Abstract
Purpose
To present our experience of cases of primary pulmonary lymphoma (PPL) found between January 2002 and July 2018, focusing on the radiological features and the differential diagnosis in order to contribute to the difficult role of the radiologist in the disease identification and to help the clinicians to reach the diagnosis.
Materials and methods
CT scans of 30 patients (14 men and 16 women, aged 58–86, mean age 72 years) with PPL were retrospectively reviewed. All patients had a histopathological confirmation of the disease: MALT lymphoma (23 patients, 76.6%); diffuse large B-cell lymphoma—DLBCL (seven patients, 23.4%). All the staging CT scans were evaluated by three experienced radiologists dedicated to thoracic disease in order to radiologically define the predominant pattern of presentation.
Results
The following parenchymal patterns were observed: 11 patients with single/multiple nodules, five with masses/mass-like consolidations, 14 with consolidations with air bronchogram, 16 with ground-glass opacity, ten with angiogram sign, 22 with perilymphatic and/or peribronchovascular spread, 15 with associated lymphadenopathies, and 13 with pleural/chest wall involvement. The main characteristics of PPLs were the presence of consolidations and ground-glass opacities, with perilymphatic and/or bronchovascular spread.
Conclusion
All the characteristics of the work should alert the radiologist to consider lymphoma among the possible differential diagnoses, always correlating the results of the CT examination with appropriate clinical laboratory evaluations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lymphoproliferative disorders of the lung can be divided into primary pulmonary lymphomas (PPLs) and secondary pulmonary involvement by lymphomas arising from adjacent nodal sites (such as mediastinal or hilar lymph nodes and thymus) or by haematogenous dissemination from an extrapulmonary site of involvement. PPL is defined as a malignant monoclonal lymphoid proliferation within the lung parenchyma, in a patient without any detectable extrapulmonary involvement at least 3 months after initial diagnosis [1]. It is a rare disease, representing approximately 3–4% of extranodal lymphomas (less than 1% of all NHL cases) and 0.5–1% of all primary malignancies involving the lung [2,3,4,5,6]. The peak incidence is in the sixth decade, and there is a slight predominance in men [1]. PPLs can be MALT lymphoma (MALToma—Low-grade marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue), other non-Hodgkin lymphomas or Hodgkin lymphoma. MALT lymphoma is the most common type, representing 58–90% of all the PPLs; it is more common in females, often associated with autoimmune disease [7]. The second most common subtype after MALT lymphoma is the more aggressive diffuse large B-cell lymphoma (DLBCL—10–20%) developing mostly in immunocompromised subjects. Others rare types of PPLs include mantle-cell lymphoma (MCL), follicular lymphoma (FL) or lymphoplasmacytic lymphoma (LL) [3, 4, 8]. Primary pulmonary Hodgkin lymphoma is the least common type of PPLs, with less than 70 cases reported in the literature since 1927 [9, 10]. In such case, parenchymal involvement usually occurs by direct extension of mediastinal pathology or it is associated with disseminating disease [5, 8,9,10]. Clinical findings are often unspecific or absent; pulmonary symptoms such as cough, dyspnoea, haemoptysis, or chest pain may occur, as well as systemic symptoms like fever, weight loss, and fatigue. In asymptomatic patients, incidental radiological findings may be the first assessment [2]. Chest X-ray usually is performed as the first radiological examination showing unspecific findings (e.g. mass, solitary or multiple nodules, pleural effusion) [2]. Otherwise, chest computed tomography (CT) examination has a crucial role for a better definition of the thoracic alterations in these patients. This study aimed to describe the predominant CT patterns of PLLs evidenced in our population of patients, focusing on the differential diagnosis to address the problematic role of the radiologist in making a correct diagnosis and to help the clinicians to set the right therapy.
Materials and methods
Inclusion criteria
Our institutional board review approved this retrospective study. Informed consent was obtained from all individual participants included in the study. We reviewed CT exams performed from January 2002 to December 2018 in 30 patients affected by PPL. Inclusion criteria were patients’ age between 18 and 90 years, acquisition of informed consent, histological diagnosis of PPL, pre-treatment chest CT with contrast medium utilization.
Reference standard
The diagnosis was histologically confirmed by lung biopsy in all cases. Clinical staging was referred to as Ann-Arbor staging, also assessing the presence of systemic symptoms (fever, night sweats, weight loss of > 10% of body weight over 6 months) [11].
Imaging protocol
Because of the length of the considered period (16 years), images were acquired by the use of different CT scanners and technique protocols (scanners: Lightspeed VCT 64, GE; Sensation 16, Siemens; Sensation 64, Siemens; Optima 64, GE; ICT 128, Philips; Somatom Definition Flash, Siemens). However, all the examinations included spiral chest scans acquired before and after intravenous administration of iodinated contrast medium. Iodinated contrast medium was administered in an antecubital vein and was injected at a flow rate of 3–3.5 ml/s. The acquisitions were all in inspiration phases, and high-resolution images were contiguously reconstructed at 1–1.5 mm using a smooth kernel for mediastinal structures and a sharp one for parenchymal evaluation.
Imaging review and data analysis
All images were stored in a picture archiving and communication system (PACS). An independent and retrospective review of chest imaging for each patient was performed by two radiologists to define the predominant pattern of PPL presentation; in case of discordance, a consensual agreement was reached. CT scans were assessed for the presence of single or multiple pulmonary nodules (diameter < 2.5 cm), monolateral or bilateral, masses (diameter > 2.5 cm) or mass-like consolidations, with or without the coexistence of a positive angiogram sign, ground-glass opacities, parenchymal distribution (bronchovascular or perilymphatic), the presence of hilar-mediastinal lymph nodes (long axis > 1.5 cm) and pleura/chest wall involvement. All thoracic images were also assessed for evidence of other associated pulmonary pathology (such as bronchiectasis/bronchiolectasis, emphysema, and fibrosis).
Results
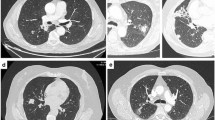
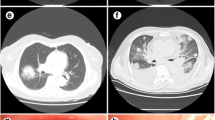
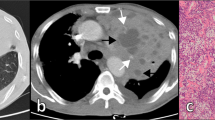
Patients were 14 males and 16 females, aged 56–86 years at diagnosis (mean age 72 years). The most common histological type was MALT lymphoma (23/30 patients, 76.6%); 7/30 patients were affected by DLBCL (23.4%). Ann-Arbor Stage was I in 17 patients (56.6%), II in 1 (3.4%), and IV in 12 patients (40%); six patients (20%) had systemic symptoms (Table 1). Lung biopsy was performed in all 30 cases (100%) (Fig. 1). Chest CT was available in all cases. Imaging features identified in CT exams are shown in Table 2. The following parenchymal patterns were observed: 11 patients with single/multiple nodules (Fig. 2), five with masses/mass-like consolidations (Fig. 3), 14 with consolidations with air bronchogram (Fig. 4), 16 with ground-glass opacity (Fig. 5), ten with angiogram sign, 22 with perilymphatic and/or peribronchovascular spread (Figs. 6 and 7), 15 with associated lymphadenopathies, 13 with pleural/chest wall involvement (Fig. 8). The most common characteristics of PPLs were the presence of consolidations and ground-glass opacities, with perilymphatic and/or bronchovascular spread. Nodules, consolidations with air bronchogram and associated lymphadenopathies are more frequent in MALT lymphomas than in DLBCL. Angiogram sign and pleural involvement were similar in both MALT and DLBCL.
Discussion
The lung is frequently involved in metastatic localization of Hodgkin and Non-Hodgkin lymphomas (up to 38% in HL and up to 24% un NHL) [7, 9, 12]. However, primary lung lymphoid tumours are rare; as discussed before, MALT lymphoma is more common than DLBCL (58–90% and 10–20% respectively). Although DLBCL can occur as a primary proliferation, it can also arise from the transformation of a MALT lymphoma into a more aggressive DLBCL [6, 13]. It has to be remembered that patients with HIV infection, a collagen vascular disease or chronic immunosuppression could develop high-grade PPL [14,15,16,17]. Imaging manifestations of PPLs may overlap with other CT features of completely different diseases and usually make a radiological diagnosis is difficult.
In our case series, the most prevalent CT findings in both MALT and DLBCL are the presence of masses and consolidations, together with diffuse/focal ground-glass opacities and with perilymphatic and peribronchovascular spread of these lesions. Also nodules (single or multiple) are common findings. Ground-glass attenuation is usually seen with less severe interstitial involvement and has more frequently a bilateral localization [18]. On imaging, the CT features of MALT and DLBCL can overlap; in both cases, patients can present nodules or consolidations, although cavitation and necrosis of the lesion are more frequent in DLBCL than in MALT lymphoma itself [2, 9]. PPL in the form of a solid lesion may be indistinguishable from metastases, bronchogenic carcinoma or sarcoidosis [19]. Instead, PPL should be considered in the differential diagnosis of non-resolving lung consolidation or ground-glass opacities, despite accurate antibiotic therapy. In a majority of patients, a follow-up CT scan shows any growth of the lesion, which can be stable for many years [20, 21]. This stability excludes other acute causes of consolidation. However, a similar pattern can be seen in other entities and the differential diagnosis, in this case, includes organizing pneumonia, eosinophilic or lipoid pneumonia, lymphoid interstitial pneumonia (LIP) or lung adenocarcinoma with lepidic growth [13, 22]. LIP and MALT lymphoma of the lung can coexist in the same patient, but it is unlikely that there is a transformation of LIP into a malignant process [9].
Usually, air bronchogram is common in PPLs because the bronchi and bronchioles tend to be unaffected from the proliferation of the tumour. Angiogram sign, that is an enhancing pulmonary vessel within a homogeneous area of consolidation, is a feature of PPL; it is a usual sign but non-specific. It could be present in other conditions such as mucinous adenocarcinoma, post-obstructive consolidation, and also in all the lymphoproliferative diseases [19, 23,24,25]. In general, a consolidation with homogeneous attenuation, air bronchograms, and angiogram signs are typical features of PPLs but non-specific; radiologist should keep high suspicion in patients with these CT features and high-risk factors (transplant, autoimmune disease, clinical indolent behaviour, HIV infection, smoke) [19, 23, 26].
The typical perilymphatic spread of PPLs’ lesions could simulate other diseases, in particular, sarcoidosis and lymphangitic carcinomatosis. All these three entities are characterized by abnormalities of the bronchovascular bundles, subpleural interstitium, and interlobular septa; the thickened interlobular septa and alterations of the subpleural interstitia are significantly greater in lymphangitic carcinomatosis than in the other two diseases [27, 28]. Lymphangitic carcinomatous often involves the peripheral interstitium at first, and then progresses towards the central area (retrograde spread); instead, lung lymphoma and sarcoidosis tend to progress from the lymph nodes to the peripheral lymphatics (anterograde spread). Furthermore, sarcoidosis tends to be involved more in the upper-middle lobes compared to pulmonary lymphoma [27, 29]. The 18FDG-PET exam does not allow a differential diagnosis between these various causes of interstitial thickening [19]. Mediastinal hilar lymphadenopathies and pleural involvement are not frequent in our case series as well as in the other most recent clinical studies [23, 30, 31]. Hilar-mediastinal lymphadenopathies may be present no more of 30% of the time [2]. Pleural involvement can be shown as a focal thickening, a secondary effusion or a pyothorax, especially in patients with Epstein–Barr virus-positive DLBCL [32, 33].
Differential diagnosis of PPLs includes also two other clinical situations: lymphomatoid granulomatosis (LG) and post-transplant lymphoproliferative disorders (PTLD). LG is a relatively rare disease characterized by an angiodestructive process with Epstein–Barr virus-positive cells, occurring in patients with autoimmune disorders [34]. Radiologically LG can be indistinguishable with PPLs but, when both cavitations and ground-glass halos are present, the disease appears similar to granulomatosis with polyangiitis (Wegener granulomatosis, especially in 30% cases here are necrotic coalescing and cavitated nodules) [35]. Thus, also in these cases, tissue sampling is required. PTLD collects a group of lymphoproliferative disorders occurring in the post-solid organ or post-stem cell transplant, varying from benign polyclonal conditions to malignant monoclonal diseases [3, 5, 9]. Also, PTLD is associated with EBV infection. Imaging manifestations are similar to DLBCL, including solid masses-nodules, consolidations, ground-glass opacities; solid masses in PTLD tend not to cavitate [19]. The main radiological differential diagnosis for PTLD is angioinvasive aspergillosis, especially in the immunosuppressed population [2]. Both LG and PTLD lesions are avidly hypermetabolic at FDG-PET [36,37,38].
Despite everything, nowadays lung biopsy is still necessary to achieve a definitive diagnosis, essentially in all cases of PPLs. Percutaneous CT-guided or bronchoscopic biopsy may be sufficient for solid lesions; in the case of ground-glass opacity, consolidation, and interstitial involvement, an open surgical biopsy may be required [3, 15, 30].
This study has some limitations. First, the retrospective nature of the analysis. Second, the statistical analysis did not reveal any significant correlation between our data, may be because of the various heterogeneity of PPLs at CT imaging and the small number of patients. Although we examined cases of PPLs in our hospital between 2002 and 2018, our sample size is rather small consisting of 30 patients. This number still needs to be increased to validate statistical results. However, to the best of our knowledge in the most recent literature, this work contains one of the numerically most significant case series of PPL [23, 30, 31, 39, 40].
In conclusion, we have described the imaging findings in a series of 30 PPLs, confirmed by lung biopsy. Although, despite the absence of specific radiological patterns, we can state that many features (such as the presence of masses and consolidations with angiogram sign, together with diffuse/focal ground-glass opacities and with perilymphatic spread) should raise the possible diagnosis of primary lymphoproliferative lung disease in an appropriate clinical setting.
All the CT features described in this work should alert the radiologist to consider lymphoma among the possible differential diagnoses, always correlating radiological results with appropriate clinical laboratory evaluations, for which a close collaboration with clinicians is required.
References
Cadranel J, Wislez M, Antoine M (2002) Primary pulmonary lymphoma. Eur Respir J 20(3):750–762
Sirajuddin A, Raparia K, Lewis VA et al (2016) Primary pulmonary lymphoid lesions: radiologic and pathologic findings. Radiographics 36:53–70. https://doi.org/10.1148/rg.2016140339
William J, Variakojis D, Yeldandi A et al (2013) Lymphoproliferative neoplasms of the lung: a review. Arch Pathol Lab Med 137(3):382–391
Koss MN (2004) Malignant and benign lymphoid lesions of the lung. Ann Diagn Pathol 8(3):167–187
Lee KS, Kim Y, Primack SL (1997) Imaging of pulmonary lymphoma. AJR Am J Roentgenol 168(2):339–345
Kurtin PJ, Myers JL, Adlakha H et al (2001) Pathologic and clinical features of primary pulmonary extranodal marginal zone B-cell lymphoma of MALT type. Am J Surg Pathol 25(8):997–1008
Restrepo CS, Carrillo J, de Christenson MR et al (2013) Lymphoproliferative lung disorders: a radiologic–pathologic overview. Part II: neoplastic disorders. Semin Ultrasound CT MRI 34:535–549
Lee WK, Duddalwar VA, Rouse HC et al (2009) Extranodal lymphoma in the thorax: cross-sectional imaging findings. Clin Radiol 64(5):542–549
Hare SS, Souza CA, Bain G et al (2012) The radiological spectrum of pulmonary lymphoproliferative disease. Br J Radiol 85(1015):848–864
Balbo Mussetto A, Savioo C, Fornari A et al (2017) Whole body MRI with qualitative and quantitative analysis of DWI for assessment of bone marrow involvement in lymphoma. Radiol Med 122(8):623–632
Cheeson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32:3059
Do KH, Lee JS, Seo JB et al (2005) Pulmonary parenchymal involvement of low-grade lymphoproliferative disorders. J Comput Assist Tomogr 29(6):825–830
Kligerman SJ, Franks TJ, Galvin JR (2017) Primary extranodal lymphoma of the thorax. Radiol Clin N Am 54:673–687. https://doi.org/10.1016/j.rcl.2016.03.002
Wannesson L, Cavalli F, Zucca E (2005) Primary pulmonary lymphoma: current status. Clin Lymphoma Myeloma 6(3):220–227
Zinzani PL, Martelli M, Poletti V et al (2008) Practice guidelines for the management of extranodal non-Hodgkin lymphomas of adult non-immunodeficient patients. Part I: primary lung and mediastinal lymphomas. A project of the Italian Society of Hematology, the Italian Society of Experimental Hematology and the Italian Group for Bone Marrow Transplantation. Haematologica 93:1364–1371. https://doi.org/10.3324/haematol.12742
Paolicchi F, Bastiani L, Guido D et al (2018) Radiation exposure in patients affected by lymphoma undergoing repeat CT examinations: how to manage the radiation dose variability. Radiol Med 123(3):191–201
Albarello F, Cristofaro M, Busi Rizzi E et al (2018) Pulmonary measles disease: old and new imaging tools. Radiol Med 123(12):935–943
Wislez M, Cadranel J, Antoine M et al (1999) Lymphoma of pulmonary mucosa-associated lymphoid tissue: CT scan findings and pathological correlations. Eur Respir J 14:423–429
Bligh MP, Borgaonkar JN, Burrel SC et al (2017) Spectrum of CT findings in thoracic extranodal non-Hodgkin lymphoma. Radiographics 37:439–461. https://doi.org/10.1148/rg.2017160077
Bae YA, Lee KS, Han J et al (2008) Marginal zone B-cell lymphoma of the bronchus-associated lymphoid tissue: imaging findings in 21 patients. Chest 133:433–440
Tomà P, Lancella L, Menchini L et al (2017) Radiological patterns of childhood thoracic tuberculosis in a developed country: a single institution’s experience in 217/255 cases. Radiol Med 122(1):22–34
Cozzi D, Moroni C, Addeo G et al (2018) Radiological pattern of lung involvement in inflammatory bowel disease. Gastroenterol Res Pract 2018:5697846. https://doi.org/10.1155/2018/5697846
King LJ, Padley SPG, Wotherspoon AC et al (2000) Pulmonary MALT lymphoma: imaging findings in 24 cases. Eur Radiol 10:1932–1938
Bicchierai G, Rigacci L, Miele V et al (2017) Role of core needle biopsy in primary breast lymphoma. Radiol Med 122(9):651–655
Yang Q, Wang Y, Ban X et al (2017) Prediction of pulmonary metastasis in pulmonary nodules (< 10 mm) detected in patients with primary extrapulmonary malignancy at thin-section staging CT. Radiol Med 122(11):837–849
O’Donnel PG, Jackson SA, Tung KT et al (1998) Radiological appearances of lymphomas arising from mucosa-associated lymphoid tissue (MALT) in the lung. Clin Radiol 53:258–263
Osamu H, Takeshi J, Kazuya I et al (1999) Comparison of high-resolution CT findings of sarcoidosis, lymphoma, and lymphangitic carcinoma: Is there any difference of involved interstitium? J Comput Assist Tomogr 23(3):374–379
Sverzellati N, Odone A, Silva M et al (2018) Structured reperting for fibrosing lung disease: a model shared by radiologist and pulmonologist. Radiol Med 123(4):245–253
Cozzi D, Bargagli E, Calabrò AG et al (2018) Atypical HRCT manifestations of pulmonary sarcoidosis. Radiol Med 123(3):174–184. https://doi.org/10.1007/s11547-017-0830-y
Ahmed S, Kussick SJ, Siddqui AK et al (2004) Bronchial-associated lymphoid tissue lymphoma: a clinical study of a rare disease. Eur J Cancer 40:1320–1326
Graham BB, Mathisen DJ, Mark EJ et al (2005) Primary pulmonary lymphoma. Ann Thorac Surg 80:1248–1253
Alexandrakis MG, Passam FH, Kyriakou DS et al (2004) Pleural effusions in hematologic malignancies. Chest 125(4):1546–1555
Valente T, Tortora G, Bocchini G et al (2017) MDCT and US of intrathoracic extrapleural space soft tissue-containing lesions: US extrapleural fat sign and MDCT fat ghost ribs sign. Radiol Med 122(7):479–486
Roschewski M, Wilson WH (2012) Lymphomatoid granulomatosis. Cancer J 18(5):469–474
Frazier AA, Rosado-de-Christenson ML, Galvin JR et al (1998) Pulmonary angiitis and granulomatosis: radiologic–pathologic correlation. Radiographics 18(3):687–710
Chung JH, Wu CC, Gilman MD et al (2011) Lymphomatoid granulomatosis: CT and FDG-PET findings. Korean J Radiol 12(6):671–678
Borhani AA, Hosseinzadeh K, Almusa O et al (2009) Imaging of posttransplantation lymphoproliferative disorders after solid organ transplantations. Radiographics 29(4):981–1000
Tomà P, Cannatà V, Genovese E et al (2017) Radiation exposure in diagnostic imaging: wisdom and prudence, still a lot to understand. Radiol Med 122(3):215–220
Ferraro P, Trastek VF, Adlakha H et al (2000) Primary non-Hodgkin’s lymphoma of the lung. Ann Thorac Surg 69:993–997
McCulloch GL, Sinnatamby R, Stewart S et al (1998) High-resolution computed tomographic appearance of MALToma of the lung. Eur Radiol 8:1669–1673
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare that they have no conflict of interest related to the publication of this article.
Ethical approval
The study protocol was submitted and approved by the Ethics Committee of our referring centre (CEAVC n. 13937). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cozzi, D., Dini, C., Mungai, F. et al. Primary pulmonary lymphoma: imaging findings in 30 cases. Radiol med 124, 1262–1269 (2019). https://doi.org/10.1007/s11547-019-01091-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-019-01091-z