Abstract
Heteroecism, or alternation between two unrelated hosts, is a widespread phenomenon among rust fungi (Pucciniales). In addition to heteroecism, rust fungi have evolved elaborate life cycles ranging from the five spore stages of macrocyclic species with many variations down to microcyclic species that may produce just two of these stages to complete their life cycles on a single host species. Considering the large number of nearly 8000 described rust fungi species and the high proportion that are host-alternating, heteroecism apparently is a successful strategy for these fungi, at least in terms of species diversity. However, the cost of maintaining a heteroecious strategy with respect to spore production and two different host plant species must be high. In Pucciniales, sister-species pairs that include one host-alternating (heteroecious) and one non-host-alternating (autoecious) species that share a common host are called correlated species. In this study, we tested Tranzschelia species for the existence of correlated species using molecular phylogenetic data. We reveal the presence of three pairs of correlated species within this single genus and suggest that this is a repeating process in the evolution of rust fungi. We show that a heteroecious macrocyclic strategy can be the starting point for deriving microcyclic autoecious species. The high cost of host alternation may be compensated by the fact that it is a facultative process in Tranzschelia with numerous strategies for the species to persist in one or the other host or as overwintering spore. Consequently, the advantage of host alternation seems higher than the cost of (facultative) heteroecism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rust fungi (Basidiomycota, Pucciniales) are obligate plant pathogens that likely evolved in the mid-Jurassic (Aime et al. 2018). They comprise the largest group of plant pathogenic fungi consisting of nearly 8000 known species (Kirk et al. 2008) which includes the single most speciose fungal family, Pucciniaceae. This dimension of speciation is astonishing considering the complexity and plasticity of the rust life cycle. For instance, a single species may possess up to five different spore states, each with a different function, and up to seven morphologically different spore types (e.g., Bruckart et al. 2010), all obligately dependent on their specific plant hosts. Moreover, most striking and nearly unique within the kingdom Fungi is the fact that the majority of species are host-alternating (heteroecious). They develop spermogonia (abbreviated 0) forming spermatia and aecia (I) forming aeciospores on a host plant herein referred to as the aecial host. Later in the vegetation season, aeciospores serve to infect another host belonging to an unrelated plant family that is referred to as the telial host. Uredinia (II) with urediniospores followed by telia (III) with teliospores are formed on the telial host. In temperate climates, thick-walled teliospores often serve as overwintering spores germinating in spring to form basidia (IV) that produce haploid basidiospores after meiosis and serve to reinfect the aecial host. Species forming all spore generations except uredinia (II) are called autopsis-forms and those forming all five spore states are called macrocyclic or eu-forms; host-alternating ones are termed opsis- and hetereu-forms, respectively. Reduced life cycles include, among others, auteu-forms (forming all spore states but not host-alternating), brachy-forms (I missing), hemi-forms (0, I missing), autopsis-forms (II missing), and micro-forms that are the most reduced life cycle type, forming only 0 (or no 0 at all), III, and IV (nomenclature of spore states after Wilson and Henderson 1966).
More than 50% of the species of Pucciniales are host-alternating (Malloch 1995), especially in temperate regions (e.g., Berndt 2012; Klenke and Scholler 2015). This proportion suggests that heteroecism is a successful strategy in the evolution of rust fungi, despite an extremely high cost according to Savile (1976), who calculated, considering host changes in both directions from aecial host to telial host and vice versa, an “annual population loss between 1/625 and 1/15,625.” De Bary (1879), Dietel (1897, 1899), Fischer (1898), and Tranzschel (1904) observed that for some micro-form rust species, the host was congeneric or conspecific with the aecial host of a hetereu-form rust species. In contrast to the popular classifications of the time, these workers hypothesized that life cycle types may not necessarily indicate taxonomic relationships. Tranzschel (1904) concluded that when heteroecious species reduce their life cycle to form a micro-form, it will be formed on the aecial host via intermediate auteu-forms. Tranzschel applied his hypothesis in searching for and describing previously unknown “parallel” species (Tranzschel, l. c.). Later refinements to this idea were initiated by Arthur (1921, 1929, 1934) and Jackson (1931), who coined the term “correlated” species for Tranzschel’s parallel species and “Tranzschel’s Law” to explain how derived auteu- and micro-forms may occur on the aecial host (and not on the telial host) of hetereu-form species. Today, Tranzschel’s Law and the concept of correlated species is widely accepted among mycologists, and some molecular phylogenetic studies, e.g., by Zambino and Szabo (1993) on grass rusts in the genus Puccinia, Roy et al. (1998) within the Puccinia monoica complex, and Vogler and Bruns (1998) on the genus Cronartium have confirmed this. While many researchers (e.g., Dietel 1897, 1899; Orton 1927; Jackson 1931; Shattock and Preece 2000) have hypothesized that micro-forms are derived from hetereu-forms the reverse hypothesis—that complex forms evolved from microcyclic rusts—has also been invoked, most frequently to explain how macrocylic species could have evolved from what were considered the most “primitive” groups of rust fungi (e.g., Dietel 1899; Hennen and Buriticá 1980).
The genus Tranzschelia includes 16 species (Scholler et al. 2014) with mainly micro- and hetereu-forms and one auteu-form (T. cohaesa (Long) Arthur, the type species), one autopsis-form (T. tucsonensis (Arthur) Dietel), and possibly several hemi-forms (T. arasbaranica M. Abbasi & M. Scholler, T. mexicana M. Scholler & M. Abbasi, T. viornae (Arthur) Arthur). It is possible that some hemi-form species may actually be hetereu-form species for which the aecial host is not known. In Tranzschelia spp., 0 and I of hetereu-form species, all spore states of auteu-form species and 0, III and IV of micro-form species are formed on Ranunculaceae. Telial hosts in hetereu-form species are stone fruit trees classified in the genus Prunus s.l. (plums and peaches, blackthorn, etc.).
Maier et al. (2005) sequenced three European Tranzschelia specimens demonstrating that T. fusca (Pers.) Dietel and T. pruni-spinosae (Pers.) Dietel (hetereu-form) on their telial hosts are indeed more closely related to each other than to another hetereu-form species, T. discolor (Fuckel) Tranzschel & M.A. Litv. In this study, we used this three-species model for molecular phylogenetic analyses including further Tranzschelia specimens from different host plant species and continents to show that the formation of correlated species was not a single but a repeated event in Tranzschelia evolution. Against this background, the function and potential advantages of this process and of heteroecism in general are discussed.
Materials and methods
Specimen sampling
Specimens of species of Tranzschelia, Leucotelium, and Ochropsora—the latter two chosen because they also utilize Ranunculaceae species as their aecial hosts—were selected from public herbaria for molecular phylogenetic analyses from as broad a geographic and host range as possible including hetereu-/hemi- and micro-form species (Table 1). Specimens were assigned to species on the basis of host plant species, morphology, geographic distribution, and the results of the molecular phylogenetic analyses. However, five specimens could not be assigned to a specific taxon (Tranzschelia spp. 1–5).
DNA extraction, PCR, and sequencing
Genomic DNA was isolated directly from herbarium specimens. For each specimen, several sori from a single leaf were excised and extracted using the UltraClean plant DNA extraction kit (MoBio Laboratories Inc., Solana Beach, California). DNA extractions were diluted 1:9 in sterile water and amplified with the rust-specific primer Rust2inv (Aime 2006) and LR6 (Vilgalys and Hester 1990) in 25 μL reaction volumes with 12.5 μL of PCR Master Mix (Promega Corp., Madison, WI), 1.25 μL of each 10 μM primer, and 10 μL of diluted DNA template with PCR parameters described in Aime (2006), amplifying approximately 1400 bp of a region of the ribosomal repeat spanning the 5.8S subunit, the internal transcribed spacer region 2 (ITS2), and the large subunit (28S) (LSU). Amplifications with weak products were cleaned and reamplified with nested primers Rust28SF (Aime et al. 2018) and LR5 (Vilgalys and Hester 1990) as described by Aime et al. (2018). PCR products were sequenced with amplification primers on an ABI3100 Automated Sequencer (Applied Biosystems, Foster City, California) after cleaning by ethanol precipitation or sent to Beckman-Coulter (Danvers, MA) for sequencing on an ABI 3730XL. Contiguous sequences were assembled and edited in Sequencher v.5.2.3 (Gene Codes Corp., Ann Arbor, MI). DNA sequences were deposited in GenBank; accession numbers are provided in Table 1 and Fig. 1.
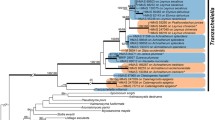
Bayesian inference of the phylogenetic relationships between the sampled Leucotelium and Tranzschelia species: Markov chain Monte Carlo analysis of an alignment of concatenated ITS + LSU base sequences using the GTR + I + G model of DNA substitution with gamma distributed substitution rates and estimation of invariant sites, random starting trees, and default starting parameters of the DNA substitution model. A 50% majority-rule consensus tree is shown computed from 75,000 trees that were sampled after the process had reached stationarity. The topology was rooted with Ochropsora ariae. Numbers on branches before slashes are estimates for a posteriori probabilities; numbers on branches after slashes are ML bootstrap support values. Branch lengths were averaged over the sampled trees. They are scaled in terms of expected numbers of nucleotide substitutions per site. Au = autoecious; HeA = hetereu-form, aecial host; HeT = hetereu-form, telial host; T. = Tranzschelia. HeT include possible hemi-form species (Tranzschelia arasbaranica, T. hyrcanica, T. mexicana)
Phylogenetic analyses
To infer the phylogenetic relationships of the examined specimens, we assembled an ITS + LSU dataset that included sequences from every species/clade for which sequence data were available. For GenBank accession numbers of the sequences used (Aime 2006; Blomquist et al. 2015), see Fig. 1.
ITS and LSU DNA regions were aligned separately. Sequence alignment was obtained using MAFFT 7.273 (Katoh and Standley 2013) using the L-INS-i option. To obtain reproducible results, manipulation of the alignments by hand and manual exclusion of ambiguous sites were avoided as suggested by Gatesy et al. (1993) and Giribet and Wheeler (1999), respectively. Instead, highly divergent portions of the alignments were omitted using GBlocks 0.91b (Castresana 2000) with the following options for the LSU dataset: “Minimum Number of Sequences for a Conserved Position”: 14, “Minimum Number of Sequences for a Flank Position”: 14, “Maximum Number of Contiguous Non-conserved Positions”: 8, “Minimum Length of a Block”: 5, and “Allowed Gap Positions” to “With half,” and for the ITS dataset: “Minimum Number of Sequences for a Conserved Position”: 6, “Minimum Number of Sequences for a Flank Position”: 6, “Maximum Number of Contiguous Non-conserved Positions”: 8, “Minimum Length of a Block”: 5, and “Allowed Gap Positions” to “With half.”
The resulting alignments [LSU dataset: new number of positions: 962 (53% of the original 1782 positions), number of variable sites: 145; ITS dataset: new number of positions: 563 (62% of the original 896 positions), number of variable sites: 219] were then concatenated. Phylogenetic analyses were computed for the dataset in maximum likelihood analyses (ML) with combined rapid bootstrapping under the GTRCAT model from 1000 runs using RAxML 8.0.17 (Stamatakis 2014). Additional posterior probability nodal support values were determined in a Bayesian phylogenetic MCMC search (BA) using MrBayes 3.2.2 (Ronquist et al. 2012) under the general time reversible model with gamma-distributed rate variation (GTR + G). Each search comprised two runs of four chains each for 10 × 106 generations sampled every 100 generations with the first 2.5 × 106 generations discarded as burn-in. This Bayesian approach of phylogenetic analysis was repeated five times to test the independence of the results from topological priors (Huelsenbeck et al. 2002).
Trees were rooted with Ochropsora ariae, which also alternates between Anemone spp. (and Aruncus spp.) and Rosaceae.
Results
Phylogenetic analyses
Bayesian (BA) and maximum likelihood (ML) analyses of ITS and LSU sequence data yielded congruent topologies (see Fig. 1 for BA analyses). These resolved Tranzschelia spp. into two major lineages, the Tranzschelia discolor clade (herein termed clade I) and the T. pruni-spinosae clade (herein termed clade II). A third clade was represented by the hetereu-form species Leucotelium cerasi, which was—although weakly supported—resolved as the earliest diverging of the three lineages.
In clade II, three pairs of correlated species were revealed: i. Tranzschelia pruni-spinosae (hetereu-form)/T. fusca (micro-form) (Eurasia); ii. T. sp. 4 (hetereu-form)/T. pseudofusca (micro-form) (North America); iii. T. hyrcanica (hetereu-form or hemi-form)/T. pulsatillae (micro-form) (Eurasia). No correlated species were found in clade I. The sister-species to micro-form species were found to be macrocyclic species except for Tranzschelia thalictri and T. sp. 3 in clade I, both of which are micro-form species.
Hetereu-form species were present in all clades.
Discussion
Phylogeny and pathways of speciation
Our phylogenetic reconstruction of species of the rust genus Tranzschelia (Fig. 2) provides evidence for Arthur’s concept of correlated species (Jackson 1931; Arthur 1934) and Tranzschel’s Law (Tranzschel 1904; Arthur 1929; Cummins 1959) within clade II for which we uncovered three pairs of correlated species, two from Eurasia and one from North America (Fig. 1). In clade I, no correlated species were discovered, maybe due to incomplete sampling. Tranzschelia sp. 3 (North America) and T. thalictri (Europe) are microcyclic species that parasitize Thalictrum spp. Although hardly distinguishable by morphology (Scholler et al. 2014), they are different in ITS 2 sequences (8 bp) and distribution (North America vs. Europe). Therefore, we consider them different species that may have speciated as a consequence of geographic isolation. Following Tranzschel’s Law, a correlated macrocyclic species for these could be sought among hetereu-forms with their aecial states on Thalictrum sp. Tranzschelia spec. has been reported from the USA with 0, I on Thalictrum purpurascens DC. (= Th. dioicum L.) (Arthur 1934; Farr and Rossman 2017). Both records are named “T. pruni-spinosae.” However, no relation to a telial host is known so far and no sequence data of the corresponding specimens are available. Sequence data of Leucotelium cerasi—a hetereu-form—are located at the base of this lineage as the earliest diverging member, suggesting a macrocyclic and heteroecious ancestry for Tranzschelia spp. The overall pattern within the group appears to be a repeated reduction from hetereu-forms to new hetereu-forms and micro-forms.
Major evolutionary and ontogenetic pathways in Tranzschelia spp. with host-alternating species as starting point. 1. Reduction via instable autoecious forms to micro-form (correlated species) on Ranunculaceae host (former aecial host) according to Tranzschel’s Law. 2. Reduction (but no speciation) on telial host (Prunoideae) via hemi-form (II, III, IV) and finally pure mitotic life cycle with uredinia (II) only as a consequence of missing aecial hosts. 3. Co-evolution with host plants (host shift) and development of new host-alternating species. Roman numerals stand for rust spore states
We suggest two major pathways of speciation within Tranzschelia, i. formation of micro-forms (0, III, IV) via auteu-forms (Fig. 2, pathway 1), and ii. formation of new host-alternating species by co-evolution/host shift (Fig. 2, pathway 3). Pathway 2 with hemi-forms (II, III, IV) and asexual uredo-forms (II) does not lead to new species but simply leads to reduced life cycles which finally (as uredo-form) prevent a return to heteroecism. Other speciation pathways such as co-speciation of auteu-forms and micro-forms with their host(s) are possible but cannot be proven by available data. Our data do not provide evidence of jumps of rust species to non-related host species as described in other studies (e.g., McTaggart et al. 2016) or for the hologenetic ladder hypothesis of Leppik (1955) and Savile (1968). It is possible that these events take place on a deeper evolutionary time scale than we have examined. Tranzschelia spp. are strictly restricted to Ranunculaceae hosts (autoecious species) and Ranunculaceae/Pomoideae (heteroecious species), respectively.
The function of heteroecism
According to Savile (1976), the cost of heteroecism in rust fungi is extremely high and he calculated a specific “loss of populations” when rust fungi infect aecial hosts with basidiospores or telial hosts with aecial spores. However, considering the high proportion of host-alternating rust species (Mulloch 1994), especially in temperate regions (Berndt 2012; Klenke and Scholler 2015), heteroecism seems a very successful strategy at least in terms of species diversity. Also for Tranzschelia, host-alternating species make up the majority of extant species (López-Franco and Hennen 1990; Scholler et al. 2014) and our study reveals that hetereu-form species are present in all recovered clades. Thus, the question remains: what may be the function of heteroecism in Tranzschelia and other rust fungi? According to Malloch (1995), two benefits for host-alternating species may be additional nutrient sources and that the additional host helps to increase the lifetime reproductive output. However, this does not explain why the many plurivorous rusts such as the grass rusts Puccinia coronata Corda and P. graminis Pers. that have hundreds of available perennial telial host species (Cummins 1971) or species of woody plants like almost all species of the family Pucciniastraceae are host-alternating.
There are simpler alternatives of disseminating rust spores, namely by transfers from telial host to nearby other telial hosts via mitotic urediniospores—regardless of whether the aecial host is present or absent. This can be shown indirectly by the number of published floristic records and specimen collections. For example, in the inventory of the fungus herbarium of the natural history museum in Karlsruhe, Germany (KR), the majority (80%) of 6078 specimens of host-alternating species are specimens on the telial host (as of 25 May 2016). In Tranzschelia pruni-spinosae, there are 30 telial and 19 aecial host specimens, in T. discolor the relation is 87:11. Tranzschelia spp. and other heteroecious species do not host-alternate in certain regions, where no aecial host population is available. So the fungus’ life cycle apparently is reduced to a hemi-form (II, III, IV) only capable of continued clonal reproduction on Prunus spp. If karyogamy and subsequent meiosis become useless in host-alternating species because of missing aecial hosts for the basidiospores to infect, teliospore/basidiospore production will finally be stopped and only mitotic urediniospores will be produced ensuring the transfer from the former telial host to telial hosts (Prunus spp.). This corresponds to path 2 in Fig. 2. This reduction to hemi-forms (II, III) and finally mere mitotic production of uredinia (II) are documented for Tranzschelia pruni-spinosae and T. discolor and may finally lead to allopatric speciation: T. discolor, with eastern Mediterranean/Near East origin, has been distributed worldwide with the cultivation of stone fruits like peaches (Prunus persica (L.) Batsch) and plums (P. domestica L.). In regions where there are no aecial hosts (Anemone coronaria L. and related Anemone spp.) available, the fungus no longer produces III and IV (e.g., Josifovič 1953; Blumer 1960; Deadman et al. 2007) allowing the species to follow its telial hosts (grown by men) and conquer areas outside their natural area of distribution. In general, fungi that rely on asexual propagation may risk extinction due to the loss of genetic diversity and the inability to adapt to changing ecological conditions (e.g., Fisher et al. 2005). But in the case of Tranzschelia discolor and T. pruni-spinosae, sexual populations still exist in Europe and Western Asia in their natural area of distribution.
Considering overwinter survival, there are alternative modes for species that rarely or not at all form teliospores, which commonly function as winter or resting spores (e.g., Kirk et al. 2008), especially in areas with temperate climate. Tranzschelia pruni-spinosae can overwinter as urediniospores and by systemic infection of the (perennial) host tissue (Blumer 1960). Blumer also found that urediniospores may tolerate low temperatures (2–3 °C) better than teliospores. The micro-form species Tranzschelia fusca (Fig. 3a) and probably all other micro-form Tranzschelia spp. on Ranunculaceae overwinter as mycelium in the rhizome buds of Anemone nemorosa near the vegetation point. Infected rhizomes may produce infected shoots again in the following year (Klebahn 1914). This mode may apply accordingly for rhizomes of Anemone ranunculoides (Fig. 3c), the aecial host of the correlated hetereu-form species Tranzschelia pruni-spinosae thus eliminating the need for overwinter survival on the telial host (Fig. 3c) or as urediniospore or teliospore. Since rhizomes are perennial, the fungus may survive in the host tissue for decades. This may be a great advantage for heteroecious species when telial hosts are rare or completely missing over long periods of time. Thus the fungus survives, producing vast quantities of aeciospores. But when telial hosts return to proximity, the fungus may host-alternate again and reproduce sexually. We do not know any “obligate” (e.g., Wilson and Henderson 1966; Zwetko 1990) or “essential” (Feau et al. 2011) heteroecism and we cannot provide any advantage or function connected with “obligate heteroecism” for hetereu-forms. Even in heteropsis-form species (0, I/III, IV) of the genus Gymnosporangium that lack a mitotic short cycle host-alternating is not obligate, because species of this genus may also survive as mycelium in the woody tissues of both hosts (e.g., Bernaux 1956; EPPO 2006; Dervis et al. 2010). In Tranzschelia spp. (and probably in all other host-alternating rust species), we consider the host-alternating process as facultative and talk about “facultative heteroecism,” a term that was used and introduced long time ago (Meinecke 1916; Meinecke 1920; group 1 in Peterson 1961).
Correlated species in Tranzschelia: a Micro-form species T. fusca forming spermogonia (0) and telia (III) on etiolated sterile Anemone nemorosa host. Background above: uninfected flowering host plants. b Rhizome of A. ranunculoides with one systemically infected sterile plant with spermogonia (0) and aecia(I) on deformed leaves and one non-infected flowering plant. c Uredinia (II) and telia (III) with teliospores on the lower side of a leaf of Prunus spinosa (telial host). Photographs taken in SW Germany by M. Scholler; b and c from Klenke and Scholler 2015
Taxonomic considerations
Our molecular phylogenetic analyses based on ITS and LSU rDNA (Fig. 1) neither support broad morphology-based species concepts of Tranzschelia fusca and T. pruni-spinosae (e.g., Hylander et al. 1953; Wilson and Henderson 1966; Urban and Markova 2009), nor do we find support for the hypothesis that life cycle types have taxonomic value in this complex as proclaimed by Schröter (1889) and Arthur (1906, 1921). Specimens of the “morphological species” Tranzschelia pruni-spinosae var. americana López-Franco & J.F. Hennen belong to three different clades in our phylogeny (Tranzschelia sp. 1, 2 and 4). Considering morphology, host plant species, geographic distribution, and our molecular phylogenetic hypothesis, six specimens (Tranzschelia sp. 1–5, see Table 1, Fig. 1) cannot be assigned to any known species. This indicates that Tranzschelia requires further taxonomic research worldwide—even in North America where Tranzschelia spp. are fairly well studied (López-Franco and Hennen 1990; Ono 1994; Scholler et al. 2014; Abbasi and Aime 2015; Blomquist et al. 2015).
Conclusion
Among the oldest extant rust fungi are heteroecious taxa, that likely evolved in conjunction with early Angiosperms during the Jurassic (Aime 2006; Aime et al. 2018). Our results confirm that correlated species evolved several times among species of Tranzschelia. In Tranzschelia, but probably in all other genera with host-alternating species, hetereu-form species do both: they most likely represent the starting point for speciation and other life cycle variations and they persist due to the plasticity of their life cycle in terms of reproduction, overwinter survival, and hosts.
References
Abbasi M, Aime MC (2015) Records of rust caused by Tranzschelia mexicana on black cherry (Prunus serotina) in the United States. Plant Dis 100:523
Aime MC (2006) Toward resolving family level relationships in rust fungi (Uredinales). Mycoscience 47:112–122
Aime MC, Bell CD, Wilson AW (2018) Deconstructing the evolutionary complexity between rust fungi (Pucciniales) and their plant hosts. Stud Mycol 89:143–152
Arthur JC (1906) Eine auf die Struktur und Entwicklungsgeschichte begründete Klassifikation der Uredineen. Résultats Scientifiques du Congrès International de Botanique Vienne 1905:331–348
Arthur JC (1921) New species of Uredineae – XIII. B Torrey Bot Club 48:31–42
Arthur JC (1929) The plant rusts (Urediniales). Wiley, Hoboken
Arthur JC (1934) Manual of the rusts in United States and Canada. Purdue Research Foundation, USA
Bernaux P (1956) Contribution l’étude de la biologie des Gymnosporangium. Ann Épiphyt 1:1–210
Berndt R (2012) Species richness, taxonomy and peculiarities of the neotropical rust fungi: are they more diverse in the Neotropics? Biodivers Conserv 21:2299–2322
Blomquist CL, Scholler M, Scheck HJ (2015) Detection of rust caused by Tranzschelia mexicana on Prunus salicifolia in the United States. Plant Dis 99:1856
Blumer S (1960) Untersuchungen über die Morphologie und Biologie von Tranzschelia pruni-spinosae (Pers.) Dietel und T. discolor (Fuck.) Tranz. et Litv. Phytopathol Z 38:355–388
Bruckart WL, Eskandari FM, Berner DK, Aime MC (2010) Life cycle of Puccinia acroptili on Rhaponticum (= Acroptilon) repens. Mycologia 102:62–68
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552
Cummins GB (1959) Illustrated genera of rust fungi. Burgess Publishing Co., Minneapolis
Cummins GB (1971) The rust fungi of cereals, grasses and bamboos. Springer, New York
De Bary A (1879) Aecidium abietum. Botanische Zeitung 37:48–52 761–774, 777–789, 801–811, 825–830, 841–847
Deadman ML, Al Sadi AM, Al Maqbali YM, Al Subhi A, Al Yahya R, Aime MC (2007) First report of rust caused by Tranzschelia discolor on peach in Oman. Plant Dis 91:638
Dervis S, Dixon L, Doğanlar M, Rossman A (2010) Gall production on hawthorns caused by Gymnosporangium spp. in Hatay province, Turkey. Phytoparasitica 38(4):391–400
Dietel P (1897) Uredinales. In: Engler A, Prantl KA (eds) Die natürlichen Pflanzenfamilien. 1. Teil, Abt. 1. W. Engelmann, Leipzig, pp 24–81
Dietel P (1899) Waren die Rostpilze in früheren Zeiten plurivor? Botanisches Centralblatt 79(81–85):113–117
EPPO (2006) Gymnosporangium spp. (non-European). EPPO Bulletin 36:441–446
Farr DF, Rossman AY (2017) Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Retrieved June 9, 2017, from https://nt.ars-grin.gov/fungaldatabases/
Feau N, Vialle A, Allaiere M, Maier W, Hamelin RC (2011) DNA barcoding in the rust genus Chrysomyxa and its implications for the phylogeny of the genus. Mycologia 103:1250–1266
Fischer E (1898) Entwicklungsgeschichtliche Untersuchungen über Rostpilze. Eine Vorarbeit zur monographischen Darstellung der schweizerischen Uredineen Beiträge zur Kryptogamenflora der Schweiz 1:1–121
Fisher MC, Hanage WP, de Hoog S, Johnson E, Smith MD, White NJ (2005) Low effective dispersal of asexual genotypes in heterogeneous landscapes by the endemic pathogen Penicillium marneffei. PLoS Pathog 1:e20
Gatesy J, DeSalle R, Wheeler W (1993) Alignment-ambiguous nucleotide sites and the exclusion of systematic data. Mol Phylogenet Evol 2:152–157
Giribet G, Wheeler WC (1999) On gaps. Mol Phylogenet Evol 13:132–143
Hennen JF, Buriticá P (1980) A brief summary of modern rust taxonomic and evolutionary theory. Rep Tottori Mycol Inst 18:243–256
Huelsenbeck JP, Larget B, Miller RE, Ronquist F (2002) Potential applications and pitfalls of Bayesian inference of phylogeny. Syst Biol 51:673–688
Hylander N, Joerstad I, Nannfeldt A (1953) Enumeratio Uredinearum Scandinavicarum. Opera Botanica 1:1–102
Jackson HS (1931) Present evolution tendencies and the origin of life cycles in the Uredinales. Memoirs of the Torrey Botanical Club 18:1–108
Josifovič M (1953) Le role du stade écidien dans l’évolution de Tranzschelia pruni spinosae (Pers.) Diet. Bulletin, Casse des Sciences Mathematiques et Sciences Naturelles. Academie Serbe des Sciences et des Arts 7:223–235
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Dictionary of the fungi, 10th edn. Cab International, Oxon
Klebahn H (1914) Uredineen. In: Dahlemer Botanischer Verein (ed) Kryptogamenflora der Mark Brandenburg, vol 5a. Bornträger, Leipzig, pp 69–946
Klenke F, Scholler M (2015) Pflanzenparasitische Kleinpilze. Bestimmungsbuch für Brand-, Rost-, Mehltau-, Flagellatenpilze und Wucherlingsverwandte in Deutschland, Österreich, der Schweiz und Südtirol. Springer-Verlag, Berlin
Leppik EE (1955) Evolution of angiosperms as mirrored in the phylogeny of rust fungi. Archivum Societatis Zoologicae Botanicae Fennicae “Vanamo” 9(suppl):159–160
López-Franco RM, Hennen JF (1990) The genus Tranzschelia (Uredinales) in the Americas. Syst Bot 15:560–591
Maier W, Begerow D, Weiß M, Oberwinkler F (2005) Phylogeny of the rust fungi: an approach using nuclear large subunit ribosomal DNA sequences. Can J Bot 81:12–23
Malloch D (1995) Fungi with heteroxenous life histories. Can J Bot 73(Suppl):S1334–S1342
McTaggart AR, Shivas RG, van der Nest MA, Roux J, Wingfield BD, Wingfield MJ (2016) Host jumps shaped the diversity of extant rust fungi (Pucciniales). New Phytol 209:1149–1158
Meinecke EP (1916) Peridermium harknesii and Cronartium quercuum. Phytopathol 6:225–240
Meinicke EP (1920) Facultative heteroecism in Peridermium cerebrum and Peridermium harknessii. Phytopathol 10:279–297
Ono Y (1994) Tranzschelia asiatica sp. nov. and its taxonomic relationship to Tranzschelia arthurii. Can J Bot 72:1178–1186
Orton LR (1927) A working hypothesis on the origin of rusts, with special reference to the phenomenon of heteroecism. Bot Gaz 84:113–138
Peterson R (1961) On “facultative heteroecism”. Phytopathol 51:266–267
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Roy BA, Vogler DR, Bruns TD (1998) Cryptic species in the Puccinia monoica complex. Mycologia 90:846–853
Savile DBO (1968) The rusts of Chelonae (Scrophulariaceae): a study in the co-evolution of hosts and parasites. Nova Hedwigia 15:369–392
Savile DBO (1976) Evolution of the rust fungi (Uredinales) as reflected by their ecological problems. Evol Biol 9:137–207
Scholler M, Abbasi M, Friedrich F (2014) Tranzschelia in the Americas revisited: two new species and notes on the Tranzschelia thalictri complex. Mycologia 106:448–455
Schröter J (1889) Pilze. In: Cohn F (ed) Kryptogamen-Flora von Schlesien. Dritter Band, Zweite Hälfte. JU Kern’s Verlag (Max Müller), Breslau
Shattock RC, Preece TF (2000) Tranzschel revisited: modern studies of the relatedness of different rust fungi confirm his law. Mycologist 14:113–117
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Tranzschel W (1904) Über die Möglichkeit die Biologie wirtswechselnder Rostpilze auf Grund morphologischer Merkmale vorauszusehen. Arbeiten der Kaiserlichen St. Petersburger Naturforschenden Gesellschaft 35:311–313
Urban Z, Markova J (2009) Catalogue of rust fungi of the Czech and Slovak Republics. Karolinum Press, Charles University, Prague
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Vogler DR, Bruns TD (1998) Phylogenetic relationships among the pine stem rust fungi (Cronartium and Peridermium spp.). Mycologia 90:244–257
Wilson M, Henderson DM (1966) British rust fungi. University Press, Cambridge
Zambino PJ, Szabo LJ (1993) Phylogenetic relationships of selected cereal and grass rusts based on rDNA sequence analysis. Mycologia 85:401–414
Zwetko P (1990) Rostpilze (Uredinales) auf Carex im Ostalpenraum. Ein neues Artenkonzept. Bibliotheca Mycologica 153:1–222
Acknowledgements
We thank the curators of the public herbaria DAOM, GLM, IRAN, MIN and PUR and collectors (listed in Table 1) for providing specimens for this study.
Funding
Molecular work was supported by the National Science Foundation Assembling the Tree of Life project (NSF DEB 0732968 to D. Hibbett and MCA), the Louisiana Board of Regents (MCA) and the German Barcode of Life (GBOL) project, supported by the German Federal Ministry of Education and Research (BMBF FKZ 01LI1501l to MS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Meike Piepenbring
This article is part of the “Special Issue on hyphomycete taxonomy and diversity in honour of Walter Gams who passed away in April 2017.”
Rights and permissions
About this article
Cite this article
Scholler, M., Lutz, M. & Aime, M.C. Repeated formation of correlated species in Tranzschelia (Pucciniales). Mycol Progress 18, 295–303 (2019). https://doi.org/10.1007/s11557-018-1417-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-018-1417-2