Abstract
Purpose
Insertion trajectory affects final intracochlear cochlear implant (CI) positioning, but limited information is available intraoperatively regarding ideal trajectory. We sought to improve intracochlear positioning CI electrodes using custom templates to specify insertion trajectory.
Methods
3D reconstructions were created from computed tomography of three cadaveric temporal bones. Trajectories co-planar with the straight segment of the cochlea’s basal turn were considered ideal. Templates were designed to fit against the drilled mastoid’s surface and convey this guided trajectory via a hollow cylinder. Templates were 3D-printed using stereolithography. Mastoidectomy was performed. Template accuracy was tested by measuring target registration error (TRE) for four templates. A novel, roller-based insertion tool (designed to fit within the template cylinder) constrained insertions to intended trajectories. Insertions were performed with MED-EL Standard electrodes in three bones with three conditions: guided trajectory with insertion tool, non-guided trajectory with insertion tool and guided trajectory with surgical forceps. For the final condition, the template was used to mark the mastoid to convey trajectory. Insertion was stopped when electrode buckling occurred.
Results
TRE ranged from 0.23 to 0.73 mm. Mean TRE ± standard deviation was 0.55 ± 0.19 mm. Insertions along guided versus non-guided trajectories averaged more intracochlear electrodes (9, 8, 8 vs. 7, 7, 8) and greater angular insertion depths (AID) (377°, 341°, 320° vs. 278°, 302°, 290°). Insertions performed with forceps using templates as a guide also achieved excellent results (intracochlear electrodes: 10, 7, 8; AID: 478°, 318°, 333°). No translocations occurred.
Conclusion
Custom mastoid-fitting templates reliably specify intended insertion trajectory and provide sufficient information for recreation of that trajectory with manual insertion after template removal. The templates can accurately target structures within the temporal bone with a TRE of 0.55 ± 0.19 mm. Our roller-based insertion tool achieves results comparable to manual insertion using surgical forceps.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cochlear implant (CI) surgery involves the insertion of an electrode array into the cochlea to directly stimulate the cochlear nerve and treat sensorineural hearing loss. The procedure continues to grow in popularity, and the indications for CI continue to expand as off-label usage has become increasingly common [1]. Hearing outcomes in CI recipients vary significantly. The cochlea is spiral-shaped organ in which three scalae wind around a central modiolus that houses the cochlear nerve endings. Electrodes are typically inserted in the scala tympani (ST) but can translocate across the basilar membrane into the scala vestibuli (SV), an outcome that is associated with worse hearing. Research suggests that the final intracochlear position of the electrode array, particularly in reference to the modiolus, is crucial in determining device performance [2, 3]. A number of factors influence the final position of CI electrodes including but not limited to electrode type (i.e., precurved or straight), angle of insertion, curl direction, depth of advance-off-stylet (when applicable) and overall depth of insertion.
Among these factors, the angle or trajectory of the CI electrode during insertion is particularly important. We hypothesize that the ideal trajectory is co-planar with the basal segment of the basal turn of the cochlea and is collinear with the middle of the ST along the straight segment (approximately the first 90° of angular depth), keeping the electrode from heading directly toward the modiolus or the lateral wall. Although the round window is visible intraoperatively, the interior of the ST is difficult to visualize. The exact orientation of the cochlea in three-dimensional space is only partially discernable to the surgeon because of the limited intraoperative field of view. The proper trajectory with which to approach the cochlea is largely determined from prior surgical experience and, perhaps in part, on mental reconstruction of the patient’s preoperative imaging with respect to the operative field.
In a recent study, our group demonstrated that the final intracochlear position of CI electrodes could be improved by providing the surgeon with better information regarding entry site, entry vector, advance-off-stylet depth and final insertion depth [4]. By choosing optimal plans, the rate of scalar translocation was reduced, and electrodes were placed in a more perimodiolar position. Templates designed to match the contour of the mastoid surface have been used by other groups in the past. Matsumoto et al. [5] developed such a template for use in image-guided CI surgery. Their goal was to allow transfer of virtual fiducial markers to a patient intraoperatively without the need for preoperative marker placement. They built templates using laser sintering and reported target registration error at the cochlea of less than 1 mm. In the present study, we describe a novel surgical template designed to convey CI insertion trajectory to a surgeon intraoperatively. We report results of experiments to validate that accuracy of the template in targeting temporal bone anatomy and in recreating intended insertion trajectory. We describe a custom-designed roller-based CI electrode insertion tool that we used to constrain electrode insertion to the template-specific trajectory. Finally, we compare insertions performed with guided insertion trajectories (i.e., trajectories co-planar with the basal segment of the basal turn of the cochlea and collinear with the middle of the ST) to insertions with non-guided trajectories.
Materials and methods
Template creation
IRB exemption for non-human subject research was obtained from our Institutional Review Board (No. 181264). Seven fresh-frozen cadaveric temporal bone specimens were procured (Science Care, Phoenix, Arizona) and thawed prior to use. Preoperative computed tomography (CT) scans were obtained on all specimens and were segmented using previously published automated methods [6,7,8,9,10] to delineate intracochlear anatomy.
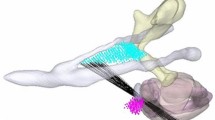
Using interactive image processing software developed in-house, custom templates were designed to fit against the rim of the drilled mastoid and extend beyond the borders of a standard mastoidectomy (Fig. 1). The templates contained a hollow cylinder in the center to demonstrate an intended trajectory for CI insertion (Fig. 2). For the purposes of experimentation, templates were designed with both guided and non-guided trajectories. Guided trajectories were both co-planar to the basal-most segment (first 90 degrees of angular depth) of the basal turn of the cochlea and collinear with the most basal portion of the basal turn so as to be halfway between the modiolar and lateral walls of the scala tympani. Non-guided trajectories were chosen manually to pass through the facial recess to the same cochlear entry point, thus falling within the range of typical clinical trajectories, but not at the guided (and presumed ideal) angle. Templates were 3D-printed using stereolithography (Form 2 SLA 3D Printer, Formlabs Inc, Somerville, Massachusetts) with an autoclavable, biocompatible resin (Dental SG Resin, Formlabs Inc). The bore holes were enlarged to fit a 1.59 mm drill bit.
The custom template mates to the skull surface and has a bore hole aligned with the planned insertion vector in which the insertion tool can be inserted (a). The same template is shown with the skull removed (b). A coronal cross section of the planning CT used to design the guide is shown (c). Also shown are the facial nerve (magenta), chorda tympani (green) and ossicles (cyan)
Targeting accuracy
To assess the accuracy of the templates, four specimens were used for measurements of target registration error (TRE). For each specimen, an arbitrary target point within the middle ear was chosen. The distance was measured from the outer surface of the template’s bore hole to the target point on preoperative 3D reconstruction. A 1.59 mm drill bit was fitted with a collar lock for each specimen so that insertion of the bit through the bore hole would stop precisely at the intended depth. (Drill bits were used to assess targeting accuracy because they end in a precise point.) The templates were placed against the corresponding specimens and the drill bits were inserted (Fig. 3). CT scan was obtained in this position. After 3D reconstruction, the actual tip of the drill bit was compared to the planned position of the tip.
Trajectory validation
To verify that the trajectories specified by the templates matched the intended trajectories in preoperative planning, two experiments were performed. First, a metal rod was inserted through the bore hole of the template while the template was in place against the mastoid surface. A CT scan was acquired in this position, and the trajectory of the metal rod was compared with the intended trajectory. (Rods were used because they were solid along their length, which facilitated trajectory comparison.) Second, the surgeon was allowed to use the template and make markings on the mastoid surface in order to understand the planned insertion trajectory. Then, the template was removed, and a metal rod was placed by the surgeon manually to reproduce the intended trajectory. Surgical absorbable gelatin sponge was packed around the rod to hold it in place. A CT scan was acquired in this position, and the trajectory of the metal rod was compared with the intended trajectory.
Insertion technique
Three specimens were used for insertion experiments. Standard mastoidectomy and drilling of the facial recess were performed for access to the round window. The round window overhang was removed with a 1 mm drill in cases where it obstructed access for electrode insertions. To ensure that electrode insertion took place with the precise intended trajectory, insertions were performed using a manually actuated, roller-based insertion tool (Fig. 4). This tool was designed in-house and advances a straight CI electrode in a linear fashion [11]. The electrode is placed in a hollow bore in the center of the tool and opposing wheels are rolled by hand to push the electrode first through a rigid shaft and then through a more flexible polyimide shaft that is inserted just inside the round window. MED-EL Standard CI electrode arrays (MED-EL, Innsbruck, Austria) were inserted into the cochlea of each specimen, and insertion was stopped when buckling of the electrode occurred outside the round window. Each MED-EL Standard electrode array contains 12 electrode contacts.
A subset of the insertions was performed manually with forceps rather than with the insertion tool. For these insertions, the mastoid template was first applied to the bone so that the surgeon could observe the intended insertion trajectory. The surgeon made markings on the mastoid surface to help indicate the intended trajectory before the template was then removed and manual electrode insertion was performed with the goal of following the intended trajectory.
Imaging
All pre- and post-insertion CT scans were obtained using a portable cone-beam CT scanner (xCAT XL, Xoran Technologies, LLC, Ann Arbor, Michigan). Postoperative scans for each specimen were registered to the preoperative images, and the implanted electrode contacts were identified. Scalar position and angular depth were calculated for each electrode contact.
Outcomes
The total number of intracochlear electrodes and the overall angular insertion depth (AID) were considered primary outcomes. Scalar location (ST vs. SV) was a secondary outcome. The data are presented with TRE measurements first to make experiments more understandable to the reader, but the experiments were actually done with insertions first. An additional specimen was used for the TRE experiments in order to increase the sample size when measuring TRE.
Results
Template creation
Approximately 4–8 h of engineer effort were required to design each template using in-house developed interactive image processing software. 3D-printing took just under 4 h and used approximately $0.96 worth of resin per template (3.2 mL of resin per template; resin priced at $299 per liter). An additional hour of work was required to finish the templates, which could be done in batches (cleaning, curing the resin, trimming the supports from the printing process and enlarging the bore holes). While all of these processes required substantial effort in our proof of concept experiments, these steps could be automated and streamlined to facilitate clinical translation.
Targeting accuracy
Three of four templates fit snugly against the mastoid surface of the corresponding temporal bone. One template had limited contact between the template and the bone as a result of an excessively wide mastoidectomy. This template was therefore more difficult to maintain in the ideal position. TRE ranged from 0.23 to 0.73 mm. Mean TRE ± standard deviation was 0.55 ± 0.19 mm.
Trajectory reproduction
Analysis of the CT scans acquired with a metal rod inserted through the template bore hole showed that the intended trajectory was reproduced with high accuracy using the templates. Overall difference in angle between the intended and actual trajectory of the metal rod was 1.29° (0.11° relative to the anterior–posterior axis and 1.28° relative to the superior-inferior axis). When the templates were used only as a guide for the surgeon and the metal rod was manually positioned by the surgeon, the actual and intended trajectories maintained high concordance with overall angle difference of 5.58° (5.50° relative to the anterior–posterior axis and 0.94° relative to the superior-inferior axis).
With insertion tool
Insertions were first performed using the insertion tool with templates in place against the mastoid cortex. Templates with guided trajectories resulted in more intracochlear electrodes (mean ± standard deviation: 8.3 ± 0.6 electrodes) than templates with non-guided trajectories (7.3 ± 0.6 electrodes). AID was also higher for guided trajectories (mean: 346° ± 29°) compared to non-guided trajectories (290° ± 12°). No scalar translocations occurred. These data are summarized in Table 1.
Without insertion tool
Insertions were also performed manually with forceps using the templates only as a guide to demonstrate guided insertion trajectory. Markings were made on the mastoid cortex with the template in place, and then the template was removed prior to attempted electrode insertion. This technique resulted in similar numbers of intracochlear electrodes (mean ± standard deviation: 8.3 ± 1.5 electrodes) when compared to insertion with the tool along a guided trajectory (8.3 ± 0.6 electrodes). AID was also similar for forceps insertions (376° ± 88°) compared to tool insertions (346° ± 29°). No scalar translocations occurred. These data are summarized in Table 1.
Discussion
Herein, we report the design and use of custom, mastoid-fitting templates for cochlear implantation. The templates can be designed easily from preoperative CT imaging and can be 3D-printed quickly and inexpensively using autoclavable materials. We sought to use mastoid-fitting templates to directly convey CI insertion trajectory information to surgeons, obviating the need for an intraoperative image-guidance system. Our results demonstrate that these templates accurately specify the intended insertion trajectory and can accurately target temporal bone structures. After application and inspection of the template, the surgeon was able to accurately replicate the intended trajectory once the template was removed. This allowed electrode insertion with forceps to occur along the intended trajectory.
The present TRE of 0.55 ± 0.19 mm approaches the accuracy of prior systems including microstereotactic frames (0.37 ± 0.18 mm) [12] and the StarFix microtargeting system (0.45 ± 0.15 mm; FDA-approved for the placement of deep brain stimulating electrodes) [13]. Future modifications to the templates could improve accuracy further by refining the fit of the templates against the bone or increasing the thickness of the templates to prevent deformation. Even in their present state, the templates fit snugly to the surface of the mastoid cortex for which they were designed, which serves as an easy safety check to verify that the correct template is being used.
It is important to note that the AIDs achieved in these specimens are much lower than the AIDs achievable in vivo. Our group has found this to be a consistent issue with cadaveric models for CI insertion. We presume that the effect is due to increased friction associated with post-mortem changes, because we were able to achieve deeper insertions when soapy water was instilled through the round window (results not reported here). Future investigation into this effect—potentially including direct measurement of intracochlear friction—would help the research community build better models for studying CI and would give surgical trainees more realistic models with which to practice CI.
An additional limitation in the application of such templates is that they require a full, standard-sized mastoidectomy. We feel this is easily achievable in the majority of adult patients, but we acknowledge that anatomic limitations in certain clinical situations (e.g., pediatric anatomy or adult anatomy with poor mastoid pneumatization) may preclude template use.
Our findings demonstrate the importance of insertion trajectory in determining final CI electrode positioning. Insertions performed along guided insertion trajectories achieved deeper angular insertion depths with more intracochlear electrodes than insertions performed with non-guided trajectories. No scalar translocations were observed in this small group; however, it is likely that insertion along the guided trajectory also helps to minimize the risk of scalar translocation. This would be particularly relevant for precurved electrode arrays given the higher translocation risk associated with that design [14, 15].
Conclusion
Custom templates designed to fit against the mastoid cortex are able to accurately convey important information to surgeons about CI insertion trajectory. In routine practice, this information is otherwise unavailable to surgeons intraoperatively. There is great potential in applying this technology—which can be built before the surgical case thereby avoiding a delay in surgical time—to standardize and improve insertion technique among CI surgeons.
References
Carlson ML, Sladen DP, Gurgel RK, Tombers NM, Lohse CM, Driscoll CL (2018) Survey of the American neurotology society on cochlear implantation: part 1, candidacy assessment and expanding indications. Otol Neurotol 39:e12–e19. https://doi.org/10.1097/MAO.0000000000001632
Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, Gotter BD, Vanderhoof SS, Mispagel K, Heydebrand G, Skinner MW (2013) Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear 34:342–360. https://doi.org/10.1097/AUD.0b013e3182741aa7
Chakravorti S, Noble JH, Gifford RH, Dawant BM, O’Connell BP, Wang J, Labadie RF (2019) Further evidence of the relationship between cochlear implant electrode positioning and hearing outcomes. Otol Neurotol 40:617–624. https://doi.org/10.1097/MAO.0000000000002204
Labadie RF, Noble JH (2018) Preliminary results with image-guided cochlear implant insertion techniques. Otol Neurotol 39:922–928. https://doi.org/10.1097/MAO.0000000000001850
Matsumoto N, Hong J, Hashizume M, Komune S (2009) A minimally invasive registration method using surface template-assisted marker positioning (STAMP) for image-guided otologic surgery. Otolaryngol Head Neck Surg 140:96–102. https://doi.org/10.1016/j.otohns.2008.10.005
Labadie RF, Balachandran R, Mitchell JE, Noble JH, Majdani O, Haynes DS, Bennett ML, Dawant BM, Fitzpatrick JM (2010) Clinical validation study of percutaneous cochlear access using patient-customized microstereotactic frames. Otol Neurotol 31:94–99. https://doi.org/10.1097/MAO.0b013e3181c2f81a
Noble JH, Gifford RH, Labadie RF, Dawant BM (2012) Statistical shape model segmentation and frequency mapping of cochlear implant stimulation targets in CT. Med Image Comput Comput Assist Interv 15:421–428. https://doi.org/10.1007/978-3-642-33418-4_52
Schuman TA, Noble JH, Wright CG, Wanna GB, Dawant B, Labadie RF (2010) Anatomic verification of a novel method for precise intrascalar localization of cochlear implant electrodes in adult temporal bones using clinically available computed tomography. Laryngoscope 120:2277–2283. https://doi.org/10.1002/lary.21104
Zhao Y, Dawant BM, Labadie RF, Noble JH (2014) Automatic localization of cochlear implant electrodes in CT. Int Conf Med Image Comput Comput Interv 17:331–338. https://doi.org/10.1007/978-3-319-24571-3_19
Noble JH, Dawant BM (2015) Automatic graph-based localization of cochlear implant electrodes in CT. Lect Notes Comput Sci 9350:152–159. https://doi.org/10.1007/978-3-319-24571-3_19
Riojas KE, Narasimhan N, Morrel WG, Mitchell J, Bruns T, Webster RJ, Labadie RF (2019) A new manual insertion tool for minimally invasive, image-guided cochlear implant surgery. In: Medical imaging 2019: image-guided procedures, robotic interventions, and modeling, vol 10951. International Society for Optics and Photonics, pp 109510J
Labadie RF, Mitchell J, Balachandran R, Fitzpatrick JM (2009) Customized, rapid-production microstereotactic table for surgical targeting: description of concept and in vitro validation. Int J Comput Assist Radiol Surg. https://doi.org/10.1007/s11548-009-0292-3
Balachandran R, Mitchell JE, Dawant BM, Fitzpatrick JM (2009) Accuracy evaluation of microTargeting platforms for deep-brain stimulation using virtual targets. IEEE Trans Biomed Eng 56:37–44. https://doi.org/10.1109/TBME.2008.2002110
Boyer E, Karkas A, Attye A, Lefournier V, Escude B, Schmerber S (2015) Scalar localization by cone-beamcomputed tomography of cochlear implant carriers: a comparative study between straight and periomodiolar precurved electrode arrays. Otol Neurotol 36:422–429. https://doi.org/10.1097/MAO.0000000000000705
O’Connell BP, Cakir A, Hunter JB, Francis DO, Noble JH, Labadie RF, Zuniga G, Dawant BM, Rivas A, Wanna GB (2016) Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol 37:1016–1023. https://doi.org/10.1097/MAO.0000000000001125
Funding
This work was supported by Grant R01 DC008408 from the National Institute on Deafness and other Communication Disorders. The content is the sole responsibility of the authors and does not necessarily reflect the views of this institute. This work was also supported by a 2018 CORE Grant award from the American Academy of Otolaryngology-Head and Neck Surgery sponsored by Xoran Technologies, LLC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RF.L. is a consultant for Advanced Bionics, Ototronix and Medtronic. No other conflicts of interest.
Human and animals rights
This work did not involve any live human participants or animals.
Informed consent
This work did not require informed consent given that no live human participants or animals were involved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morrel, W.G., Riojas, K.E., Webster, R.J. et al. Custom mastoid-fitting templates to improve cochlear implant electrode insertion trajectory. Int J CARS 15, 1713–1718 (2020). https://doi.org/10.1007/s11548-020-02193-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-020-02193-0