dimensions.
Galeropsis andina was originally described by Singer in 1963 based on collections from Peru and Argentina (Singer 1963) and further finds of this species were unknown. The isotype study revealed minor differences in basidiospore size with descriptions in protologue, viz. we did not see those large and giant spores which pointed by Singer (18–25.8 × 11–13.3 μm).
The results of our molecular phylogenetic reconstruction indicate that there is one species characterized by a wide range of morphological variation and encompassing at least three previously known species of Galeropsis. This taxon should be called hereafter Panaeolus plantaginiformis.
Agrocybe angusticeps (Peck) Watling, in Watling & Gregory, Bibliotheca Myleg. 82: 26 (1981).
≡ Galera angusticeps Peck, Bull. Torrey Bot. Club 24: 143 (1897).
≡ Conocybe angusticeps (Peck) Murrill, Mycologia 4(5): 248 (1912).
≡ Galerula angusticeps (Peck) Murrill, N. Amer. Fl. (New York) 10(3): 168 (1917).
≡ Galeropsis angusticeps (Peck) Singer, Sydowia 15(1–6): 83 (1962).
The species was originally described by Peck as Galera angusticeps in 1897 based on material collected on lawns and pastures in Pasadena, Los Angeles, and Compton (USA). Besides the type specimen, there are known some other collections (all from North America) (Singer and Ponce De Leon 1982).
We were not able to study the type collection ourselves because the specimen is probably lost (personal message from the Curator of NYS Herbarium), but the most comprehensive description of the species was given by Singer (Singer 1963; Singer and Ponce De Leon 1982) who emphasized the following characters as the main for the taxon: conical or subcylindrical pileus with viscid surface; developed more or less anastomosing brown lamellae; thin stipe; gelatinous pileipellis with clavate to utriform cystidia-like elements; numerous heteromorphic cheilocystidia with subcapitate apex, 28–55 × 5.5–15.5 μm; 2- and 4-spored basidia; dark-colored (like Agrocybe) ellipsoid basidiospores, (10)11.5–18(20) × 7–11(12) μm; the presence of caulocystidia.
In 1962, Singer placed Galera angusticeps to the genus Galeropsis. He also noticed later (Singer and Ponce De Leon 1982) that the species was morphologically closest to Galeropsis liberata (Kalchbr.) R. Heim.
Heim (1950) considered Galera angusticeps a synonym of Galeropsis besseyi and also indicated its similarity with G. liberata.
Our morphological examination of Bolbitius liberatus type specimen corroborated the close affinity of both species, and we consider this to be a sufficient argument to accept a combination Agrocybe angusticeps previously proposed by Watling (Watling and Gregory 1981).
Ecology Gregarious (in small to large groups); on soil; on lawns and pastures or in any grassy places.
Distribution North America.
Agrocybe besseyi (Peck) E.F. Malysheva, G. Moreno & M. Villarreal, comb. nov. (Fig. 13).
Basionym: Galera besseyi Peck, Bulletin of the New York State Museum 131: 35, Pl. V, Figs. 15–20 (1909).
≡ Galerula besseyi (Peck) Murrill, N. Amer. Flora (1917).
≡ Conocybe besseyi (Peck) R. Heim, Comptes rendus hebdomadaires des séances de l’Académie d’Agriculture de France. Paris 192: 294 (1931).
≡ Cyttarophyllum besseyi (Peck) Singer, Annales Mycologici 34: 344 (1936).
≡ Galeropsis besseyi (Peck) R. Heim, Revue de Mycologie (Paris) 15: 11 (1950).
MycoBank No. MB 829489
The species was described by Peck in 1909 based on specimen from El Paso (Colorado, USA) and named after collectors C.E. and E.A. Bessey. Peck (1909) indicated an unusual shape of pileus (permanently close with abruptly contracted and often attached to stipe margin) as the most typical and constant character for the species, not found in other known species of the genus Galera.
Murrill (1917) regarded the species to belong to Karsten’s genus Galerula, together with some species of Conocybe. Similarly to this view, Heim (1931) placed Galera besseyi in a separate section Cyttarophyllum within the genus Conocybe. Afterwards, Singer (1936) has elevated this section to the level of genus, Cyttarophyllum (R. Heim) Singer, pointing after Heim to its gasteroid morph and similarity to species assignable to Galeropsis.
However, later, Heim (1950) reconsidered the species concept and attributed it to Galeropsis by proposing a new combination Galeropsis besseyi (Peck) R. Heim. In his work, he studied some other North American collections in addition to the type of Galera besseyi, specimens of Patouillard (treated by Patouillard (1927) as Galera besseyi var. madagascariensis) and similar materials from Madagascar (1931–1935). He assigned all of them to Galeropsis besseyi based on spore shape and size and treated Galera angusticeps Peck (together with a little-known taxon Galera dakotensis Brenckle) as synonyms for G. besseyi on grounds of spore morphology as well, stressing greater variability of this feature for G. angusticeps. Heim also did not exclude the identity of G. besseyi and Galera paradoxa Matt. described from Ethiopia (Mattirolo 1924).
In contrast to Peck’s opinion, Heim proposed not to consider pileus shape as intrinsic taxonomic feature of the species, assuming the possibility of its variation depending on the ecological factors—from conical-elongate to almost globose: “La variabilité dans la forme du chapeau, qui résulte des nombreuses récoltes faites dans le Sud de Madagascar, constitue une particularité spécifique, qui nous évite d’attacher à une silhouette précise une valeur absolue” (Heim 1950: 14). On the other hand, he supposed that size and shape of spores with large germ pore, their almost axial symmetry, absence of prominent hilar depression, and frequent occurrence of giant or monstrous spores are typical characteristics of the species. In accordance with this view, Heim identified all American and African collections as Galeropsis besseyi after their morphological examination, although subsequently Singer (1963) raised the status of a variety Galera besseyi var. madagascariensis to the species rank and treated it as a separate taxon belonging to the genus Galeropsis—Galeropsis madagascariensis Pat. ex Singer.
After Heim’s work (1950), the taxonomic position of the species in the genus Galeropsis has not been disputed, though Watling and Martin (Watling and Martín 2003) supposed close affinity of G. besseyi with Agrocybe gasteromycetoides Watling and considered the pileus structure and spore color as characters rather related to the genus Agrocybe.
Our morphological and molecular study of the type collection and some additional specimens greatly expanded all data on Galera besseyi available so far. It also demonstrated G. besseyi was closely allied to Agrocybe species and henceforward it should be considered as a member of this genus.
Ecology The species is known for its preference for sand areas but can occasionally occur in meadows, pastures, or woodland (parks). It grows on sandy soil, gravelly soil, or loam.
Distribution So far, it has been collected only from North America (USA, Mexico).
Additional studied collections MEXICO, Valle de la Trinidad, Km 120 Ensenada to San Felipe road, Ensenada, Baja California, in humus and remnants of wood of Lycium californicum, leg. G. Moreno, A. Altés, F. Esteve-Raventós, N. Ayala & C. Ochoa, 29-X-1991, AH 14096, AH 14268.
Agrocybe deceptiva (T.J. Baroni) E.F. Malysheva & G. Moreno, comb. nov.
Basionym: Gastrocybe deceptiva T.J. Baroni, Mycologia 73(1): 181 (1981).
≡ Galeropsis deceptiva (T.J. Baroni) G. Moreno, Heykoop & Illana, Mycotaxon 36(1): 66 (1989).
MycoBank No. MB 829490
It is a North American species described by Baroni in 1981 (Baroni 1981) as a consequence of the revision of Bartholomew’s collection (labeled Bolbitius tener var. incarnata) kept in the Farlow Herbarium. The holotype specimen was found on wet ground in a pasture in Kansas (USA). Baroni attributed this new species to the genus Gastrocybe because of the combination of microscopic features bringing it closer to another known species of the genus—Gastrocybe lateritia. The species is characterized by parabolic conic pileus up to 16 mm high, crowded dark brown lamellae, thin (less than 1 mm) and long stipe (to 80 mm), hymeniform pileipellis made up of sphaeropedunculate or pyriform elements, the presence of clavate and pigmented hymenial cystidia, 2-spored basidia, thick-walled and dark cinnamon brown basidiospores, broadly ellipsoid-ovoid, often malformed, 14–17.5(20) × 8.5–11(12.5) μm, with large (up to 2.5 μm), often protruding, germ pore.
In 1989, Moreno with co-authors (Moreno et al. 1989) proposed a combination Galeropsis deceptiva (T.J. Baroni) G. Moreno, Heykoop & Illana supposing the whole genus Gastrocybe is synonymous of Galeropsis.
We did not examine the type by ourselves, but morphologically Gastrocybe deceptiva is evidently closely related to other North American species, Galeropsis angusticeps and likewise G. besseyi, on the grounds of 2-spored basidia, large basidiospores, and the presence of cheilocystidia, though it differs from them in its pileipellis structure which is much like that of G. desertorum and G. iberica.
Although the sequencing of the type specimen has not yet been implemented, taking into account the geography of the species along with the combination of morphological characters and the result of our phylogenetic reconstruction, we incline to consider this taxon to belong to the genus Agrocybe.
Ecology Solitary or in small groups; on wet soil in pastures.
Distribution North America (USA).
Agrocybe liberata (Kalchbr.) E.F. Malysheva, comb. nov.
Basionym: Bolbitius liberatus Kalchbr., Bulletin de la Société Impériale des Naturalistes de Moscou: no 1302 (1879).
≡ Galeropsis liberata (Kalchbr.) R. Heim, Revue de Mycologie (Paris) 15: 10 (1950).
≡ Cyttarophyllum liberatum (Kalchbr.) Singer, Lilloa 22: 481 (1951).
MycoBank No. MB 829491
The type from South Africa (South African Republic) was collected by P. Mac Owan in 1877, and the species was described in exsiccate series of Thümen (1879) accompanied by Kalchbrenner’s brief description. It is especially characterized by the predominance of 2-spored basidia, numerous subcapitate cheilocystidia, and spores 11–13(14.5) × (6)7–9(11) μm.
The species was originally described belonging to the genus Bolbitius, and only in 1950, R. Heim (Heim 1950) began to treat it as a member of the genus Galeropsis. In his work (1950), Heim particularly mentioned the similarity between B. liberatus, Galera besseyi, and Galera angusticeps on the basis of spore shape and size. The characteristics of spores were also considered by him as the key features to distinguish B. liberatus from two other secotioid species—Secotium polytrichoides and Bolbitius cucullatus. On the whole, in his comprehensive work, Heim drew important conclusions about significant similarity between many secotioid species (eventually considered within the genus Galeropsis) based on morphological characters. After Heim’s paper, the species concept of B. liberatus has not been reviewed and the taxon has long been neglected by researchers, not including a brief reference in some papers of R. Singer (Singer 1949, 1963). In the present paper, we provide the first most detailed microscopic description of the species.
The morphological revision of the type shows the species combines some characters of Bolbitius (presence of pseudoparaphyses) with some of Agrocybe (subcapitate cheilocystidia, color of spores). Based on molecular data, it most closely matches Agrocybe pediades and is placed within the Agrocybe clade on the phylogenetic tree (Fig. 1). The nrITS sequences of B. liberatus and A. pediades are quite similar (genetic distance < 1%). However, it was shown that for fine resolution of closely related species in Agrocybe, nrITS has its limits (Malysheva and Kiyashko 2011). To unify sequence-based and classical morphological concepts of species, confirmation using other genes is needed. Presently, a new combination, Agrocybe liberata, is proposed.
Two additional collections studied (AH 14268, AH 14096), originally described as Galeropsis besseyi, are also nested in the B. liberatus-A. pediades clade. Morphologically, they cannot be distinguished from B. liberatus, but there are some differences in the nucleotide structure of their nrITS and nrLSU sequences. Therefore, we do not consider them to belong to B. liberatus.
Ecology The ecological preferences of the species are still unknown.
Distribution South Africa. Known only from the type locality so far.
Additional studied collections Isotype (LE 11322).
Conocybe deliquescens Hauskn. & Krisai, Öst. Z. Pilzk. (No. 15): 206 (2006).
= Galeropsis lateritia (Watling) G. Moreno, Heykoop & Illana, Mycotaxon 36(1): 66 (1989).
= Gastrocybe lateritia Watling, Michigan Bot. 7: 20 (1968).
Originally described from North America (Watling 1968), the species was subsequently found also in Europe, and many records indicate its really wide distribution. For a long time, the species was placed in the genus Gastrocybe, then in Galeropsis (Moreno et al. 1989) until the work of A. Hausknecht and I. Krisai-Greilhuber (Hausknecht and Krisai-Greilhuber 2006), where the authors established its affiliation to the genus Conocybe and proposed a new name Conocybe deliquescens, which is accepted to this day. The placement of this species in the genus Conocybe was also proved using molecular data (Hallen et al. 2003; Tóth et al. 2013).
The main diagnostic “secotioid” features that differ it from other species of Conocybe are partial deliquescent basidiocarps, conical, and slimy pileus with margin usually touching stipe at least partially, anastomosing lamellae, basidia with long sterigmata, and the presence of pseudoparaphyses.
Ecology Solitary or in small to large groups; on different types of soil or dung; in lawns, gardens or in grassy unshaded places.
Distribution North America, Europe, Africa (Hausknecht 2009).
Leratiomyces cucullatus (Shope & Seaver) Beever & D.C. Park, Mycotaxon 103: 116 (2008).
≡ Bolbitius cucullatus Shope & Seaver, Mycologia 27(6): 649 (1935).
≡ Galeropsis cucullata (Shope & Seaver) Singer, Beih. Botan. Centralbl., Abt. B 56: 150 (1936).
≡ Cyttarophyllum cucullatum (Shope & Seaver) A.H. Sm. ex Singer, Lilloa 22: 481 (1951).
≡ Weraroa cucullata (Shope & Seaver) Thiers & Watling, Madroño 21(1): 2 (1971).
= Secotium longipes Zeller, Mycologia 33(2): 209 (1941).
This species was described by Shope and Seaver as Bolbitius cucullatus in 1935 (Seaver and Shope 1935) based on a collection from Wyoming. Today, there are known several finds of this species from various parts of the USA (from Oregon to Wyoming and California).
In the original description, the authors emphasized the closest similarity between this taxon and the European species Bolbitius tener Berk., which differ from each other, in their opinion, only in the appearance of the pileus. Subsequently, some authors (Singer 1936; Pilát 1948; Vassilkov 1954) believed that the species belongs to the genus Galeropsis and must be either identical to Galeropsis desertorum or Psammomyces plantaginiformis. However, a thorough study of some specimens of B. cucullatus, including type collection, has allowed Thiers and Watling (Thiers and Watling 1971) to conclude that the species most likely belongs to another secotioid genus Weraroa, close to strophariaceous fungi. The most distinctive characters of the species (following Singer 1963; Thiers and Watling 1971) are conical pileus with fibrils of veil at the margin; long thin stipe (50–110 × 1–4 mm); lamellae anastomosing, dark colored with white margin; 4-spored basidia; ellipsoid or amygdaliform basidiospores, 11.5–14.5 (15.7) × 6.5–8(8.8) μm, with wide truncate germ pore, deep honey in KOH; numerous cylindrical cheilocystidia and lageniform caulocystidia.
Recent investigation of stropharioid and related taxa based on molecular data (Bridge et al. 2008) showed that Weraroa cucullata was placed in the Leratiomyces clade together with other secotioid and agaricoid taxa. Hence, the current taxonomic position of the species is described by the combination Leratiomyces cucullatus.
Ecology Gregarious to scattered; on soil, in grassy places, boggy, or marshy areas.
Distribution North America (USA).
Taxa excluded from our consideration
Galeropsis madagascariensis (Pat. ex Heim) Singer, Sydowia 15(1–6): 83 (1962).
≡ Galera besseyi var. madagascariensis Pat. ex Heim., Rev. Myleg. 15: 11, 13 (1950).
≡ Cyttarophyllum madagascariense (Pat. ex Heim) Watling [as ‘madagascariensis’], in Watling & Gregory, Bibliotheca Mycologica 82: 151 (Watling and Gregory 1981).
It is known that some Patouillard’s collections from Eastern Africa (Madagascar) (Galera besseyi var. madagascariensis Pat.) were studied and determined by Heim (1950) as Galeropsis besseyi, though he did not distinguish them from North American collections of G. besseyi based on morphology (see detailed discussion for Agrocybe besseyi).
Singer (1963) raised the status of a variety Galera besseyi var. madagascariensis to the species rank and treated it as a separate taxon belonging to the genus Galeropsis—G. madagascariensis—but in his work, he did not provide any morphological description of the species.
The most detailed definition of the taxon thereby we can find only in Heim’s work (1950) but due to the fact that he was unable to differentiate between three taxa (Galera angusticeps, G. besseyi, and G. besseyi var. madagascariensis), then the information is not enough to make any conclusion on the actual taxonomic status of the latter species.
Galeropsis mitriformis (Berk.) R. Heim [as ‘mitraeformis’], Revue de Mycologie (Paris) 15: 23 (1950).
≡ Bolbitius mitraeformis Berk., London J. Bot. 3: 186 (1844).
The species was originally described by J. Berkeley in 1844 (Berkeley 1844) under the name Bolbitius mitraeformis based on a collection of W. H. Harvey from the Cape of Good Hope (South Africa). In his rather scarce description, Berkeley wrote about mitriform (acuminated) striated pileus, twisted stipe, anastomosing lamellae, and cymbiform spores. Further study of the type collection by Heim (Heim 1950) led him to the conclusion about possible identity of the latter (he considered it as Galeropsis mitraeformis) with G. besseyi and G. madagascariensis due to the similar appearance, and likewise, the large variability in the spore size in all species of Galeropsis. In addition, Singer (1963) directed his attention to the similarity between Galeropsis andina and G. mitriformis.
Unfortunately, we could not examine the type specimen under this study. Since all known morphological descriptions of the species are insufficient, further studies (taking into account the collection of molecular data) are needed to define its true taxonomic status.
Galeropsis paradoxa (Mattir.) Pilát, Stud. Bot. Čechoslav. 9: 184 (1948).
≡ Galera paradoxa Mattir., Atti Accad. Sci. Torino 59: 714 (1924).
≡ Conocybe paradoxa (Mattir.) R. Heim, C. r. hebd. Séanc. Acad. Sci., Paris 192: 294 (1931).
It is a tropical African species described by O. Mattirolo as Galera paradoxa in 1924 based on specimens collected on pastures in Ethiopia. The species is characterized by short claviform pileus 10–20 mm high, lamellae with numerous anastomoses forming something like lacunae, and basidiospores, 16–20 × 10–12 μm, without germ pore.
In 1931, Heim created a section Cyttarophyllum in the genus Conocybe (Heim 1931) in which secotioid fungi like Conocybe besseyi (Peck.) R. Heim and Conocybe paradoxa (Mattir.) R. Heim were included. Pilát (1948) made a combination Galeropsis paradoxa and considered the taxon as belonging to the subgenus Cyttarophyllum in the genus Galeropsis along with the second species Galeropsis besseyi. Furthermore, Heim in his work (Heim 1950) did not exclude the identity of the Ethiopian fungus with Galeropsis besseyi.
The type specimen has not been re-examined using modern microscopy and molecular techniques so far. Without obtaining such additional data expanding the scanty extant description, it is impossible at present to speak with certainty about the taxonomic status of this fungus.
Galeropsis polytrichoides (Zeller) Zeller, Mycologia 35 (4): 410 (1943).
≡ Secotium polytrichoides Zeller, Mycologia 33 (2): 211 (1941).
≡ Cyttarophyllum polytrichoides (Zeller) Singer, Lilloa 22: 481 (1951).
It was originally collected on moist soil among grass by W.B. Cooke from California and then was described by Zeller under the name Secotium polytrichoides (Zeller 1941). Zeller pointed out that the species has a peculiar shape of its pileus (ellipsoid or conical with acute apex), the presence of long whitish fibrils at the margin of the pileus, anastomosing lamellae, and dark brown rather small (8.7–10 × 5.8–6.3 μm) spores with germ pore. Afterwards, he placed the taxon in the genus Galeropsis (Zeller 1943) and emphasized its close affinities with G. desertorum. Subsequent studies (Heim 1950; Singer 1963; Thiers and Watling 1971; Pomarico and Rath 1991) confirmed the unique combination of features of this species (such as well-developed veil and relatively small spores—up to 9.3–12.6 × 5.7–7.2 μm according to Heim 1950, 11–12.5 × 6.8–8.3 μm according to Singer 1963, and 10–13 × 5–6 × 6–6 μm according to Thiers and Watling 1971), and all mentioned authors were inclined to treat the taxon as a separate species in the genus Galeropsis, rather not related to G. desertorum.
Despite the presence of good morphological descriptions of the species in the literature, its actual taxonomic position seems still unclear, since the taxon is not very similar to any of the species of Galeropsis. Unfortunately, we could not examine the type specimen under this study. Therefore, this issue needs further investigation using molecular technique.
Discussion and conclusion
In the past centuries, before the molecular era in mycology, the basidiocarp shape (cylindrical-conical enclosed pileus with margin clasping the stipe), undeveloped and anastomosing lamellae, presumably statismosporic basidia, rusty brown spores and growth in arid areas were the main basis for classifying species as belonging to the secotioid genus Galeropsis.
Recent phylogenetic results highlighted the polyphyletic nature of secotioid morph of basidiocarp at the genus or family level, and the molecular data unequivocally demonstrated the evolutionary process of “gasteromycetation” occurred many times independently within Agaricomycetidae (Matheny et al. 2006) that was foreseen much earlier by some authors (Heim 1971; Singer 1986). Thus, it is now conclusively known that many secotioid taxa have evolved among the euagarics (Hopple and Vilgalys 1994; Hibbett et al. 1997; Miller et al. 2001; Peintner et al. 2001; Geml 2004; Binder and Hibbett 2006; and many other papers). Our study showed that the obscure genus Galeropsis was also no exception. The present results imply that the latter is an artificial taxon representing a complex of several divergent lineages, where neither the relationships between the lineages nor the direction of evolution are known. The generic type, G. desertorum, is shown to be placed within Panaeolus clade, whereas other species belong to Agrocybe, Parasola, Conocybe, and Leratiomyces. However, the issue of phylogenetic content of the whole genus Galeropsis in its wide traditional concept still remains finally unresolved. This is complicated by the fact of rarity of finds of these fungi in nature, as well as a very old type material for most known species impedes a complete molecular study.
New evidences should also re-focus how we define the taxonomic value of morphological characters. The species delimitation in Galeropsis was previously determined by pileus shape and size, the presence and density of lamellar anastomoses, the presence or absence of cheilocystidia, and mainly the morphology of the spores. Nevertheless, these features were not always unanimously recognized as systematically important by all authors. Heim (1950) stressed extraordinary morphological variability of the group that, he concluded, could entail the apparent overestimation of species diversity in Galeropsis by the various authors. He believed that some described species may represent a single one but range of morphological variety remained ambiguous because of inadequate taxon sampling and lack of modern collections. This assumption got partial support from our phylogenetic analyses showing synonymy of G. andina, G. bispora, and G. plantaginiformis.
Another progressive idea that has put forward by Heim was that the genus Galeropsis may be only an ecological group. Obviously, there are several strategies in adaptation to the certain ecological conditions in Galeropsis. Steppe species possess almost enclosed pileus but have functional lamellae and can give a spore-print, a character attributable to the agaricoid morph, dark-colored spores and developed stipe. Yet, not all species of Galeropsis were found in totally arid conditions, viz. G. desertorum, G. angusticeps, G. deceptiva, G. lateritia, G. cucullata, and G. aporos were constantly or occasionally found in wet grassy places. Some of them have peculiar features such as deliquescent basidiocarps, which can give an advantage for spore dispersal by drops of rain or dew in humid areas. However, not all morphological features can be unambiguously interpreted as just a response to environmental factors. Remarkable example of plasticity of morph was demonstrated for Galeropsis lateritia (= Conocybe deliquescens) and, as Hallen et al. (2003) hypothesized, this may be due to a permanent infection of basidiocarps with Chryseobacterium which is capable to cause such a peculiar morphology.
Nonetheless, consideration of the ecological circumstances in comparison with morphological features can help to comprehend the trends of convergent evolution in phylogenetically different lineages of euagarics.
References
Baroni TJ (1981) Collections in the Farlow Herbarium: new species of Melanophyllum and Gastrocybe, type studies on Armillaria and Stropharia. Mycologia 73(1):181–197
Baroni TJ, Matheny PB (2011) A re-evaluation of gasteroid and cyphelloid species of Entolomataceae from eastern North America. Harv Pap Bot 16:293–310. https://doi.org/10.3100/0.25.016.0205
Berkeley MJ (1844) Decades of fungi. Lond J Bot III 186
Běťák J, Čapoun M (2015) Špičatička stepní (Galeropsis desertorum) znovu objevena v České Republice. Thayensia (Znojmo) 12:119–127
Binder M, Hibbett DS (2006) Molecular systematics and biological diversification of Boletales. Mycologia 98(6):971–981. https://doi.org/10.1080/15572536.2006.11832626
Braaten CC, Matheny PB, Viess DL, Wood MG, Williams J, Bougher NL (2014) Two new species of Inocybe from Australia and North America that include novel secotioid forms. Botany 92:9–22. https://doi.org/10.1139/cjb-2013-0195
Bridge PD, Spooner BM, Beever RE, Park DC (2008) Taxonomy of the fungus commonly known as Stropharia aurantiaca, with new combinations in Leratiomyces. Mycotaxon 103:109–121
Clémençon H (2009) Methods for working with macrofungi. Laboratory cultivation and preparation of large fungi for light microscopy. IHW-Verlag
Courtecuisse R (1993) Galeropsis aporos sp. n., genre et espece nouveaux pour la France. Doc Mycol 22:1–6
Donk MA (1941) Nomina generica conservanda and confusa for Basidiomycetes (Fungi). Bull Bot Gard Buitenz 17(1):155–197
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Mol Ecol 2:132–118. https://doi.org/10.1111/j.1365-294x.1993.tb00005.x
Geml J (2004) Evolution in action: molecular evidence for recent emergence of secotioid genera Entoptychum, Gyrophragmium and Longula from Agaricus ancestors. Acta Microbiol Immunol Hung 51(1–3):97–108. https://doi.org/10.1556/AMicr.51.2004.1-2.7
Gorovoi LF, Batyrova GS (1986) Some characters of developing fruit bodies of Galeropsis desertorum Vel. et Dvor. (Podaxales, Basidiomycetes). Mikol Fitopatol 20(6):451–455
Hallen HE, Watling R, Adams GC (2003) Taxonomy and toxicity of Conocybe lactea and related species. Mycol Res 107(8):969–979. https://doi.org/10.1017/S0953756203008190
Hausknecht A (2009) A monograph of the genera Conocybe Fayod and Pholiotina Fayod in Europe. Fungi Europaei 11. Alassio: Candusso
Hausknecht A, Krisai-Greilhuber I (2006) Infrageneric division of the genus Conocybe - a classical approach. Österr Z Mykol 15:187–212
Heim R (1931) Sur les liens pliyletiques entre les Agarics Ochrospores et certains Gasteromycetes. Comptes r'endus Ac Sc 192:291–293
Heim R (1950) Le genre Galeropsis Velenovsky. Revue de Mycologie 15:3–28
Heim R (1971) The interrelationships between the Agaricales and Gasteromycetes. In: Petersen RH (ed) Evolution in the higher Basidiomycetes. The University of Tennessee Press, Knoxville, pp 505–534
Hibbett DS, Pine EM, Langer E, Langer G, Donoghue MJ (1997) Evolution of gilled mushrooms and puffballs inferred from ribosomal DNA sequences. Proc Natl Acad Sci U S A 94:12002–12006. https://doi.org/10.1073/pnas.94.22.12002
Hopple JS Jr, Vilgalys R (1994) Phylogenetic relationship among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA. Mycologia 86:96–107
Kotlaba F, Pouzar Z (1959) Nový nález vzácné houby špičatičky stepní – Galeropsis desertorum Velen. et Dvoř. v Československu a poznámky k rodu Galeropsis Velen. Česká Mykologie 13(4):200–211
Krüger D, Binder M, Fischer M, Kreisel H (2001) The Lycoperdales. A molecular approach to the systematics of some gasteroid mushrooms. Mycologia 93:947–957 http://hdl.handle.net/10255/dryad.5790
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lebedeva LA (1932) On the new fungus of the family Secotiaceae Ed. Fisch Bull Pl Prot (II) 5:111–118
Li Y, Li TH, Yang ZL, Dai YC, Tolgor B (2015) Atlas of Chinese macrofungal resources. Central China Farmers’ Publishing House, Zhengzhou
Liu L-N, Razaq A, Atri NS et al (2018) Fungal systematics and evolution: FUSE 4. Sydowia 70:211–285. https://doi.org/10.12905/0380.sydowia70-2018-0211
Malysheva EF, Kiyashko AA (2011) Contribution to the study of Agrocybe pediades complex (Agaricales) in Russia based on nrITS sequences. Mycol Balc 8:115–124
Matheny PB, Curtis JM, Hofstetter V et al (2006) Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98:982–995
Mattirolo O (1924) Funghi africani raccolti del Dolt. Giovanni Negri nella Etiopia Meridionale (Scioa-Galla). Atti della Reale Accademia delle Scienze di Torino 59:374–377
Miller SL, McClean TM, Walker JF, Buyck B (2001) A molecular phylogeny of the Russulales including agaricoid, gasteroid and pleurotoid taxa. Mycologia 93:344–354
Moncalvo JM, Vilgalys R, Redhead SA et al (2002) One hundred and seventeen clades of euagarics. Mol Phylogenet Evol 23:357–400. https://doi.org/10.1016/S1055-7903(02)00027-1
Moreno G, Illana C, Heykoop M (1987) Gastrocybe iberica sp. nov. in Spain (Bolbitiaceae, Agaricales). Criptogamie Myleg 8(4):321–327
Moreno GM, Heykoop M, Illana C (1989) Studies on Galeropsis and Gastrocybe (Bolbitiaceae, Agaricales). Mycotaxon 36(1):63–72
Murrill WA (1917) The taxonomy of the Agaricaceae. AmJ Bot 4(6):315–326
Patouillard N (1927) Contribution a l’etude des Champignons de Madagascar. Mem Acad Malgache 6:30
Peck СH (1909) Report of the State Botanist, 1908. Bull N Y State Museum 181:35
Peintner U, Bougher NL, Castellano MA et al (2001) Multiple origins of sequestrate fungi related to Cortinarius (Cortinariaceae). Am J Bot 88:2168–2179. https://doi.org/10.2307/3558378
Pilát A (1948) On the genus Galeropsis Velenovský. Stud Bot Čechosl (Praha) 9:177–185
Pomarico O, Rath F (1991) Nota sul ritrovamento di Galeropsis polytrichoides (Zeller) Zeller in Italia – Val Del Riso (BG). Rivista di Micologia 34(3):231–238
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67(5):901–904. https://doi.org/10.1093/sysbio/syy032
Ronquist F, Teslenko M, van der Mark P et al (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61(3):539–542. https://doi.org/10.1093/sysbio/sys029
Seaver FJ, Shope PF (1935) New or noteworthy Basidiomycetes from the Central Rocky Mountain Region. Mycologia 27:649–650
Singer R (1936) Studien zur Systematik der Basidiomyceten. Beih Bot Cbl (B) 56:137–156
Singer R (1949) The “Agaricales” (mushrooms) in modern taxonomy. Lilloa 22:1–832
Singer R (1950) Type studies on Basidiomycetes, IV. Lilloa 23:147–247
Singer R (1963) Notes on secotiaceous fungi: Galeropsis and Brauniella. Kon Nederl Akad Wet Proc, C 66:106–117
Singer R (1986) The Agaricales in modern taxonomy, 4th edn. Koeltz Scientific Books, Koenigstein, Germany
Singer R, Ponce De Leon P (1982) Galeropsidaceae west of the Rocky Mountains. Mycotaxon 14:82–90
Spegazzini C (1913) Mycetes argentinenses. Anales del Museo Nacional de Historia Natural de Buenos Aires 24:167–185
Szarkándi JG, Schmidt-Stohn G, Dima B et al (2017) The genus Parasola: phylogeny and the description of three new species. Mycologia 109(4):620–629. https://doi.org/10.1080/00275514.2017.1386526
Thiers B (2016) Index Herbariorum: a global directory of public herbaria and associated stuff [continuously updated]. Botanical Garden’s Virtual Herbarium, New York. http://sweetgum.nybg.org/ih [Accessed 30 Oct. 2018]
Thiers HD, Watling R (1971) Secotiaceous fungi from Western United States. Madrono 21(1):1–9
Thümen F (1879) Mycotheca Universalis. Bulletin de la Société Impériale des Naturalistes de Moscou. Centurie 14:1301–1400
Tóth A, Hausknecht A, Krisai-Greilhuber I et al (2013) Iteratively refined guide trees help improving alignment and phylogenetic inference in the mushroom family Bolbitiaceae. PLoS One 8(2):e56143. https://doi.org/10.1371/journal.pone.0056143
Vassilkov BP (1954) O nyekotorykh intyeryesnykh I novykh vidakh gasteromicetov SSSR. Acta Inst Bot Komarov Acad Sci URSS (Pl Crypt) Ser II 9:447–464
Velenovský J (1930) Galeropsis gen. nov. Mykologia 7:105–106
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4239–4246
Wasser SP (1974) Modern view on taxonomic position of the genus Galeropsis Vel. Emend S. Wasser. Ukrainian Botanical Review 31(5):567–577
Watling R (1968) Observations on the Bolbitiaceae IV. A new genus of gasteromycetoid fungi. Mich Bot 7:19–24
Watling R, Gregory NM (1981) Census catalogue of the world members of the Bolbitiaceae. Bibl Mycol 82:1–224
Watling R, Martín MP (2003) A sequestrate Psilocybe from Scotland. Bot J Scotl 55(2):245–257
Watling R, Quadraccia L, Tabares M, Rocabruna A (1986) Gastrocybe in Europe. Notes Roy Bot Gard Edinb 43(2):307–311
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Zeller SM (1941) Further notes on Fungi. Mycologia 33:196–214
Zeller SM (1943) North American species of Galeropsis, Gyrophragmium, Longia and Montagnea. Mycologia 35:409–421
Acknowledgments
We are very grateful to the curators of herbaria LIP, S, NYS, BAFC, BRNM, and AH for providing specimens for loan, and personally Dr. Régis Courtecuisse, Dr. Pierre-Arthur Moreau, Dr. Lorinda Leonardi, Dr. Andrea I. Romero, Dr. Susana Pereira, Dr. V. Spirin, Dr. Hana Ševčíková, and Dr. Javier Rejos for their help with specimens. Yury Rebriev (Russia) and Irina Gorbunova (Russia) kindly shared with us their collections and photos.
Funding
The research was partly supported by project АААА-А19-119020890079-6 of the Komarov Botanical Institute of the Russian Academy of Sciences (the authors EM, VM).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Zhu-Liang Yang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malysheva, E., Moreno, G., Villarreal, M. et al. The secotioid genus Galeropsis (Agaricomycetes, Basidiomycota): a real taxonomic unit or ecological phenomenon?. Mycol Progress 18, 805–831 (2019). https://doi.org/10.1007/s11557-019-01490-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-019-01490-6