Abstract
Boletus longipes was described in 1909 by George Massee from a specimen collected in Singapore. The species was not mentioned again until E.J.H. Corner’s book in 1972 on Malaysian boletes. Corner had collected it many times during his tenure in Singapore, and he synonymized Boletus tristis with B. longipes described nine years after B. longipes by Patouillard and Baker from the same site. Among the distinguishing characters of B. longipes were deep vinous red spore deposit, red oxidation reaction of the hymenophore when bruised, and spores that displayed a strong violet color reaction in contact with KOH. C.B. Wolfe ultimately moved both species to a new genus Austroboletus. During recent efforts to circumscribe Austroboletus in Australasia using morphological and molecular phylogenetic inferences, it became clear that B. longipes was neither in harmony with Boletus, Porphyrellus, nor Austroboletus. Rather, it is a new genus, which we describe here as Ionosporus, allied to Borofutus, Spongiforma, and Rhodactina of subfamily Leccinoideae. In addition, recent collections from Queensland and New South Wales, Australia, that are morphologically similar to I. longipes, are inferred to be a separate new species, I. australis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Massee (1909) first described Boletus longipes based on a collection sent to Kew by H. Ridley from the Gardens’ Jungle, Singapore. Morphologically, it was observed to have a long, slender, and flexuous stem similar to Boletus fuligineus Fr. in structure (Massee 1909, p 207). Boletus longipes was noted to most likely be gregarious, since at the site of collection, there were nine other well-preserved specimens along with a watercolor drawing.
Corner (1972) placed B. longipes in subg. Tylopilus of Boletus. He also treated Boletus tristis Pat. & C.F. Baker collected from the same locality (Patouillard and Baker 1918) as a synonym. He made comparison to B. griseipurpureus Corner and Porphyrellus atrofuscus E.A. Dick & Snell as similar or almost identical species. Corner commented that the spore print was red to deep wine color and the spores appeared smooth, narrowly boletoid and turned purplish in KOH. Along with the type specimen from Singapore, Corner cited two of his own collections gathered at that site during 1929 and 1931 plus another collection from 1930 at Negri Sembilan, Malaysia. Later, Corner (1980) noted that he had found B. longipes repeatedly in the Gardens’ Jungle, Singapore from 1929 to 1944.
Singer (1945) not only recombined B. tristis in Porphyrellus based primarily on macromorphology but also noted that the spore wall was “…cloudy punctulate…” (p 22). Later, Wolfe and Petersen (1978) used spore ultrastructure in their effort to sort out supraspecific taxa of Porphyrellus. They hypothesized that B. longipes and B. tristis were contaxic, which was supported by scanning electron micrographs (SEMs) of the spores of the type specimens. Those illustrations showed that both taxa appeared to be “…ornamented with complex ridges and shallow pits…” (p 154). Thus, using the earliest basionym, they recognized Porphyrellus longipes (Massee) Wolfe & Petersen as the legitimate name to circumscribe this taxon.
Based on detailed type studies of B. longipes and B. tristis, Wolfe (1979) reevaluated the conspecificity of the two taxa. He noted differences in EM (median length/width ratio) values of the spores and observed a significant difference between the two in the thicknesses of the subhymenium and hymenium. This led to the proposal of Austroboletus tristis (Pat. & C.F. Baker) Wolfe as a distinct species. The position of B. longipes was also reevaluated, and the combination Austroboletus longipes (Massee) Wolfe was proposed. Austroboletus was previously recognized as a subgenus of Boletus by Corner (1972), but Wolfe (1979) modified the concept and elevated the subgenus to generic rank based on unique spore ornamentation, stipe ornamentation, and pileipellis differences.
Corner (1980) refuted the hypotheses of Wolfe (1979) and defended the original placement of the species in Boletus subg. Tylopilus because the spore print color did not match that typical of species placed in subg. Austroboletus; the spore shape was observed to be boletoid as common in Tylopilus and not the amygdaliform shape characterizing the subg. Austroboletus sensu Corner (1972). Further, the pleurocystidia, cheilocystidia, and tube shape all showed characteristics typical of Tylopilus (Corner 1980). Corner also stated that the variation in spore size and shape, hymenial thicknesses, and terminal cells in the pileipellis as observed in the type of B. tristis by Wolfe (1979) were due to developmental stages and referred to his original hypothesis that the latter is a later synonym of B. longipes. In addition, Corner (1980, Table 1) noted 12 disparate and overlapping spore size measurements for B. longipes and B. tristis to support synonymy. Singer (1986), on the other hand, endorsed Wolfe’s distinction of B. tristis and B. longipes based on perceived differences in stipitipellis structure.
Watling and Li (1999) referred to a specimen lacking field data from Queensland, Australia (JECA 86/64, BRIP19815 in BRI) that could possibly be allied to B. longipes or B. tristis. Their observation was based on the purple color reaction of the spores to KOH. Differences in stipe ornamentation and colors were noted to be in disagreement with either species however. Watling (2000) summarized the historical coverage of the two taxa up to that point and made a reference to the aforementioned Queensland material. Recently, one of us (NF) verified that the BRIP collection does indeed have spores with a purple reaction in KOH and that they are minutely roughened on the surface when observed by light microscopy.
Chan (2010) provided a description of Malaysian Austroboletus longipes based on four recent collections under Shorea sp. from the Pasoh Forest Reserve. Chan’s material matched Corner’s hypothesis of B. longipes as presented in subgen. Tylopilus, including the synonymy of B. tristis.
Horak (2011) disputed the placement of B. longipes in Tylopilus because the verruculose spores did not fit the usual morphology for Tylopilus. This was based on his examination of the holotype. He further accepted, with hesitation, the placement in Austroboletus as proposed by Wolfe and Petersen (1978) (sic; placed in Austroboletus by Wolfe 1979). Horak (2011) also stated that the spores of the types of B. longipes and B. tristis were identical and that the latter would be a later synonym. However, in the next sentence (Horak 2011, p 197), he noted that based on the protologues of B. longipes and B. tristis, they could not be contaxic and referred to Corner (1980) for more data. Based on another specimen collected by Corner in Sarawak during 1972 (P 143, E) and cited in a later paper (Corner 1974), Horak (2011) suggested that B. longipes ss Corner was not A. longipes s.str. due to smaller spores and the large lanceolate hymenial cystidia. Yet, in that 1974 paper, Corner noted three other specimens that would have the appropriate spore size range.
Desjardin et al. (2009) and Hosen et al. (2013) discussed the similarities of Spongiforma Desjardin, Manfr. Binder, Roekring & Flegel, and Borofutus Hosen & Zhu L. Yang to A. longipes and A. tristis especially with regard to spore ornamentation and reaction of the spores to dilute solutions of KOH. Of particular note is that the apices of the spores in Spongiforma are truncated with a depressed pore, a very obscure feature noted by Horak (2011) for the type of B. longipes and just barely noticeable in light micrographs obtained when viewed with Nomarski optics (Fig. 1). It is rarely apparent in SEMs (Fig. 2b). Another species of Spongiforma was described by Desjardin et al. (2011), with reference to Desjardin et al. (2009) for comparison to other bolete genera. Hosen et al. (2013) described the specific morphological features of A. longipes and A. tristis, based on observations of one of the types (A. tristis) and material from Corner (1972). In a summary comparison, Hosen et al. (2013) noted that both A. longipes and A. tristis showed spores that were “slender boletoid” and appeared verrucose with light microscopy and roughened or pitted under SEM. Further reference was given to the unique KOH reaction (Corner 1972; Wolfe and Petersen 1978; Wolfe 1979; Horak 2011), but no evidence was proposed for maintaining the separation of the two species as different taxa. Another macroscopic feature described and illustrated by Hosen et al. (2013, Fig. 5e) was the red bruising reaction of the hymenophore of Borofutus as noted by Corner (1972) and Chan (2010) in their respective concepts of A. longipes. That oxidation reaction also appears in the novel Australian taxon described below. In Fig. 4b of Hosen et al. (2013), a spore of Borofutus in adaxial view appears to be obscurely truncated possibly indicating the presence of a germ pore. Our examination of a Thai specimen of B. dhakanus (OR 352) shows a clear purple-violet reaction in KOH and an obvious germ pore in equatorial view and the pitted wall in surface view (Fig. 3).
Light micrographs of spores from type specimens in KOH: B. longipes and B. tristis. a Spores of B. longipes in equatorial view showing faint germ pore (arrows). b Spore of B. longipes showing faint surface ornamentation. c Spores of B. tristis in equatorial view showing faint germ pore (arrows). d Spores of B. tristis showing faint surface ornamentation. Scales bars = 10 μm
Recently, Vadthanarat et al. (2018) revised the sequestrate genus Rhodactina Pegler & T.W.K. Young inferring its placement in subfam. Leccinoideae G. Wu & Zhu L. Yang. They noted that the genus, along with sister genera, Borofutus and Spongiforma, has the same purple color reaction to KOH as reported previously for A. longipes (and A. tristis). Those authors also suggested that A. longipes, A. tristis, and A. malaccensis (Pat. & C.F. Baker) Wolfe would likely belong to this group. However, according to Corner (1972) and Horak (2011), the latter species lacks the purple reaction of the spores in KOH, and the protologue of B. malaccensis succinctly stated that the stipe is very coarsely sulcate scrobiculate (Patouillard and Baker 1918).
Since 2006, we have made six collections of a bolete in eastern Australia with a range from northern Queensland to the central coast of New South Wales which we originally identified as A. longipes. These conclusions were based solely on basidiome morphology, spore print color, and the purple reaction of the spores in KOH. Fortunately, one of us (SL) obtained recent, fresh material of A. longipes from Singapore that could be sequenced. Regrettably, another specimen from peninsular Malaysia (KUM 90057) collected by H.T. Chan would not provide DNA. In this study, we use molecular data from the rpb2 protein-coding locus (RNA polymerase II second largest subunit) to infer the phylogenetic position of Malaysian A. longipes and the recent collections from Australia.
Materials and methods
Morphological studies
Macromorphological data were obtained from fresh specimens. General color terms are approximations, and the color codes (e.g., 7D8) are page, column, and grid designations from Kornerup and Wanscher (1983). All microscopic structures were observed and measured with an Olympus BHS compound light microscope equipped with Nomarski Differential Interference Contrast (DIC) optics from dried material revived in 3% KOH. The abbreviation Q refers to the mean length/width ratio measured from n basidiospores, observed from p collections, and x refers to the mean length × mean width. Light micrographs were obtained via Spot 5.3 Imaging software using a SPOT Insight Gigabit digital camera from Diagnostic Instruments. For observation of spores under scanning electron microscope (SEM), hymenophoral fragments were removed from dried basidiomata, mounted directly on aluminum stubs with carbon adhesive tabs, and coated with 10 nm of gold with a Hummer II sputter coater. SEM micrographs were captured digitally from a Hitachi S-2700 scanning electron microscope operating at 20 kV. Herbarium codes (Thiers 2018) are cited for all collections from which morphological features were examined. Novel names are registered with MycoBank.
DNA isolation, PCR amplification, and DNA sequencing
Genomic DNA was extracted from dried basidiomata following extraction protocols outlined in Hosaka and Uno (2011) and Kluting et al. (2014). A portion of the rpb2 gene was amplified with the primers bRPB2-6F and bRPB2-7.1R (Matheny 2005). The cycling conditions included an initial denaturation at 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 58 °C for 1 min, 72 °C for 1 min, and a final extension at 72 °C for 7 min. PCR products were sequenced in both directions by Macrogen (http://macrogenusa.com/). ITS sequence data were gathered according to protocols used by Bonito et al. (2017), except 5 mm3 of dried basidiomata was the source of the genomic DNA.
Alignment and phylogenetic analyses
Novel rpb2 sequences generated for this study were proofread and aligned with FinchTV v1.4 (Geospiza 2009) and BioEdit v7.0.5 (Hall 1999). Novel sequences were aligned with additional exemplars from GenBank based on previous generic placement of the target taxa having rpb2 data, appropriate exemplars used by Vadthanarat et al. (2018), Wu et al. (2018), and a BLAST search on GenBank using the isotype of Ionosporus australis. An alignment of partial sequences of rpb2 was performed with MUSCLE via the EMBL-EBI website (http://www.ebi.ac.uk/). The intron was removed from the rpb2 alignment prior to phylogenetic analysis. The final alignment contained 35 taxa and 695 nucleotide sites/characters. Bayesian analysis was performed with MrBayes 3.2.6 (Ronquist et al. 2012) on the CIPRES Science Gateway 3.1 (Miller et al. 2010) using a generalized time-reversible model (GTR) with gamma-distributed rate variation between sites. Since the RAxML manual recommends a GTR model, we ran a GTRGAMMA model for both MrBayes and RAxML runs. The priors utilized were defaults in MrBayes. Metropolis-coupled Markov chain Monte Carlo analyses were run with default settings (nruns = 2, nchains = 4, swapfreq = 1, nswaps = 1) for 20,000,000 generations, which was sufficient for convergence (average standard deviation of split frequencies ca. 0.0025). The average estimated sample size (ESS) for each parameter ranged between 8000 and 14,685. The proportion of samples discarded as burnin was 25%. Maximum likelihood (ML) analyses were conducted with 1000 MLBS replications using the rapid bootstrap algorithm (Stamatakis et al. 2008). The clades and levels of support were inferred with a ML analysis and ML bootstrap values (MLBS) generated with RAxML-HPC 8.0.24 (Stamatakis 2006; Stamatakis 2014) on the CIPRES gateway (Miller et al. 2010). Chalciporus africanus and C. aff. piperatus were used for outgroup taxa following Vadthanarat et al. (2018). Alignment is deposited in Dryad (https://datadryad.org/). Novel rpb2 sequences generated from this study are deposited in GenBank under accession numbers MH712031–MH712037. Novel ITS sequences are deposited under accession numbers MK248172–MK248176.
Results
Molecular data
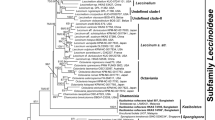
A total of seven rpb2 sequences were newly generated and deposited in GenBank (Table 1). All selected specimens of I. australis formed a monophyletic group sister to I. longipes in the Ionosporus clade (Fig. 4). Pairwise alignment of the rpb2 sequences from the two species revealed a total of 26 base pair differences. Results of the phylogenetic analyses showed that Ionosporus was closely related to Spongiforma and Borofutus in subfam. Leccinoideae inferring further support to some of the morphological similarities among the genera. The clade containing all the genera with spores that have a purple reaction in KOH (Ionosporus, Rhodactina, Borofutus, and Spongiforma) is well resolved with Bayesian posterior probability support of 1 and ML 100% boot strap support (Fig. 4). Five novel ITS sequences were generated, and the GenBank numbers are listed with the appropriate species (below).
Taxonomy
Ionosporus O. Khmelnitsky, gen. nov.
MycoBank: MB 827132
Diagnosis: Basidiomata epigeous, dry, dark gray to sooty gray-brown on pileus and stipe; hymenophore tubulose with angular pores, whitish to grayish yellow to pale greenish yellow, staining red when bruised; stipe usually central, finely but conspicuously reticulate and densely finely subpruinose, conspicuously white at the base; context white or very pale yellow, unchanging when exposed. Spores pale violet to reddish brown in deposit, deeply purple-violet in dilute KOH solutions, dextrinoid in Melzer’s reagent, fusoid to elongate, appearing smooth with bright field light microscopy, barely granulose with Nomarski DIC optics, irregularly and finely granulose to pitted granulose with SEM, sometimes with a faint germ pore. Clamp connections absent
Typus: Boletus longipes Massee
Etymology: Ion (ιον, Greek for violet) + spora (σπορα, Greek for spore); in reference to the violet reaction of the spores in contact with KOH
Key to species of Ionosporus
1 Spores purple in deposit; basidiospore Q = 2.7; Malaysia ………………………………………………...… I. longipes
2 Spores red-brown in deposit; basidiospore Q = 2.15; Australia ………………………………………... I. australis
Ionosporus longipes (Massee) O. Khmelnitsky, Davoodian, Raspé, S. Lee & Halling, comb. nov. Figures 1, 2a–b, 5, 6
Light micrographs of I. longipes spores in KOH (a, b, d) and in Melzer’s (c). a Spores of I. longipes (Lee 1180) in equatorial view. b Spores of I. longipes (Lee 1180) showing roughened spore surface. c Dextrinoid spores of I. longipes (Lee 1208) in equatorial view showing striations (arrow). d Spores of I. longipes (Lee 1208) showing germ pore (arrow). Scale bars = 10 μm
≡ Boletus longipes Massee, Bull. Misc. Inform. 207. 1909
≡ Porphyrellus longipes (Massee) Wolfe & R.H. Petersen, Mycotaxon 7: 160. 1978
≡ Austroboletus longipes (Massee) Wolfe, Biblio. Mycol. 69: 105. 1979 (1980)
TYPE: SINGAPORE. Ridley 81 (holotype: K-M000167119!)
= Boletus tristis Pat. & C.F. Baker, J. Straits Branch Roy. Asiat. Soc. 78: 70. 1918
≡ Porphyrellus tristis (Pat. & C.F. Baker) Singer, Farlowia 2: 118. 1945
≡ Austroboletus tristis (Pat. & C.F. Baker) Wolfe, Biblio. Mycol. 69: 127. 1979 (1980)
TYPE: SINGAPORE. Botanic Gardens, Aug '17, C.F. Baker no. 4995 (holotype: FH!)
MycoBank: MB 827133
GenBank ITS: MK248172
The following description is modified with permission from that in Chan (2010): Pileus (3) 4.4–6 (7.3) cm broad, convex, dry, velvety, even to uneven, brown (5E4, 5F7), with decurved to slightly uplifted margin, not bruising, turning red with NH4OH. Context 5–9 mm thick, soft and spongy, white to yellowish white, no color change when exposed, red with NH4OH. Hymenophore tubulose, adnexed to adnate, light yellow (3A5, 4A3), with concolorous pores less than 1 mm broad, bruising red, sometimes with fine black discoloration, red with NH4OH. Stipe 5.5–8 (11.6) cm long, 4–7 (9) mm broad, central, equal to slightly subclavate, dry, finely (shallowly) costate to finely sublacunose or finely elongate reticulate, otherwise subpruinose, brown to dark brown (5E4, 6F3), white at the base, no color change when bruised, red with NH4OH, with white basal mycelium; interior white with chalky consistency, red with NH4OH
Basidiospores purplish in deposit, (13) 14–19 (22) × (5) 6–7 μm, x = 16.5 × 6.22 μm, Q = 2.7, n = 135, p = 5, ellipsoid to subfusoid, often with an obscure germ pore, smooth under light microscope, obscurely granulose pitted under Nomarski differential interference contrast optics, dextrinoid, translucent purple in KOH, obviously granulose pitted with SEM. Basidia (20) 22–27 × 10–12 (15) μm, 4-sterigmate, short-clavate, hyaline, inamyloid. Cheilocystidia (40) 49–65 (95) × (13) 16–20 (22) μm, ventricose to subventricose to subfusoid, hyaline, inamyloid, thin-walled. Hymenophoral trama divergent (boletoid), with hyaline elements, inamyloid. Pileus trama interwoven with hyaline, thin-walled hyphae, 4–10.4 (16) μm. Pileipellis a trichodermium, composed of erect to suberect cylindrical elements, (60) 65–108 (118) × (9) 10–15 (24) μm, smooth, thin-walled, hyaline or pale brown content, inamyloid. Stipitipellis a trichodermium of caulocystidia 29–43 × 9–15 μm, subcylindric to clavate, smooth, thin-walled, inamyloid, with brown content in KOH, subtended by longitudinally oriented, hyaline, cylindrical tramal hyphae. Clamp connections absent
Distribution: Currently known from Singapore and peninsular Malaysia
Habit and habitat: Gregarious on soil under Dipterocarpaceae (Shorea sp.). According to Lee (2017), the species occurs in groups during the rainy season in Kepong, Malaysia, growing under various dipterocarps, including Shorea acuminata Dyer and S. macroptera Dyer.
Material examined: Malaysia. Negeri Sembilan. Pasoh, Hutan Simpan. 2° 58′ 7″ N, 102° 17′ 49″ E, 111 m, 20 Sep 2006, H.-T. Chan, ex KUM 90057 (NY). Singapore. Bukit Timah Nature Reserve, Jungle Fall Path, 1° 21′ 16″ N, 103° 46′ 34″ E, 172 m, 29 Aug 2017, S. Lee 1180 (leg. B.C. Ho, S. Lee) (BR, SING); 22 Mar 2018, S. Lee 1208 (leg. X.Y. Ng, R.C.J. Lim, S. Teo) (SING)
Comments: The long slender stipe of I. longipes is a feature noted by Massee (1909) but is not always present (Fig. 5b, Lee 1180). However, the purple-violet discoloration of the spores when in contact with KOH solution distinguishes it from the typical features of the genera it had been placed in by previous authors. That character can also serve as an additional indicator of the placement of other epigeous species in Ionosporus and the sister clades including Borofutus, Spongiforma, and Rhodactina. While B. tristis and B. longipes were said to differ based on differences in hymenial thicknesses and spore morphology (Wolfe 1979), Corner’s (1980) closer study of the morphological features and keen observations of live material suggest little significant distinction. Closer observations of the spores of I. longipes (Lee 1180, Lee 1208) showed that a minority displayed striations, especially when mounted in Melzer’s, a feature reminiscent of Horak’s (2011) evaluation of B. tristis (Fig. 6c). The presence of obscure germ pores on some spores of both taxa supports synonymy (Fig. 1). The contaxic relationship between the two species is strengthened when comparing SEMs of the spores of B. tristis (Hosen et al. 2013, Fig. 4g, h) and I. longipes (Fig. 2a, b), which show little to no difference in spore ornamentation. The recent specimen from Singapore, Lee 1180, had a faint but clearly deep purple spore deposit with spore size, shape, and KOH reaction strongly reminiscent of B. tristis (Fig. 1c, d). At this point, besides the phylogenetic inference and spore size and shape differences, the other microscopic features are reminiscent of I. australis sp. nov. described below. More collections in SE Asia are needed for critical evaluation. A Malaysian specimen (KUM 90057) that we treat as a specimen of A. longipes could not be sequenced due to highly degraded genomic DNA.
Ionosporus australis O. Khmelnitsky & Halling, sp. nov. Figures 2c–d, 7, 8
Light micrographs (Nomarski DIC) of microscopic features from Ionosporus australis. a Halling 10131, equatorial view of basidiospores in KOH showing faint germ pore (arrow). b Halling 10131, surface view of basidiospores in KOH showing faint granulose ornamentation. c Halling 10131, pileipellis trichodermium. d Halling 10131, stipitipellis with fertile basidium. e Halling 10131, cheilocystidia. Scale bars = 5 μm in a, b; 20 μm in c–e
MycoBank: MB 827179
GenBank ITS: MK248713–MK248716
Type: Australia, New South Wales, Central Coast, Strickland State Forest, Ridge to Rainforests Track, 33° 22′ 18.12″ S, 151° 19′ 19.2″ E, 160 m, 9 Mar 2017, Halling 10116 (holotype: DAR #83338!; isotype: NY #02686001!)
Etymology: From Latin “auster” meaning south, referring to Australia
Pileus (2) 5.7–6.2 (8) cm broad, convex becoming plano-convex, to plane with uplifted margin and depressed center, densely matted subtomentose, then finely subtomentose, becoming finely areolate, dry, black at first, then gray to gray-brown, becoming brown to cocoa brown (6E6) to red-brown (7F7), fading to light grayish yellow (4C5) with age, with even margin, sometimes remaining red-brown; context white, unchanging, or pale yellow to dull pinkish tan when exposed; red where insect-damaged. Odor mild; taste sometimes slightly bitter. Tubes adnexed, white when young, pale yellow to olive yellow to dark grayish yellow (4C4), staining red, up to 1 cm long, with pores angular, pale yellow, staining red, 1–2 mm in diameter, golden blonde (4A4) becoming light grayish yellow (4B5) in age, staining red-brown. Stipe (2.9) 3.5–5 (7) cm long, 5–10 mm broad, equal to subclavate, then tapered at base, dry, finely but clearly reticulate nearly to base, usually densely subpruinose granulose, black to dark gray, dark grayish to brownish near base, white at the base, sometimes with copious basal mycelium; context white, slowly pale to dull pinkish tan. NB: One basidiome (Halling 9784-non) had a finely scissurate scabrous stipe, otherwise similar to those with reticulate stipe.
Basidiospores reddish brown in deposit, (11) 12–13 × 4.8–5 μm, x = 12.17 × 5.04 μm, Q = 2.15, n = 140, p = 7, subfusiform, smooth when viewed with bright field light microscopy, irregularly and obscurely pitted granulose under Nomarski differential interference contrast optics, obviously granulose pitted with SEM, translucent purple to pale violet in KOH, a minority dextrinoid. Basidia (15) 20–22 (24) × (4.6) 7–9 (11) μm, clavate, 4-sterigmate, hyaline in KOH. Cheilocystidia 33–47 × 6.4–11.2 μm, ventricose to subventricose to obclavate or occasionally aculeate, with an obtuse apex, hyaline, inamyloid, thin-walled. Pileus trama interwoven with hyaline, thin-walled hyphae, 5.6–11.2 μm. Pileipellis a trichodermium of erect to suberect cylindrical hyphae, (54) 64–112 (136) × (3.2) 5.6–9.6 (12.8) μm, smooth, thin-walled, with homogenous brown contents or sometimes hyaline, inamyloid. Hymenophoral trama divergent (boletoid) from a central strand, with hyaline elements, inamyloid. Stipitipellis a hymeniform layer of basidioles, occasional basidia, and caulocystidia, 24–36 (42) × 7.2–10.4 (12) μm, clavate to ventricose, smooth, thin-walled, inamyloid, with homogenous brown content in KOH, subtended by longitudinally oriented, hyaline, cylindrical tramal hyphae. Clamp connections absent
Known distribution: Currently known from the central coast of New South Wales to southern Queensland and the Atherton Tableland of northern Queensland
Habit and habitat: Sclerophyll forest, gregarious to scattered, on soil dominated by Eucalyptus spp., Allocasuarina, Angophora spp., Angophora costata, Syncarpia glomulifera (Sm.) Nied
Material examined: Australia. New South Wales, North Coast, Yarrahapinni (Yarriabini) National Park, Way Way Creek Road, 30° 47′ 43.44″ S, 152° 58′ 12″ E, 211 m, 22 Apr 2015, Halling 10015 (NY, MEL); Central Coast, Strickland State Forest, Strickland Falls Track, 31° 22′ 22.44″ S, 151° 19′ 19.2″ E, 149 m, 10 Mar 2017, Halling 10131 (NY, DAR); Queensland, Herberton Shire, Mt. Baldy, Mt. Baldy road ± 5.3 km from Atherton–Herberton Highway, 17° 18′ 51.12″ S, 145° 24′ 10.8″ E, 1040 m, 15 Feb 2006, Osmundson 1128, (NY, BRI); Springbrook National Park, tracks near Tallanbana Picnic Area, 28° 13′ 28.92″ S, 153° 16′ 15.6″ E, 810 m, 13 Feb 2013, Halling 9784, 9784-non (NY, BRI); D’Aguilar National Park, Mt. Mee, Pegg’s Road, 27° 5′ 48.84″ S, 152° 42′ 36″ E, 524 m, 21 Feb 2013, Halling 9814, (NY, BRI).
Comments: Based strictly on morphological features, we previously considered the Australian collections to represent A. longipes (now I. longipes). However, material (Lee 1180) collected in the Bukit Timah Nature Reserve (± 8 km NNW of the Singapore Botanic Gardens), provided quality rpb2 data to support I. longipes as distinct from the Australian material, herein proposed as the new species I. australis. Another specimen (Lee 1208) collected from the same locality a year later is a morphological match to Lee 1180. Additionally, differences in the Q values indicate a distinguishing characteristic between the two species; I. longipes (Fig. 1; Fig. 2a, b) has spores which are longer and narrower than those of I. australis (Fig. 2c, d; Fig. 8a, b) which tends to have shorter and wider spores. One of the exemplars that we sequenced, Halling 9784 non (without reticulum, arrow, Fig. 7e), was noted in the field to be non-reticulate, inconsistent with other basidiomata growing side by side. However, the molecular data inferred no variation between the reticulate and non-reticulate specimens. It is also worth noting that Corner (1972, p 165) described B. longipes as “…rarely without a reticulum.”
The phylogram (Fig. 4) indicates that all Australian specimens form a monophyletic group strongly supported by molecular data, spore morphology (size and color), purported mycorrhizal associates (Myrtaceae versus Dipterocarpaceae), and geographical observations.
Discussion
Ionosporus has a combination of morphological traits that distinguish it from any currently known genus. The reaction of the spores that turn purple-violet in KOH and the irregular but finely granulose ornamentation of the spore wall are distinctive characteristics that set I. australis and I. longipes apart from the typical traits of the genera in which they were previously placed. After being described in Boletus (Massee 1909), moved to Porphyrellus (Wolfe and Petersen 1978), and Austroboletus (Wolfe 1979), the molecular data show that all selected sequences form a monophyletic group that does not share a recent common ancestor with any of those genera. Bayesian and ML analyses (Fig. 4) infer that specimens we collected from Australia resolve as a different species compared to an exemplar collected in Singapore, the type locality of I. longipes (and B. tristis). They differ by 26 base pairs at the rpb2 locus. Ionosporus is most closely related to S. thailandica and Borofutus all of which are in the subfamily Leccinoideae. Notably, all the genera in the subfamily Leccinoideae with the purple-violet reaction of spores in KOH (Borofutus, Rhodactina, Spongiforma, and Ionosporus) are grouped together as a clade with high support (Fig. 4; Vadthanarat et al. 2018, Wu et al. 2018).
Rhodactina is clearly distinguished from others with the purple KOH reaction by the sequestrate habit, a cartilaginous gleba, and thick-walled spores with a stellate appearance in polar view because of seven to ten prominent ridges. While Spongiforma has a sequestrate, epigeous habit, it lacks a peridium and has a rubbery-pliant texture that will regain shape if compressed while in the fresh state. Although the spore ornamentation is somewhat similar to that of Ionosporus, those spores become smooth when mounted and viewed in KOH solution and are statismosporic versus ballistosporic. The germ pore is quite obvious in Spongiforma (Desjardin et al. 2009; Hosen et al. 2013), but not so in Ionosporus (Fig. 2). Finally, even though Borofutus has an obviously epigeous, mushroom-like habit, that habit is quite small; the hymenophore is decurrent and appears to contribute to the subdecurrent ridges on the surface of the stipe. That hymenophore also is relatively shallow and broadly poroid, with large hexagonal pores which can be duplex. Microscopically, the spores are fairly thick-walled, have a conspicuous pore, and an ornamentation that is quite smooth with scattered, isodiametric to irregular pores rather than completely, irregularly granulose-pitted. In addition, the cheilocystidia in Borofutus are often variously thick-walled, with pigmented contents or thickenings as opposed to the thin-walled, hyaline elements in Ionosporus (Fig. 8e).
Along with the long-standing debate on the classification of I. longipes, there has also been disagreement regarding the relationship between I. longipes and B. tristis. There is sufficient evidence to conclude that B. tristis is synonymous with I. longipes. Aside from producing the unique purple chemical reaction in KOH, it was previously noted that both B. longipes and B. tristis produce similar basidiospores. SEM micrographs of B. tristis and B. longipes show a fusoid shape and a rough surface as noted by Wolfe and Petersen (1978) and Pegler and Young (1981). Also, Corner’s (1980) observations of fresh specimens and developmental morphology suggest that the two species are synonymous.
References
Binder M, Larsson KH, Matheny PB, Hibbett DS (2010) Amylocorticiales ord. nov. and Jaapiales ord. nov.: early diverging clades of Agaricomycetidae dominated by corticioid forms. Mycologia 102:865–880. https://doi.org/10.3852/09-288
Bonito G, Hameed K, Toome-Heller M, Healy R, Reid C, Liao HL, Aime MC, Schadt CW, Vilgalys R (2017) Atractiella rhizophila, a novel endorrhizal fungus within the rust lineage (Puccinomycotina) adapted to the root microbiome of Populus and a broad diversity of plant hosts. Mycologia 109:18–26
Chan H-T (2010) Diversity of Boletaceae in peninsular Malaysia. MA thesis, University of Malaya, Kuala Lumpur
Corner EJH (1972) Boletus in Malaysia. Government Printer, Singapore
Corner EJH (1974) Boletus and Phylloporus in Malaysia: further notes and descriptions. Gardens’ Bulletin, Singapore (part I) 27:1–16
Corner EJH (1980) Boletus longipes Mass., a critical Malaysian species. Gardens’ Bulletin, Singapore (part II) 33:290–296
Desjardin DE, Binder M, Roekring S, Flegel T (2009) Spongiforma, a new genus of gastroid boletes from Thailand. Fungal Divers 37:1–8
Desjardin DE, Peay KG, Bruns TD (2011) Spongiforma squarepantsii, a new species of gasteroid bolete from Borneo. Mycologia 103:1119–1123
Geospiza (2009) Finch TV 1.4.0 for Windows (https://digitalworldbiology.com/FinchTV)
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Horak E (2011) Revision of Malaysian species of Boletales s.l. (Basidiomycota) described by Corner EJH (1972, 1974). Forest Research Institute and Ministry of Natural Resources and Environment, Kepong
Hosaka K, Uno K (2011) Assessment of the DNA quality in mushroom specimens: effect of drying temperature. Bulletin of the National Museum of Nature and Science, Series B 37:101–111
Hosen MI, Feng B, Wu G, Zhu X-T, Li Y-C, Yang ZL (2013) Borofutus, a new genus of Boletaceae from tropical Asia: phylogeny, morphology and taxonomy. Fungal Divers 58:215–226
Kluting KL, Baroni TJ, Bergemann SE (2014) Toward a stable classification of genera within the Entolomataceae: a phylogenetic re-evaluation of the Rhodocybe-Clitopilus clade. Mycologia 106:1127–1142
Kornerup A, Wanscher JH (1983) Methuen handbook of colour, 3rd edn. Eyre Methuen, Ltd., London
Lee SS (2017) A field guide to the larger fungi of FRIM. Research Pamphlet No. 135. Maziza Sdn. Bhd., Kepong
Massee G (1909) XXIV.–Fungi Exotici: IX. Bull Misc Inf 1909:204–209
Matheny PB (2005) Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe, Agaricales). Mol Phylogenet Evol 35:1–20
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees in Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, Louisiana p1–8
Patouillard N, Baker CF (1918) Some Singapore Boletinae. J Straits Branch Roy Asiat Soc 78:67–72
Pegler D, Young TWK (1981) A natural arrangement of the Boletales, with reference to spore morphology. Trans Br Mycol Soc 76:103–146
Raspé O, Vadthanarat S, De Kesel A, Degreef J, Hyde KD, Lumyong S (2016) Pulveroboletus fragrans, a new Boletaceae species from Northern Thailand, with a remarkable aromatic odor. Mycol Prog 15:38. https://doi.org/10.1007/s11557-016-1179-7
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Largeta B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Singer R (1945) The Boletineae of Florida with notes on extralimital species. I. The Strobilomycetaceae. Farlowia 2:97–141
Singer R (1986) The Agaricales in modern taxonomy, 4th edn. Koeltz Scientific Books, Koenigstein
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Stamatakis A (2014) RAxML 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for RAxML web servers. Syst Biol 57:758–771. https://doi.org/10.1080/10635150802429642
Thiers B (2018) Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium http://sweetgum.nybg.org/science/ih/. Accessed 5 Aug 2018
Vadthanarat S, Raspé O, Lumyong S (2018) Phylogenetic affinities of the sequestrate genus Rhodactina (Boletaceae), with a new species, R. rostratispora from Thailand. MycoKeys 29:63–80. https://doi.org/10.3897/mycokeys.29.22572
Watling R (2000) Bresadola, Cesati and Patouillard’s old world boletes. In: Papetti C, Consiglio G (eds) Mycologia Duemila. Associazione Micologica Bresadola, Trento
Watling R, Li T-H (1999) Australian boletes: a preliminary survey. Royal Botanic Garden, Edinburgh
Wolfe CB Jr (1979 [1980]) Austroboletus and Tylopilus subg. Porphyrellus with emphasis on North American taxa. Bibl Mycol 69:1–148
Wolfe CB Jr, Petersen RH (1978) Taxonomy and nomenclature of the supraspecific taxa of Porphyrellus. Mycotaxon 7:152–162
Wu G, Feng B, Xu J, Zhu X-T, Li Y-C, Zeng N-K, Hosen MI, Yang ZL (2014) Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers 69:93–115
Wu G, Lee SML, Horak E, Yang Z-L (2018) Spongispora temasekensis, a new boletoid genus and species from Singapore. Mycologia 110:919–929
Acknowledgments
The Queensland Herbarium (BRI) collaborated generously with assistance and support for herbarium and field studies. Fieldwork in New South Wales was facilitated with guidance and support from Pam O’Sullivan, Teresa and John Van Der Heul, and Ian Dodd. The curators of herbaria at FH and K provided the loan of valuable type specimens. Hong-Twu Chan provided permission to use and modify her descriptive data of A. longipes. Confirmation of specimen data lodged at the Forestry Research Institute of Malaysia (FRIM) was offered by Su See Lee and Patahayah Mansor. We are indebted to Mike Baxter for expertise and access to the SEM facility at the CUNY-Lehman College campus (Bronx, NY, USA). OR is grateful to Amy Choong Mei Fun for her invitation to study boletes of Singapore. We are grateful to Julian Liber of Michigan State University for obtaining and assembling the ITS sequences.
Funding
The last author was partially supported by the National Science Foundation (USA) with funds from grants DEB 0414665 and DEB 1020421. The National Geographic Society Committee for Research and Exploration provided funding via grant 8457-08. OR received traveling grants to Thailand and Singapore from F.N.R.S. (Belgium) and the National University of Singapore. Research support from the Friends of the RBG Victoria, Melbourne and a Helen McLellan Research Grant to TL is appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Zhu-Liang Yang
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khmelnitsky, O., Davoodian, N., Singh, P. et al. Ionosporus: a new genus for Boletus longipes (Boletaceae), with a new species, I. australis, from Australia. Mycol Progress 18, 439–451 (2019). https://doi.org/10.1007/s11557-018-01463-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-018-01463-1