Abstract
Objectives
The aim of this study was to report our experience with early stage glioblastoma (e-GB) and to investigate the possible clinical and imaging features that may be helpful to the radiologist to correctly diagnose this entity.
Methods
We performed a retrospective research of patients diagnosed with glioblastoma at two hospitals during a 10-year period. We reviewed all pre-operative MR and included only patients with early stage GB lesions, characterized by hyperintense on T2-weighted signal, with or without contrast-enhancement at post-contrast T1-weighted images, without “classic” imaging appearance of GB (necrosis, haemorrhage, oedema). All preoperative MR were evaluated by an experienced neuroradiologist and information on patients’ demographics, clinical presentation, follow-up, and histopathology results study were collected. When available, preoperative CT examination was also evaluated.
Results
We found 14 e-GBs in 13 patients (9 males, 4 females, median age 63 years) among 660 patients diagnosed with GB between 2010 and 2020. In 10 lesions, serial imaging revealed the transformation of e-GB in classic glioblastoma in a median time of 3 months. Clinical presentation included stroke-like symptoms, vertigo, seizures and confusion. Preoperative plain CT was performed in 8/13 cases and in 7 e-GBs presented as a hyperdense lesion. Ten out of 14 lesions transformed in classic GB before surgical intervention or biopsy. All lesions revealed typical immunohistochemical pattern of primary glioblastoma.
Conclusions
E-GB is a rare entity that can often lead to misdiagnosis. However, the radiologist should be aware of its imaging appearance to suggest the diagnosis and to request close imaging follow-up, hopefully improving the prognosis of this very aggressive disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GB) corresponds to grade IV astrocytoma in 2016 World Health Organization Classification of Tumors of the Central Nervous System (2016 CNS WHO) and represents the most common malignant brain tumour [1]. Identification and diagnosis of brain tumours has been subjected to a renovation, switching from the traditional principle of neuropathological diagnosis to a molecularly oriented diagnosis. Thus, according to 2016 CNS WHO, GB is now categorized in IDH wild type and IDH mutant, with IDH gene status being the major criterion for classification [2]. GB IDH wild type corresponds with de novo or primary GB, while GB IDH mutant corresponds to the secondary glioblastoma, deriving from a prior lower grade diffuse glioma. GB IDH wild type is the most common GB (nearly 90%), and it has the most aggressive behaviour, with a mean length of clinical history of 4 months and a median overall survival of 9.9–15 months when surgery, radiotherapy and chemotherapy are administered [2].
In addition to IDH status, other genetic aberrations have been searched and catalogued by The Cancer Genome Atlas (TCGA) Research Network [3], in order to find the ones that may be associated with higher malignant potential, better response to treatment, and targeted therapies.
Immunohistochemistry tests are routinely performed, in addition to IDH status, to research the most common alterations, such as loss of heterozygosis (LOH) on chromosome 10q, epidermal growth factor receptor (EGFR) amplification, platelet-derived growth factor receptor (PDGFR) amplification, tumour protein 53 (TP53) mutation, cyclin-dependent kinase inhibitor 2 A/B (CDKN2A/B) deletion, phosphatase and tensin homolog (PTEN) mutation or deletion, O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation [4].
Despite the wide variety of genetic profiles, at imaging and particularly on MRI, GB typically reveals an heterogeneous lesion with ring-like enhancement around necrotic central components, with surrounding T2 fluid-attenuated inversion recovery (FLAIR) signal abnormality, corresponding to infiltrating tumour and oedema [5].
Besides this classic imaging presentation, there is a subset of GB that is found before it develops central necrosis and mass effect. It presents mostly as an ill-defined T2 weighted (T2w) hyperintense lesion, with or without focal/nodular enhancement and can be misdiagnosed as non-neoplastic lesions such as encephalitis, seizure changes, infarction, venous thrombosis and demyelinating process [6,7,8,9,10]. These GBs with unusual characteristics were defined as early stage glioblastomas (e-GB) [10]. At present, our knowledge about e-GB imaging findings derives mostly by its description in case reports and case series with a modest number of patients. Among them, one of the most comprehensive analysis, by Toh et al. [11], was based on 26 cases. In their work, they proposed a classification for e-GB and described the pattern of growth of these atypical cortical lesions.
We believe that further research about e-GB is necessary, firstly to raise awareness about this entity, because it may be encountered during routine emergency practice by the radiologist and neuroradiologist, as happened in our case series; secondly to help building a comprehensive and more coherent picture about this topic, considering that the rarity of e-GB makes the literature fragmentary.
The aim of this case series is to share our experience, through the retrospective analysis of e-GBs that underwent serial MR examinations and to investigate the possible clinical and imaging red flags that may be helpful to the radiologist to give these patients the correct diagnosis.
Materials and methods
Patient population
We performed a retrospective analysis at two reference centres for neurological surgery. The study was approved by our institutional review board and the need for informed consent was waived.
We searched for patients diagnosed with GB from a prospectively maintained database of the Pathology department of both hospitals between January 2010 and January 2020. We excluded patients with GBs arising from histology-proved low-grade gliomas or relapsing GBs. For all selected patients we performed a research in the Picture Archiving and Communication System (PACS) of both institutions, and we excluded all patients without a preoperative imaging available. All preoperative studies (MR and CT) were reviewed by a neuroradiologist with more than 15 years of experience, who selected only patients with e-GB findings at preoperative study. We considered as e-GB circumscribed hyperintense areas on T2-weighted and FLAIR imaging, with or without enhancement at T1-weighted (T1w) images, without “classic” imaging appearance of GB (necrosis, haemorrhage, oedema, heterogeneous enhancement). We collected information on patients’ demographics, clinical presentation, imaging follow-up, clinical management and histologic and immunohistochemistry study.
Histology and immunohistochemistry
All cases were confirmed by histopathology according to 2016 CNS WHO [2]. Immunohistochemistry was performed with specific antibodies (Ab) against GFAP, R132H mutation in isocitrate dehydrogenase 1 (IDH1-R132H) and EGFR. The status of MGMT promoter was also evaluated.
Imaging analysis and clinical management
All preoperative examinations were evaluated for lesion size, location, involvement of grey matter (GM) and/or white matter (WM), presence of restricted diffusion, enhancement pattern and CT appearance, if available. All lesions were categorized following the Toh et al. classification that divides lesions in three groups: type I, lesion involving predominantly GM without any enhancement; type II, lesions involving the cortex and subcortical WM, without enhancement, and type III, lesions involving both cortex and subcortical WM with small focal areas of enhancement at GM/WM junction [11].
MR imaging protocol differs from the two medical centres; however, there are basic sequences that were acquired in all examinations and were used for the evaluation. MR scanner used were a 3 T and 1.5 T. The sequences analysed acquired at 3 T scanner included T1w and T2w turbo-spin-echo (TSE) images, FLAIR, DWI sequence (b = 0 and b = 1000), contrast-enhanced (CE) SE T1w and T1w 3D MPRAGE. All sequences were acquired on axial or coronal plane with 3 mm of slice thickness, and T1w 3D MPRAGE sequence acquired with 1 mm slice thickness.
The sequences acquired at 1.5 T scanner included T1w spin-echo (SE) images, FLAIR, T2w TSE images, DWI sequence (b = 0, b = 1000) and CE SE T1w sequence. All sequences were acquired on axial or coronal plane with 5 mm of slice thickness.
A standard dose of 0.1 mmol/kg of gadoterate meglumine (Dotarem) or gadobutrol (Gadovist) was injected intravenously for CE sequences.
MR studies were also classified as incomplete if CE T1w sequences were lacking. Emergency or elective setting of imaging was also evaluated.
We collected data about morphologic changes, lesion enlargement and time to transformation in classic GB by revising follow-up imaging. Information about initial diagnosis and clinical management were also recorded.
Statistical analysis
Given the small number of patients enrolled in the study, we performed only descriptive statistics. Continuous variables were presented as mean or median and categorical values as absolute values and percentages. All statistical analysis was performed by using software MedCalc (version 15.6.1; MedCalc Software bvba, Ostend, Belgium).
Results
Patient population
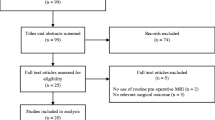
We found 660 patients diagnosed with GB between 2010 and 2020. Among these, 87 were excluded because they were secondary or relapsed GBs treated at other institutions. Among the 573 patients enrolled, 430 had an available preoperative MRI on PACS. After revision of all preoperative MRI examinations, 13 patients (9 males, 4 females) fulfilled the imaging criteria for e-GB (Fig. 1). Median age was 63 years, age range 48–86. One patient presented at preoperative imaging study with two lesions, with a final of 14 e-GBs in our case series. In 10/14 lesions, serial imaging revealed the transformation of e-GB in classic GB. Ten patients presented to the emergency department and underwent the first MR examinations in an emergency setting. Among the preoperative MR examinations, 8/13 were performed with a complete protocol.
As for clinical presentation, three patients presented with unilateral hemiparesis/hemiplegia/hyposthenia, two with aphasia, two with dysarthria and two with seizures (one in combination with speech disturbances, one with right facial hypoesthesia). Other presenting symptoms were vertigo, confusion and disorientation. Clinical onset was not available in three cases. Table 1 summarizes patients’ demographic and clinical information, management and immunohistochemistry results of each lesion.
Immunohistochemistry and histopathology
All lesions revealed typical findings of pseudopalisading necrosis and microvascular proliferation.
All cases resulted IDH wild type, consistent with a diagnosis of de novo type glioblastoma. Seven patients presented EGFR amplification, and two resulted positive for glial fibrillary acidic protein (GFAP). MGMT promoter was methylated in 9 patients (Table 1).
Preoperative imaging analysis
Twelve lesions were localized in the left hemisphere (4 frontal, 3 parietal and 4 temporal lobe, 1 insula) and 2/14 lesions in the right hemisphere (1 frontal and 1 temporal lobe). All lesions were hyperintense at FLAIR images and had a mean maximal diameter of 16 mm (range 4–40 mm). Six lesions involved predominantly GM, seven lesions involved both GM/WM, and one lesion involved only WM. E-GBs were classified into three types using the Toh classification [11]: one lesion resulted type I, two lesions type II and five lesions type III.
In five cases, it was not possible to classify the lesion, because of incomplete MR protocol. One lesion did not match the classification criteria as it involved only WM (Fig. 2).
Case 2, a 60-year male presenting with vertigo. Pre-operative MR shows a hyperintense lesion on FLAIR image (a), involving white matter in the left frontal lobe, without any clear extension to the cortex neither diffusion restriction on ADC map (c). He comes back to emergency department 6 months later for apraxia of speech, and MR examination shows a heterogeneous lesion with necrotic centre on FLAIR image (b) and post-contrast T1 weighted image (d)
Seven lesions showed restriction of diffusion with low ADC values. Preoperative plain CT was performed in 8/13 patients and 7 e-GBs presented as a hyperdense lesion (Fig. 3). Table 2 shows findings on preoperative imaging studies and MR findings at diagnosis.
FLAIR images (a, d, g) DWI images (b, e, h) and plain CT (c, f, i) of case 13 (a-c) and case 6 (d-i). All lesions showed hyperdensity on plain CT (c, f, i, white arrows). Lesion 13 demonstrates hyperintense signal on both FLAIR and DWI sequences, while lesions 6a (d-f) and 6b (g-i) do not present restricted diffusion on DWI (e, h)
Follow-up imaging analysis and clinical management
In the initial MR report diagnosis was subacute ischemic lesion for 5 patients, venous thrombosis for one patient, non-specific gliotic lesions suggestive for chronic vascular leukoencephalopathy for 2 patients, neoplastic lesion for 2 patients and encephalitis for 2 patients. In one patient no diagnosis was mentioned in the report. Ten lesions transformed in classic GB before surgical intervention or biopsy. Median time of transformation from e-GB to classic GB at MR serial imaging was 3 months, with a maximum of 12 months and a minimum of 4 days (Fig. 4). One lesion transformed from type II to type III, one lesion transformed from not classifiable to a type III lesion and five not classifiable lesions transformed into classic GBs. Mean maximal diameter at diagnosis was 30 mm (range 13–51 mm) with a mean increase of 11 mm [95% confidence interval (C.I.): 7–15] corresponding to a mean increase of 80% (95% C.I.: 39–122) of the original maximal dimension.
(Case 3) a 45-year-old woman with dysarthria and vertigo, at first MR showed GM/WM hyperintensity on FLAIR image in left temporal lobe (a, white arrows); 4 days later MR was repeated for worsening of symptoms, showing a necrotic lesion (b, c) with blood barrier damage and contrast enhancement at T1w image acquired after contrast injection (d)
Discussion
In our retrospective analysis, we found 13 patients with 14 e-GBs that underwent serial imaging revealing the transformation from early stage to classic GB appearance.
In our case series, we had predominant involvement of left hemisphere (12/14 lesions), with prevalence of frontal (5/14) and temporal lobe (5/14, 2 of them in the hippocampus), while parietal (3/14), and insula regions were less affected.
Even if it has not been demonstrated that early clinical presentation of cerebral tumours corresponds to specific brain localisations, in our experience 11 out of 14 e-GBs were in eloquent areas, such as motor and language control cortex; accordingly, patients presented with acute onset of aphasia, dysarthria, hemiparesis, seizures. Given the variety of presenting symptoms, the first proposed diagnosis ranged from ischemic stroke to encephalitis to venous thrombosis, when malignancy was not considered.
Our data are consistent with other studies, describing frontal lobe as the first most common location of e-GB [12] and a first radiologic diagnosis that was often wrong or at least very challenging [10]. However, literature on this topic is mostly composed by several case reports [6, 9, 13,14,15], with only a few studies attempting at characterizing this atypical entity and discussing its best management [10, 11, 16].
The immunohistochemistry findings of our case series raised few considerations. Nine lesions out of 14 e-GB demonstrated MGMT promoter methylation, seven lesions presented EGFR amplification and two GFAP expression. EGFR amplification is associated with high-grade malignancy and a good response for EGFR-targeted therapies and MGMT promoter methylation is a marker of favourable prognosis linked to better response to Tamizolide therapy [4].
Unfortunately, most of e-GB case reports lack of information about molecular profile [6, 14, 17,18,19]. When described, it presents some similarities to our results as for IDH status, EGFR amplification and GFAP overexpression [9,10,11, 13].
There have been several attempts at correlating genomics to imaging findings such as radiogenomics studies trying to correlate semiquantitative (i.e. enhancement or T2 flair hyperintensity) and quantitative (i.e. cerebral blood volume or MR intensity texture) attributes to genetic profiles [20,21,22]. However, this topic is beyond the scope of the article, and the population is too small to find significant correlations.
In our analysis, we tried to categorize lesions according to the classification proposed by Toh et al. [11], which includes 26 patients and represents one of the most comprehensive description of e-GB available at present. This classification is based on the epicentre of the lesion (grey matter, or grey/white matter junction) and its contrast enhancement. In our study, most of the lesions had epicentre in GM/WM junction (6 out of 14), while seven lesions showed a predominant involvement of the cortex, and one lesion involved only white matter without any clear spread to cortical ribbon on preoperative MR.
However, apart from the epicentre, we could not fit 6 out of 14 lesions in any category. The first most important hurdle was the lack of CE sequences in MR protocol performed in the emergency setting that made impossible to classify five lesions. Besides the incomplete imaging protocol, one lesion (case 2) did not match any criteria for Toh et al. classifications, because the lesion involved WM without a clear extension to grey matter or GM/WM junction [11]. To our knowledge, e-GBs with exclusive involvement of white matter with intact cortex at imaging were not previously described [6, 9, 13,14,15]. With such atypical epicentre, the lesion was considered as part of the mild leukoencephalopathy that affected the patient and he was discharged without scheduling any follow-up.
Overall, our results suggest that in a in a real-world setting, especially in an emergency one, the Toh et al. classification [11], based on the largest number of e-GB collected in a case series, may be difficult to apply because of the need of contrast administration.
Secondly, we found one case with imaging findings not consistent with the pattern of a small cortical lesion that is the most widely accepted description of e-GB. Therefore, we cannot exclude that there is a subset of e-GB with different imaging characteristics that may be considered in order to build a more comprehensive vision.
Reviewing all preoperative imaging studies, we believe there are some clinical and imaging red flags that may help the neuroradiologist to propose the early the correct diagnosis.
As regards imaging red flags, plain CT may add some useful clues to the neuroradiologist.
In our case series, 7/8 CT demonstrated regions of hyperattenuation corresponding to the lesion. As described by Wang et al. [16] in the analysis of 8 cases of e-GBs, a combination of DWI and unenhanced CT findings may improve diagnostic accuracy. In fact, even if lesions as encephalitis, infarction, demyelinating or degenerative disease may be associated to hypoattenuation, they do not show hyperattenuation. The interpretation of the hyperdense appearance of the gliomas may be explained by an increased cellularity and vascularisation of the tumoural lesion [18].
In our experience, DWI was not such a discriminating sequence to characterize e-GB, as 10 out of 14 lesions presented hyperintense signal in DWI images, and only seven lesions corresponded to restriction of apparent diffusion coefficient, the other corresponding to T2 ‘shine-through’ effect. This discordance between DWI and ADC map in the diagnosis of glioblastomas has been described [23] as the result of several signal changes, deriving from tumour vasogenic oedema, tumour cellularity, degenerative changes and compression of normal structures. Moreover, diffusion restriction may also be present in other neoplastic pathologies, such as lymphoma or metastasis, or in non-neoplastic diseases, such as brain abscess, encephalitis, and stroke.
Concerning CE T1w sequences, in literature several lesions are described as non-enhancing [6, 11, 14, 24], so the neuroradiologist may suggest the right diagnosis even with a MR study performed without contrast injection in the emergency setting.
Interestingly, three lesions (case 1, case 3 and case 4) changed MR appearance from e-GB to classic GB in few days. This short time MR follow-up was demanded by clinical worsening, and it demonstrated an increase in dimension of the lesion, and heterogeneous signal due to a central higher signal intensity in FLAIR/T2w sequence, representing necrosis, and an increase in mass effect on the near cortical tissue and oedema (as showed on Fig. 4). In all these cases, contrast media was not administered in the first examination, but in the follow-up examination, CE T1w sequence confirmed the central initial necrosis, with blood-barrier damage and ring-enhancement. Unfortunately, there is no possibility to know if contrast-media administration at the first examination would have shown blood barrier at the beginning, however, necrotic fluid-filled areas appeared on T2w images at the second examination. The change from ill-defined small cortical/subcortical lesion, to heterogeneous lesion with oedema, central necrosis and haemorrhage, helped to suggest the right diagnosis in short time. In literature, it is described the short interval of time between e-GB and classic GB appearance [6, 9,10,11, 13,14,15, 17,18,19]; however, these three cases suggest the possibility to shorten the time of follow-up MR examination, especially when new symptoms are present.
Other important red flags concern the discordance between imaging and clinical setting.
Two of our cases (case 6 and 13) presented with seizures. The association between seizures and e-GB has been reported: infiltration of the cortex may result in earlier onset of symptoms and possibly focal neurologic deficits, if the malignancy develops in eloquent areas of the brain [25]. MR signal changes are usually present after prolonged seizure activity, or status epilepticus [26]. Transient seizure-induced signal changes on MR in patients with first seizures has been described by Kim et al. [27] as peri-ictal changes involving both cortex and thalamus or hippocampus, rather than only unilateral cortex. Seizures combined to with ill-defined hyperintensities on MR or hyperattenuating CT lesions in adult patients are red flags and require close follow-up because of the possibility of high-grade glioma.
As for the differential diagnosis between subacute stroke and e-GB at preoperative imaging, neuroradiologists should consider both onset of symptoms and imaging findings. T2-FLAIR images are positive 6–12 h after onset of symptoms, and ADC require at least 10 days to normalize [28]. It is not uncommon for e-GB to mimic ischemia, as reported by Nishi et al. [15]. In our case reports, reviewing the five cases misdiagnosed as stroke (cases 1, 6, 7, 10, 11), we noticed they presented with cortical ribbon “blurring” of GM/WM junction, and only three cases presented heterogeneous diffusion restriction. On plain CT, both case 6 and 11 showed hyperattenuation. Neurologic deficits with ill-defined prevalent cortical lesions, with no correct correlation between the onset of symptoms and the imaging findings on MR, or hyperattenuation on CT are red flags, and early stage GB should be considered as possible diagnosis.
In this context, the neuroradiologist has the chance to report the lesion at its very early stage, as acute symptoms like seizures or focal neurological deficits are often approached with imaging. Unfortunately, early appearance of glioblastoma is rare. In our research, only 13 patients out of 430 with preoperative imaging in our databases presented as e-GB at imaging. This give us a partial idea of how rare the possibility is to catch the disease in advance. As described in other reports [9, 14, 29], we also observed a rapid growth of the lesions from nodular to the development of central necrosis.
However, it is uncertain if an earlier diagnosis of GB has a true impact on patients’ prognosis. GB is characterized by poor prognosis, with medial survival of about 15 months if surgery, radiotherapy and chemotherapy are all performed [2]. To this aggressive behaviour, correspond an aggressive surgical treatment that aims at performing a maximal and safe resection of the tumour. It was shown that maximal resection prolongs overall survival [30,31,32,33,34]. It was also observed that preoperative MR showing little or no necrosis, little tumour enhancement and a lesser degree of peritumoral oedema are associated to a better prognosis [35]. Reporting glioblastoma at its early stage, when the lesion is still small, especially in young patients, may therefore provide a survival prolongation thanks to a more radical resection.
Limitation of our study is the impossibility to assess the true prevalence of e-GB in our cohort, because not all patients with a definite diagnosis of GB had imaging records available in our PACS. In addition, a proportion of patients presenting with imaging findings consistent with e-GB does not receive a pathologically confirmed diagnosis, because of the advanced age and/or comorbidities. For these reasons, further studies that combine pathologic data with clinical and imaging data are required.
Conclusions
Awareness of the early presentation of GB is growing; however, we are still far from a comprehensive imaging description of the e-GB. Given the rarity of the condition, case reports and case series will help to give a more detailed picture of this entity, and to catch characteristic imaging findings.
In our case series, we found a combination of clinical settings and relevant imaging findings that enables the radiologist to suggest e-GB as a possible diagnosis and to request close imaging follow-up to exclude the presence of a neoplastic lesion, hopefully improving the prognosis of this very aggressive disease.
Availability of data and material
The authors confirm that all data and materials support their published claims and comply with the field standards.
Abbreviations
- e-GB:
-
Early stage glioblastoma
- GB:
-
Glioblastoma
- T1w:
-
T1-weighted
- T2w:
-
T2-weighted
- MPRAGE:
-
Magnetization Prepared Rapid Acquisition Gradient Echo
- GM:
-
Grey matter
- WM:
-
White matter
References
Wesseling P, Capper D (2018) WHO 2016 Classification of gliomas. Neuropathol Appl Neurobiol 44:139–150. https://doi.org/10.1111/nan.12432
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. https://doi.org/10.1007/s00401-016-1545-1
Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455(7216):1061–1068. https://doi.org/10.1038/nature07385
Montemurro N (2020) Glioblastoma Multiforme and genetic mutations: the issue is not over yet. An overview of the current literature. J Neurol Surg A Cent Eur Neurosurg 81(1):64–70. https://doi.org/10.1055/s-0039-1688911
Guerin JB, Eckel LJ, Kaufmann TJ (2016) Updates to the WHO Brain Tumor Classification System: What the Radiologist needs to know. RadioGraphics 37:2164–2180. https://doi.org/10.1148/rg.2017170037
Jung T-Y, Jung S (2007) Early neuroimaging findings of glioblastoma mimicking non-neoplastic cerebral lesion. Neurol Med Chir (Tokyo) 47:424–427. https://doi.org/10.2176/nmc.47.424
Nam TS, Choi KH, Kim MK, Cho KH (2011) Glioblastoma mimicking herpes simplex encephalitis. J Korean Neurosurg Soc 50(2):119–122. https://doi.org/10.3340/jkns.2011.50.2.119
Thaler PB, Yi J, Isakov Y et al (2012) Normal or non-diagnostic neuroimaging studies prior to the detection of malignant primary brain tumors. J Clin Neurosci 19:411–414. https://doi.org/10.1016/j.jocn.2011.09.002
Oyama H, Ando Y, Aoki S et al (2010) Glioblastoma detected at the initial stage in its developmental process. Neurol Med Chir 50:414–417. https://doi.org/10.2176/nmc.50.414
Ideguchi M, Kajiwara K, Goto H, Sugimoto K (2015) MRI findings and pathological features in early-stage glioblastoma. J Neurooncol. https://doi.org/10.1007/s11060-015-1797-y
Toh CH, Castillo M (2017) Early-stage Glioblastomas: MR Imaging-based classification and imaging evidence of progressive growth. AJNR Am J Neuroradiol 38:288–293. https://doi.org/10.3174/ajnr.A5015
Larjavaara S, Mäntylä R, Salminen T et al (2007) Incidence of gliomas by anatomic location. Neuro Oncol 9:319–325. https://doi.org/10.1215/15228517-2007-016
Hishii M, Toshiharu M, Arai H (2019) Diagnosis and teatment of early-stage Glioblastoma. Asian J Neurosurg 14:589–592. https://doi.org/10.4103/ajns.AJNS_18_19
Cohen-Gadol AA, DiLuna ML, Bannykh SI et al (2004) Non-enhancing de novo glioblastoma: report of two cases. Neurosurg Rev 27:281–285. https://doi.org/10.1007/s10143-004-0346-5
Nishi N, Kawai S, Yonezawa T (2009) Early appearance of high grade Glioma on Magnetic Resonance Imaging. Neurol Med Chir 49:8–12. https://doi.org/10.2176/nmc.49.8
Wang H, Liu Z, Zhang Y, Hou F, Fu W, Lin J, Liu Y, Liu X (2020) Additional diagnostic value of unenhanced computed tomography plus diffusion-weighted imaging combined with routine magnetic resonance imaging findings of early-stage gliblastoma. Biomed Res Int 2020:1672736. https://doi.org/10.1155/2020/1672736
Nishi N, Kawai S, Yonezawa T, Fujimoto K, Masui K (2009) Early appearance of high grade glioma on magnetic resonance imaging. Neurol Med Chir (Tokyo) 49(1):8–12. https://doi.org/10.2176/nmc.49.8
Rossi R, Figus A, Corraine S (2010) Early presentation of de novo high grade glioma with epileptic seizures: Electroclinical and neuroimaging findings. Seizure 19:470–474. https://doi.org/10.1016/j.seizure.2010.07.001
Zhang YY, Ruan LX, Zhang S (2016) Rapid progression of glioblastoma multiforme: a case report. Oncol Lett 12(6):4803–4806. https://doi.org/10.3892/ol.2016.5228
Hong EK, Choi SH, Shin DJ et al (2018) Radiogenomics correlation between MR imaging features and major genetic profiles in glioblastoma. Eur Radiol 28(10):4350–4361. https://doi.org/10.1007/s00330-018-5400-8
Hu LS, Ning S, Eschbacher JM et al (2017) Radiogenomics to characterize regional genetic heterogeneity in glioblastoma. Neuro-Oncology 19:128–137. https://doi.org/10.1093/neuonc/now135
Ellingson BM (2015) Radiogenomics and imaging phenotypes in glioblastoma: novel observations and correlation with molecular characteristics. Curr Neurol Neurosci Rep 15(1):506. https://doi.org/10.1007/s11910-014-0506-0
Baehring JM, Bi WL, Bannykh S et al (2007) Diffusion MRI in the early diagnosis of malignant glioma. J Neurooncol 82:221–225. https://doi.org/10.1007/s11060-006-9273-3
Utsuki S, Oka H, Miyajima Y et al (2012) Glioblastoma without remarkable contrast enhancement on Magnetic Resonance Imaging. Int J Clin Med 03:439–445. https://doi.org/10.4236/ijcm.2012.36082
Koeller KKHJ (2001) From the archives of the AFIP: superficial gliomas: radiologic-pathologic correlation. Radiographics 21:1533–1536. https://doi.org/10.1148/rg.246045146
Friedman E (2014) Epilepsy imaging in adults: getting it right. Am J Roentgenol 203:1093–1103. https://doi.org/10.2214/AJR.13.12035
Kim SE, Lee BI, Shin KJ et al (2017) Characteristics of seizure-induced signal changes on MRI in patients with first seizures. Seizure 48:62–68. https://doi.org/10.1016/j.seizure.2017.04.005
Allen LM, Hasso AN, Handwerker J, Farid H (2012) Sequence-specific MR imaging findings that are useful in dating ischemic stroke. Radiographics 32:1285–1297. https://doi.org/10.1148/rg.325115760
Landy HJ, Lee TT, Potter P et al (2000) Early MRI findings in high grade glioma. J Neurooncol 47:65–72. https://doi.org/10.1023/A:1006494604527
Yamaguchi S, Kobayashi H, Terasaka S et al (2012) The impact of extent of resection and histological subtype on the outcome of adult patients with high-grade gliomas. Jpn J Clin Oncol 42:270–277. https://doi.org/10.1093/jjco/hys016
Stark AM, Van De BJ, Hedderich J et al (2012) Glioblastoma: clinical characteristics, prognostic factors and survival in 492 patients. Clin Neurol Neurosurg 114:840–845. https://doi.org/10.1016/j.clineuro.2012.01.026
Jovčevska I (2019) Genetic secrets of long-term glioblastoma survivors. Bosn J Basic Med Sci 19(2):116–124. https://doi.org/10.17305/bjbms.2018.3717
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198. https://doi.org/10.3171/jns.2001.95.2.0190
Sanai N, Polley MY, McDermott MW, Parsa ATBM (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8. https://doi.org/10.3171/2011.2.jns10998
Hammoud MA (1996) Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol 27:65–73. https://doi.org/10.1007/BF00146086
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submissions made a substantial contribution to the conception or design of the work, the acquisition analysis and interpretation of data. All authors revised it critically for important intellectual content. All author approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
The authors of this manuscript declare that they have no competing interests.
Ethics approval
Institutional review board approval was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ceravolo, I., Barchetti, G., Biraschi, F. et al. Early stage glioblastoma: retrospective multicentric analysis of clinical and radiological features. Radiol med 126, 1468–1476 (2021). https://doi.org/10.1007/s11547-021-01401-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-021-01401-4