Abstract
Bjerkandera is a worldwide polypore genus characterized by the pileate grayish basidiomes, often becoming darker when dried, and monomitic hyphal system. Tyromyces atroalbus is a rare Brazilian species re-discovered in recent surveys in São Paulo State. Even though it has been placed in Tyromyces, it presents a characteristic darkening of basidiomes when touched or dried, a feature absent in other species of the genus. In order to understand the evolutionary relations of T. atroalbus with Bjerkandera and Tyromyces and find its proper phylogenetic placement, morphological and molecular studies were carried out. Specimens from Brazil and Mexico were studied morphologically and selected specimens were used for DNA sequence analyses of internal transcribed spacer (ITS) and large subunit (LSU) of ribosomal RNA genes and translation elongation factor 1-alpha genes. Type specimens of T. atroalbus and T. humeana were also morphologically examined to confirm their possible synonymy as well as to confirm the identity of the recent collections. Molecular and morphological characters of T. atroalbus place the species in Bjerkandera. Our results also revealed that the specimens from Brazil and Mexico represent two distinct species. Therefore, the new combination Bjerkandera atroalba and description of new species B. centroamericana is published. In addition, the type study of T. atroalbus and T. humeana supports their synonymy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Bjerkandera P. Karst. is characterized by species with pileate basidiomes, monomitic hyphal system, and hyaline basidiospores. Although there are several names associated with Bjerkandera in Mycobank database (http://www.mycobank.org/), most of those are currently treated as synonyms or are older records with very little data available. In recent works, only two species are included in the genus and registered frequently in different regions of the globe, B. adusta (Willd.) P. Karst. and B. fumosa (Pers.) P. Karst. (Bernicchia 2005; Gilbertson and Ryvarden 1986; Ryvarden and Gilbertson 1993; Nuñez and Ryvarden 2001; Baltazar and Gibertoni 2009). Both species are rather similar morphologically and can be differed by the size of pores and presence of paler tubes separated from the context by a dark line in the later (Ryvarden and Gilbertson 1993).
Tyromyces atroalbus (Rick) Rajchenb. is a rare species described by Rick (1935) and later validated by Rajchenberg (1987), who selected a lectotype from the PACA herbarium collection. Tyromyces humeana (Murrill) J. Lowe, described from North America (Lowe 1975), was proposed as a synonym of this species (Rajchenberg 1987). It is characterized by the white basidiomes that become dark when dried or bruised, with large pores (2–5/mm), monomitic hyphal system and hyaline, thin-walled, ellipsoid basidiospores. The genus Tyromyces P. Karst. is generally characterized by the pileate, usually white, short-lived basidiomes with monomitic hyphal system (or with skeletal hyphae in trama), generative hyphae with clamps and lack of true cystidia (Ryvarden 1991), and most species with such features are placed in this genus. However, the type species of the genus, T. chioneus (Fr.) P. Karst., presents dimitic hyphal system and molecular data show that it is related to Skeletocutis Kotlaba & Pouzar and the Antrodia clade (Binder et al. 2013). In addition, species with different characteristics, as presence of gloeocystidia or inflated hyphae, have also been placed in the genus, case of T. aquosus (Henn.) Ryvarden (Ryvarden 2012) and T. hypocitrinus (Berk.) Ryvarden (Ryvarden 1984). Tyromyces atroalbus differs from other species of the group by the darkening of the basidiomes when dried and distinctly monomitic hyphal system, characteristics common in species of Bjerkandera. However, it differs in the white basidiomes, since both B. adusta and B. fumosa present gray pore surface. In order to check the proper placement of T. atroalbus and clarify its relation with Tyromyces and Bjerkandera, morphological and molecular studies were carried out. Specimens identified as T. atroalbus from Brazil and Mexico were studied morphologically and selected specimens were used for DNA extraction. In addition, specimens of B. adusta from Europe and South America and B. fumosa from Europe were added to the phylogenetic analysis dataset. Morphological and molecular data on all species are presented, corroborating to the description of a new species and a new combination. Descriptions, comments, illustrations, and an identification key are presented.
Material and methods
Specimens of T. atroalbus from SP herbarium in Brazil and three collections from Mexico (J. Kout, personal collection) were used for morphological and molecular analysis. Additional specimens of B. adusta and B. fumosa from BRNM herbarium were also included to molecular analysis. In addition, the types of Polyporus atroalbus Rick (as P. albo-ater) from PACA herbarium and Trametes humeana Murrill from NY herbarium were studied for morphological comparison and confirmation. For microscopic analysis, hand-cut sections of the basidiomes were mounted on microscope slides with a drop of 3 % KOH solution and 1 % aqueous phloxine solution. All microscopic structures were measured with aid of an eyepiece micrometer and 20 to 30 measurements were taken from each structure present. Drawings of the microstructures were made with the aid of a drawing tube. For DNA extraction, the first 10 steps of DNeasy Plant Mini Kit (QIAGEN) protocol were followed. The samples were then sent to Department of Chemistry and Biochemistry of Mendel University in Brno (Czech Republic), where DNA extraction was continued using magnetic-bead technology of MagNA Pure compact system. When needed, DNA was purified using DNA Clean & Concentration Kit (Zymo Research). DNA amplification of the regions ITS and LSU were performed using ITS5/ITS4-Basidio (Nikolcheva and Bärlocher 2004) primer combination for ITS and LR0R/LR6 that for LSU. The PCR regimes followed Tomšovský et al. (2010a). For tef1-alpha (primers: 983 F/2218R) region, touchdown PCR with gradually reduced annealing temperature (60–50 °C) was performed (Rehner and Buckley 2005). In some samples where PCR did not present good results, a nested PCR was performed according to Tomšovský et al. (2010b).
Amplification products were sent for sequencing in MacroGen Ltd. (Korea). The sequences obtained were initially edited in the BioEdit software (Hall 1999) and aligned using the MAFFT online server and adjusted manually. Reference sequences were chosen based on Moreno et al. (2011), Miettinen and Rajchenberg (2012); Miettinen et al. (2012); Jung et al. (2014) and through BLAST searches in the NCBI (National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov). The sequences used in this study are summarized in Table 1. The dataset of sequences was aligned using MAFFT online server and used for the construction of phylogenetic trees applying Bayesian inference in the software MrBayes 3.2.2 (Ronquist et al. 2012). The evolutionary models were inferred with the jModelTest 2.c1.4 (Darriba et al. 2012).
Results
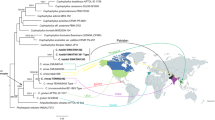
Molecular analysis confirmed that Tyromyces atroalbus belongs to Bjerkandera and is separated from Tyromyces (Figs. 1 and 2), which is supported by the morphological features, such as the pileate basidiomes that become dark when dried and monomitic hyphal system. In addition, the studied Mexican specimens identified as T. atroalbus represent a distinct species, which is characterised by different DNA sequences and remarkably smaller pores. The analysis of types of Polyporus atroalbus and Trametes humeana support their synonymy and confirmed the identity of the new Brazilian collections. Full descriptions on the two neotropical Bjerkandera are presented below. The morphological characters of B. adusta and B. fumosa followed data published by Ryvarden and Gilbertson (1993) and Bernicchia (2005) and supported placement of T. atroalbus in this genus. The detailed morphological analysis of B. adusta and B. fumosa was not conducted due to incomplete sampling (more comments in Discussion).
The phylogenetic tree of ITS-LSU region conducted by Bayesian analysis (for legends to numbers, see Table 1). Numbers at branches indicate maximum likelihood bootstrap proportion and Bayesian posterior probability values. The asterisk (*) marks different topology in both analyses. The bar indicates number of expected substitutions per position
The phylogenetic tree of ITS-LSU-tef1 region conducted by Bayesian analysis (for legends to numbers, see Table 1). Numbers at branches indicate maximum likelihood bootstrap proportion and Bayesian posterior probability values. The asterisk (*) marks different topology in both analyses. The bar indicates number of expected substitutions per position
Bjerkandera atroalba (Rick) Westphalen & Tomšovský, combination novum, MB 813009 (Figs. 3 and 4)
≡ Polyporus atroalbus Rick, Brotéria, Ci. nat.: 25 (1935) [MB#374944] basionym
≡ Tyromyces atroalbus (Rick) Rajchenb., Nordic Journal of Botany 7 (5): 558 (1988) [MB#134032]
= Trametes humeana Murrill, Bulletin of the Torrey Botanical Club 65: 656 (1938) [MB#267422]
≡ Tyromyces humeana (Murrill) J. Lowe, Mycotaxon 2 (1): 25 (1975) [MB#325209]
Basidiomes annual, pileate, sessile to effused-reflexed, dimidiate, often imbricate, soft and somewhat flexible when fresh, becoming corky after dried, up to 8 cm wide and 3 cm thick; pileus white to cream, becoming dark when touched and after dried, then pale brownish-gray to dark gray, flattened to convex, surface glabrous to somewhat velutinate and azonate; margin thin, acute, becoming black on dried specimens; pore surface white to cream, becoming dark when touched or after dried, pores large, regular, round or more commonly angular, with thin dissepiments, sometimes partially dilacerate after dried, 2–5/mm; tubes concolorous with the pore surface, slightly darker than the context in dried specimens, up 1.5 cm deep; context white to cream, homogeneous or with interwoven thin black lines, somewhat fibrous, up to 1.5 cm thick.
Hyphal system monomitic; generative hyphae clamped, smooth and hyaline, 3–6 μm wide, frequently branched, interwoven, in the trama thin to slightly thick walled, up to 4 μm wide, in the context with somewhat thicker walls, but never resembling skeletal hyphae, up to 6 μm wide; inflated hyphal ends present in the dissepiments, club-shaped to capitate. Fusoid cystidioles present in the hymenium, 8–15 × 3–5 μm. Basidia clavate, 4-sterigmate, 10–15 × 4–5 μm; basidiospores widely ellipsoid to narrowly ellipsoid, hyaline, smooth, thin-walled, IKI-, 4–5 × 3–4 μm.
Ecology: Growing on dead hardwood logs in areas of Atlantic Rainforest (Dense Ombrophilous Forest) in South Brazil.
Remarks: B. atroalba is characterized by the whitish basidiomes that become darker after dried, large pores, the monomitic hyphal system, and ellipsoid basidiospores. Even though this species has been treated as Tyromyces, both morphological and molecular data support that Bjerkandera is the proper genus to accommodate it. It differs from B. adusta and B. fumosa by the larger pores and whitish basidiomes when fresh. Lowe (1975) comments on the strong odor of anise as a characteristic of T. humeana, but we believe this is not a good character to add to the species definition as some specimens may not present this odor and it cannot be felt in dried materials. The darkening of the basidiomes is rather variable and often is more prominent on the margins. While some specimens may become dark brown to almost black, others present only a slight darkening, becoming beige to straw coloured.
Examined specimens: Brazil, Rio Grande do Sul, Pouso Novo, leg. J. Rick FR18397, 1932 (PACA lectotype of P. atroalbus) – São Paulo, Riberião Grande, Parque Estadual Intervales, leg. M.C. Westphalen 425/13, 05.2.2013 (SP 446205) – São Paulo, Parque Estadual da Cantareira, leg. M. Capelari and J.J.S.Oliveira 4626, 7.XI.2011 (SP 445630); leg. V. Motato-Vásquez 59, 6.XII.2011 (SP 445628); leg. V. Motato-Vásquez 158, 7.III.2012, (SP 445629); leg. V. Motato-Vásquez, M.C. Westphalen and A.C. Bolaños 248, 27.VI.2012 (SP 445530); leg. V. Motato-Vásquez and M.C. Westphalen 266, 30.VII.2012, (SP 445672) – USA, Florida, Barnesville, W.A. Murril (NY 00705029, holotype of T. humeana).
Bjerkandera centroamericana Kout, Westphalen & Tomšovský, sp. nov., MB 813443 (Figs. 3 and 4)
Holotype: Mexico, Veracruz, San Andrés Tuxtla municipality, near Montepío. Dead hardwood; 18.5750000° N, 095.0408333 °W; 14.X.2006, leg. Jiří Kout 0610/A7 (Holotype: SP 466336, Isotype: BRNM 771949.)
Etymology: centroamericana - refers to the region where the species was found.
Basidiomes sessile, pileate, effused-reflexed, reflexed up to 3 cm, up to 7 cm wide and 1 cm thick at the base, but generally not over 0.5 cm thick; upper surface of pileus white when fresh, cream to brownish in dry condition, dark on the base, slightly tomentose, velutinate, azonate, margin blunt in young, becoming sharp; pore surface sordid white, becoming dark brown or grey to black when wounded, pores angular, 7–11/mm, dissepiments thin, entire; context pale buff, sometimes with interwoven black lines that can form a thin dark layer on the pilear surface, up to 5 mm thick; tubes with one or more layers, then separated by black lines, white to cream when fresh, becoming slightly darker than the pores after dried, distinct from context by a thin unremarkable layer (slightly darker than context) at the base of tubes.
Hyphal system monomitic; hyphae with clamps (some septa without clamps also seen, but rare), hyaline, smooth, occasionally branching, interwoven, 2–5 μm wide, in the trama thin to slightly thick-walled, in the context similar, but sometimes with somewhat thicker walls. Cystidioles fusoid, bottle-shaped, 10–25 × 4–5 μm. Basidia clavate, tetrasterigmatic, 10–14 × 5–6 μm. Basidiospores subglobose up to widely ellipsoid, hyaline, smooth, with small apiculus, IKI-, 4–5 × 3–4.5 μm.
Ecology: growing on dead hardwood at man-made habitats (municipal park or edge of path in cultivated landscape).
Remarks: B. centroamericana has a very similar morphology to B. atroalba and can be differed only by the fairly smaller pores (Fig. 3b). Microscopically, the basidiospores of B. centroamericana are slightly wider, subglobose to widely ellipsoid, while in B. atroalba they can be narrowly ellipsoid. However, there are cases of overlapping in basidiospores shape and size and only a detailed analysis of both species can reveal this difference (Fig. 4).
Bjerkandera atroalba (a–e) and B. centroamericana (f–i). a. Basidiospores. b. Basidium. c. Cystidioles. d. Sterile elements from the dissepiments. e. generative hyphae. f. Basidiospores. g. Basidiole and basidium. h. Cystidioles. i. Generative hyphae. Illustrations by M.C. Westphalen (a–e) and J. Kout (f–i)
Examined specimens (paratypes): Mexico, Veracruz, San Andrés Tuxtla municipality, near Montepío, Dead hardwood; 18.5750000° N, 095.0408333° W; leg. Jiří Kout 0610/A10, 14.X.2006 (BRNM 771945) – Tabasco, Villahermosa. Dead hardwood; 17.9891667° N, 092.9280556° W; leg. Jiří Kout 0610/A13, 18.X. 2006 (BRNM 771950).
Additional examined specimens: Bjerkandera adusta: Argentina, Patagonia, Trevelin, National Park Los Alerces, Puorto Chucao, dead hardwood; 42.730043° S, 71.757992° W; leg. Matěj Pánek, det. Michal Tomšovský MT 48/2014, 15.XI.2014 (BRNM 771946) – Czech Republic, Brno, City Centre, Mendel’s Square, stump of Aesculus hippocastanum; 160 m a.s.l., 49.1908661° N, 16.5948944°E; leg. et det. Michal Tomšovský MT 49/2014, 18.XII.2014 (BRNM 771948) – Bjerkandera fumosa: Slovakia, Trenčín, Skalka nad Váhom, NW margin of village, bank of the Súčanka creek, stump of a hardwood (Alnus or Populus), 227 m a.s.l.; 48.9283056° N, 018.0628889° E; leg. et det. Michal Tomšovský MT 51/2013, 10.X.2013 (BRNM 771947).
Identification key to species of Bjerkandera
-
1 Pore surface white to cream becoming dark when touched or after dried, tubes concolorous or only slightly darker than the context
-
2 Pores 2–5/mm, South American species B. atroalba
-
2* Pores 7–11/mm, Central American species B. centroamericana
-
1* Pore surface buff to grayish, somewhat darker after dried, tubes darker gray contrasting with the white context
-
3 Pores 2–5/mm, pore surface buff to smoky gray B. fumosa
-
3 Pores 6–7/mm, pore surface gray to almost black, cosmopolitan species B. adusta
Discussion
The phylogenetic analysis revealed the placement of samples identified as T. atroalbus from Brazil and Mexico in the genus Bjerkandera and that the two different lineages represent different species. Our results also show their relation to traditionally recognized B. adusta and B. fumosa and other genera form the Phlebioid clade. Bjerkandera atroalba and B. centroamericana are closely related, which is also supported by their very similar morphological characters. Both species are distinctive by the white to cream basidiomes that become dark when touched or after dried and they can be well differed only by the size of pores (2–5/mm in the former and 7–11/mm in the latter). The type revision of Polyporus atroalbus and Trametes humeana did not find any distinguishing characteristics that would support separating both species; therefore, we choose to keep them as synonyms, as previously suggested by Rajchenberg (1987). More molecular data with the addition of DNA sequences of materials from the USA could clarify if B. atroalba has in fact a wider distribution over the American continent or if the North American species may present differences at the molecular level. In addition, we added to our dataset sequences available in GenBank of two specimens of endophyte fungi identified as Bjerkandera cf. adusta (Martin et al. 2015), which are closely related to B. atroalba. These sequences were obtained from endophytic fungi isolated from leaves of Hevea brasiliensis in Peru and may represent a local population differing from Brazilian one in ITS sequence or a new species in the genus. The endophytic phase of life cycle of Bjerkandera was also confirmed by Yuan et al. (2011). Genera Tyromyces and Bjerkandera were in the past treated as closely related, and Pouzar (1966) proposed Bjerkandera as a subgenus of Tyromyces. However, recent results of molecular phylogeny (e.g. Binder et al. 2013) clearly confirm independent taxonomic positions of the two genera. All this may indicate that the genus Bjerkandera has been overlooked and that it in fact encompasses more species and has a wider circumscription than as it has been traditionally treated. In addition, B. adusta and B. fumosa were described from temperate Europe and are reported throughout the globe. For example, our record of B. adusta from temperate Argentina has almost identical sequences as samples from Europe. Therefore, more work including molecular data is needed to confirm their diversity in other regions.
The micromorphological characters of B. adusta and B. fumosa are rather uniform. The basidiospore dimensions of B. adusta are 4.5–5.5(6) × 2.3–3(3.5) μm, while in B. fumosa they are usually longer, 5–6.5(7) × 2.5–3.5 μm. Our observations in micromorphology of both species follow those by Bernicchia (2005) and Ryvarden and Gilbertson (1993). More discriminative characters can be seen in macromorphology: B. fumosa has in general larger, more robust basidiomes and paler brownish to light gray pore surface, while in B. adusta pore surface varies from gray to almost black with paler margins. The number of pores is (5)6–7 per mm in B. adusta and (2)3–5 per mm in B. fumosa, but in older parts of the basidiomes that are attached to the substrate pores can be split (our observations, Bernicchia 2005; Ryvarden and Gilbertson 1993). Nevertheless, the morphological diversity within the whole area of distribution of both species may be high as indicated by Jung et al. (2014) and worth studying in detail.
References
Baltazar JM, Gibertoni TB (2009) A checklist of the aphyllophoroid fungi (Basdiomycota) recorded from the Atlantic Rain Forest. Mycotaxon 109:439–442
Bernicchia A (2005) Polyporaceae s.l., Fungi Europaei, vol 10. Candusso, Alassio
Binder M, Justo A, Riley R, Salamov A, López-Giráldez F, Sjökvist E, Copeland A, Foster B, Sun H, Larsson E, Larsson KH, Townsend J, Grigoriev IV, Hibbett DS (2013) Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 105:1350–1373. doi:10.3852/13-003
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9(8):772–772. doi:10.1038/nmeth.2109
Gilbertson RL, Ryvarden L (1986) North American polypores. Abortiporus – Lindtneria, v.1. Fungiflora, Oslo, 433pp
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Jung PE, Fong JJ, Park MS, Oh SY, Kim C, Lim YW (2014) Sequence Validation for the Identification of the White-Rot Fungi Bjerkandera in Public Sequence Databases. J Microbiol Biotechnol 24(10):1313–1319. doi:10.4014/jmb.1404.04021
Lowe JL (1975) Polyporaceae of North America. The genus Tyromyces. Mycotaxon 2(1):1–82
Martin R, Gazis R, Skaltsas D, Chaverri P, Hibbett D (2015) Unexpected diversity of basidiomycetous endophytes in sapwood and leaves of Hevea. Mycologia 107(2):284–297. doi:10.3852/14-206
Miettinen O, Rajchenberg M (2012) Obba and Sebipora, new polypore genera related to Cinereomyces and Gelatoporia (Polyporales, Basidiomycota). Mycol Prog 11:131–147. doi:10.1007/s11557-010-0736-8
Miettinen O, Larsson E, Sjökvist E, Larsson KH (2012) Comprehensive taxon sampling reveals unaccounted diversity and morphological plasticity in a group of dimitic polypores (Basidiomycota, Polyporales). Cladistics 28:251–270. doi:10.1111/j.1096-0031.2011.00380.x
Moreno G, Blanco MN, Checa J, Platas G, Pelaez F (2011) Taxonomic and phylogenetic revision of three rare irpicoid species within the Meruliaceae. Mycol Prog 10:481–491. doi:10.1007/s11557-010-0717-y
Nikolcheva LG, Bärlocher F (2004) Taxon-specific primers reveal unexpectedly high diversity during leaf decomposition in a stream. Mycol Prog 3:41–49. doi:10.1007/s11557-006-0075-y
Nuñez M, Ryvarden L (2001) East Asian Polypores, vol 2. Fungiflora, Oslo
Pouzar Z (1966) Studies in the taxonomy of the Polypores II. Folia Geobotanica et Phytotaxonomica 1(1):356–375. doi:10.1007/BF02854587
Rajchenberg M (1987) Type studies of Polyporaceae (Aphyllophorales) described by J. Rick. Nord J Bot 7:553–568
Rehner SA, Buckley EP (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97:84–98. doi:10.3852/mycologia.97.1.84
Rick J (1935) Polysticti Riograndenses. Brotéria Série Trimest: Ciências Naturais 4:121–138
Ronquist F, Teslenko M, van der Mark P, Ayres D, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.3: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. doi:10.1093/sysbio/sys02
Ryvarden L (1984) Type studies in the Polyporaceae. 16. Species described by J.M. Berkeley, either alone or with other mycologists from 1856 to 1886. Mycotaxon 20(2):329–363
Ryvarden L (1991) Genera of Polypores: nomenclature and taxonomy. Synop Fungorum 5:1–363
Ryvarden L (2012) Type studies in Polyporaceae 27. Species described by P. Hennings. Czech Mycol 64:13–21
Ryvarden L, Gilbertson RL (1993) European Polypores, Synopsis Fungorum 6, vol 1. Fungiflora, Oslo, 387pp
Tomšovský M, Menkis A, Vasaitis R (2010a) Phylogenetic relationships in European Ceriporiopsis species inferred from nuclear and mitochondrial ribosomal DNA sequences. Fungal Biol 114:350–358. doi:10.1016/j.funbio.2010.02.004
Tomšovský M, Sedlák P, Jankovský L (2010b) Species recognition and phylogenetic relationships of European Porodaedalea (Basidiomycota, Hymenochaetales). Mycol Prog 9(2):225–233. doi:10.1007/s11557-009-0628-y
Yuan ZL, Rao LB, Chen YC, Zhang CL, Wu YG (2011) From pattern to process: species and functional diversity in fungal endophytes of Abies beshanzuensis. Fungal Biol 115(3):197–213. doi:10.1016/j.funbio.2010.11.002
Acknowledgments
The authors would like to thank MSc. Ricardo Mateus Pires for the assistance in the computational analysis. FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo, Brazil. Grant number: 2011/17219-0) and European Social Fund and the state budget of the Czech Republic (Project Indicators of trees vitality Reg. No. CZ.1.07/2.3.00/20.0265) are acknowledged for finacial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Franz Oberwinkler
Rights and permissions
About this article
Cite this article
Westphalen, M.C., Tomšovský, M., Kout, J. et al. Bjerkandera in the Neotropics: phylogenetic and morphological relations of Tyromyces atroalbus and description of a new species. Mycol Progress 14, 100 (2015). https://doi.org/10.1007/s11557-015-1124-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-015-1124-1