Abstract
Introduction
Radiation therapy plays an important role in the management of SCLC both in curative and palliative setting, however, conflicting data from clinical trials incite debate over the appropriate use of radiation therapy regarding prophylactic cranial irradiation (PCI) and/or thoracic consolidative in extensive-stage SCLC (ES-SCLC). This survey is conducted to evaluate the current pattern of care among Italian radiation oncologists.
Methods
In June 2016, all Italian radiation oncologists were invited to a web-based survey. The survey contained 34 questions regarding the role of RT in SCLC. Questions pertaining the role of RT in the clinical management of both limited-stage (LS) and ES-SCLC were included.
Results
We received 48 responses from Italian radiation oncologists. More than half of respondents had been practicing for more than 10 years after completing residency training and 55% are subspecialists in lung cancer. Preferred management of LS-SCLC favored primary concurrent chemoradiotherapy (89%), even if the 36.9% usually delivered RT during or after the cycle 3 of chemotherapy, due to organizational issues. The most common dose and fractionation schedule in this setting was 60 Gy in 30 once-daily fractions. Furthermore, almost all respondents recommended PCI in patients with LS-SCLC. For ES-SCLC scenario, chemotherapy was defined the standard treatment by all respondents. PCI was recommended in ES-SCLC patients with thoracic complete remission (63% of respondents), with thoracic partial response (45%) and with thoracic stable disease (17%) after first-line chemotherapy. Lastly, the thoracic consolidative RT was recommended by 51% of respondents in patients with ES-SCLC in good response after first-line chemotherapy and a great variability was shown in clinical target volume definition, doses and fractionation schedules.
Conclusions
Our analysis showed a high adherence to current guidelines among the respondents in regard to chemoradiation approach in LS-SCLC patients and to PCI indications and doses. The great variability in radiation therapy doses and volumes in the thoracic consolidative radiotherapy in ES-SCLC is concerning. Future clinical trials are needed to standardize these treatment approaches to improve treatment outcomes among patients with ES-SCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small cell lung cancer (SCLC) accounts for about 10–15% of all lung cancer cases. It is usually related to smoking habits and it tends to show a rapid systemic spread, meaning it is often diagnosed in advanced stages [1].
Radiation therapy (RT) plays an important role in the management of SCLC, both in curative and palliative setting. Concurrent chemoradiotherapy (CCRT) in limited-stage SCLC (LS-SCLC) is widely adopted due to the strong evidence supporting the use of thoracic RT concurrently with platinum-based chemotherapy to improve overall survival (OS) [1,2,3,4]. Although the role of thoracic RT is well-established in the management of LS-SCLC, the optimal radiation dose and fractionation schedule is still under investigation [5, 6], as well as target delineation and optimal timing of concurrent chemotherapy.
On the other hand, chemotherapy is the cornerstone treatment for extensive stage (ES-SCLC) and four to six cycles of platinum-based systemic therapy represent the current standard of care [2, 3]. However, ES-SCLC prognosis is still poor, with a median OS of 7–11 months [2, 3]. Studies investigating other systemic approaches, as target therapy or maintenance chemotherapy, have not shown significant improvement [6, 7]. Consolidative thoracic radiotherapy (CTRT) seems to be beneficial in patients with ES-SCLC who achieved a response after primary chemotherapy, as shown by a recently published phase III randomized trial conducted on 498 adults with advanced SCLC from 42 hospitals [8].
In addition, prophylactic cranial irradiation (PCI) following response to induction chemotherapy provided a survival benefit in a phase III trial [9] and it is currently recommended for responders with either LS- or ES-SCLC [2, 3]. However, the risk for late radiation effects and other clinical factors might impact on its routinary use [6, 10, 11].
For all these reasons, the management of LS- and ES-SCLC can be highly variable between different Oncology Centers and it is likely to be guided by clinician experience and local patterns of practice.
The aim of this survey was to evaluate the current management of SCLC in Italy and to highlight areas of potential improvement, if needed. In particular, we designed this survey to assess Italian Radiation Oncologists attitude regarding both PCI and CTRT in LS-SCLC and ES-SCLC, and what factors influence physicians’ clinical recommendations.
Methods
The study was approved by Associazione Italiana di Radioterapia Oncologica (AIRO). An online web-based survey was planned and then developed using Survey Monkey software (http://www.surveymonkey.com). An e-mail with the link to the web-based questionnaire was sent to all AIRO members. A first call was originally sent on June 6 2016, and a reminder 1 month later, to maximize response rate.
The survey contained 34 questions regarding the management of SCLC, and particularly the role of CTRT and PCI in ES-SCLC. Physicians were first asked details about demographics and clinical experience: years since residency completion, number of patients affected with SCLC treated in the past years, practice setting and basic knowledge. Questions about the role of RT in the clinical management of both LS- and ES-SCLC were then presented. Factors influencing the use of PCI, the timing and choice of RT regimens, the role of multidisciplinary case discussion and RT planning techniques and delivery were also evaluated. Table 1 provides a complete list of all questions. The survey was intentionally structured to keep an average completion time within 10 min. All answers (including partial responses) were deemed eligible for analysis using descriptive statistics. Ethical approval was not required for this study.
Results
Demographics
The survey was sent to 812 addresses. Forty-eight responses were received, representing 6% of all Italian radiation oncologists registered as member of AIRO.
The level of experience of the respondents varied: three radiation oncologists (6%) have been working in a radiation oncology department for less than 5 years, 15 (32%) for 5–10 years and 29 (61.7%) for more than 10 years. The median number of newly diagnosed SCLC patients seen annually was more than 20 for only 4 (8.33%) radiation oncologists, less than 10 for 19 (39.58%) and between 10 and 20 for over half of respondents. 55% are subspecialists in lung cancer.
The rate of multidisciplinary case-discussion was 82.98%.
Management of limited-stage SCLC
Radiation oncologists were asked to select the chemoradiation approach in “fit” patients with LS-SCLC. The majority (89.58%) indicated that they would offer CCRT, but only 27.08% use this approach in all fit patients; 31.25% of respondents offer CCRT in less than 20% of all fit patients with LS-SCLC and 18.75% in no more than a half of the fit patients. When a concurrent approach is used, most clinicians usually deliver RT during cycle 1 or 2 of chemotherapy (56.2%), rather than during cycle 3 (36.9%). We asked to define the cause of radiation delay: more than half of the respondents declared to have organizational issues.
Concerning the definition of clinical target volume (CTV), 22.9% answered that they would include the entire pre-chemotherapy disease extent in the CTV, while 27% would outline only the current measurable disease (post-chemotherapy residual disease, identified by CT scan and/or 18 FDG PET-CT) and 50% would define the CTV somewhere “in between” the pre-chemotherapy and the residual disease, often with two separated volumes and with different dose levels. PET-CT was commonly used (95.3%) to delineate target volume.
Thoracic RT fractionation schedule was 60 Gy in 30 fractions for more than 54% of respondents. 3D-CRT, IMRT and VMAT were used by 42.55, 19 and 38.3%, respectively.
Recommendation of PCI Almost all radiation oncologists (97.92%) usually recommend PCI to their patients. Respondents were more keen to perform PCI in LS-SCLC patients who achieve only complete (38.3%) or complete and/or partial clinical response (72.34%) after systemic chemotherapy. Thirty-four percent recommends PCI also in patients with stable disease after first-line chemotherapy; regarding ES-SCLC, 63.83% suggests PCI in patients with complete thoracic remission after chemotherapy, 44.68% also in patients with partial thoracic response and 17.02% in patients with stable thoracic disease too. Only one radiation oncologist would perform PCI in all patients irrespective of response to chemotherapy. PCI schedule is 25 Gy in 10 daily fractions for the majority of respondents.
Role of thoracic consolidative RT in ES-SCLC
Almost all clinicians (97.87%) recommend 6 cycles (46.7%) or 4–5 cycles (48.89%) of cisplatin and etoposide as first-line chemotherapy in patients with ES-SCLC.
Among respondents, 51% estimated a frequency of radiotherapy consultation for CTRT in more than 70% of patients with ES-SCLC responding to systemic therapy, as shown in Table 2.
Moreover, we asked to estimate the number of patients with ES-SCLC undergoing CTRT. The majority of radiation oncologists (71.7%) treats on average less than 10 patients per year; only 6.52%, as showed in Table 3, treats more than 20 patients per year.
Fifty-one percent would offer CTRT in all ES-SCLC patients with good response (complete or partial) after first-line chemotherapy. The responses, divided into different clinical scenarios, are listed in Table 4. Respondents were allowed to choose more than one option for this question.
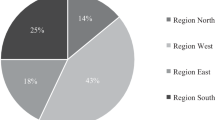
Regarding the definition of clinical target volume (CTV), 8.89% of respondents asserted that they would include the entire pre-chemotherapy thoracic disease extent in the CTV, 55.56% would treat only the post-chemotherapy residual disease and 35.56% would define the CTV somewhere “in between” the pre-chemotherapy and the residual disease, with two separated volumes and different dose levels. PET-CT appeared to be commonly used (79.2%) to delineate target volume. The survey allowed respondents to pick the following thoracic doses and fractionation schedule: 30 Gy in 3 Gy per fraction, 45 Gy in 3 Gy per fraction, 54–60 Gy in 2 Gy per fraction, > 60 Gy in 2 Gy per fraction and 45 Gy in hyperfractionation. The distribution of recommended schedule by survey respondents was showed in Fig. 1.
Discussion
The results of this radiation oncology Italian survey show the variability of practice occurring in the treatment of both LS and ES- SLCL. Historical trials have established CCRT as the mainstay of treatment for LS-SCLC and two meta-analyses published in 1992 reported an absolute survival benefit of 5.4% at 3 years and an improvement in local control from 24 to 47% at 2 years with CCRT [2,3,4,5, 12].
In our survey, we observed a general consensus on concurrent chemoradiation approach, although the proportion of treated CCRT patients in the actual clinical practice is quite low.
The timing of RT was not unanimous, with CCRT starting early, within the 2nd cycle, for 55.32% of respondents. Furthermore, there is no accordance in literature regarding CCRT timing [13, 14]. De Ruysscher et al. [15] have conducted a systematic review and literature-based meta-analysis to establish the most effective way of combining chest RT with chemotherapy for patients with LS-SCLC. The authors conclude that “when platinum-based chemotherapy concurrently with chest RT is used, the 2- and 5-year survival rates of patients with LS-SCLC may be in favor of early chest radiotherapy, with a significant difference if the overall treatment time of chest radiation is less than 30 days”.
The increasing use of dose escalation was also demonstrated (25% of respondents would offer > 60 Gy), according to previous prospective trials [16, 17]. A number of studies have shown that hyperfractionated radiotherapy was associated with promising results [18, 19]. The Intergroup 0096 trial showed that 45 Gy/1.5 Gy per fraction, delivered twice-daily over 3 weeks with concurrent cisplatin–etoposide, was superior to 45 Gy/1.8 Gy per fraction, once a day over 5 weeks [20]. Twice-daily radiotherapy improved 5 year survival from 16 to 26% at the cost of an increase in grade 3–4 oesophagitis from 16 to 32%. In our survey, 81.25% of respondents recommended a conventionally fractionated radiotherapy; only 12.5% would offer hyperfractionation. The poor adoption of this regimen in routine practice is probably due to logistical issues and increased toxicity.
A large multicenter international randomized phase III trial of CCRT in LS-SCLC (CONVERT TRIAL) comparing 45 Gy in 30 twice-daily fractions with 66 Gy in 33 daily fractions has been completed and preliminary results were presented at the 2016 ASCO Annual Meeting [21]. In this study, there was no statistically significant OS difference between the RT groups. One–year survival was 83% for the twice-daily RT arm and 76% for the daily arm. These inconclusive results suggest that both the schedules can be used in this setting. The ongoing randomized trial CALGB 30610 should shed more light on this issue. This randomized phase III trial is comparing three different chest radiation therapy regimens (70 Gy/2 Gy daily over 7 weeks, 61.2 Gy/1.8 Gy daily for 16 days followed by 1.8 Gy twice-daily for 9 days and 45 Gy/1.5 Gy twice-daily over 3 weeks),to investigate their safety and efficacy in LS-SCLC.
The use of PCI for LS-SCLC entered routine practice in 1999 after the publication of an individual patient’s data meta-analysis of seven trials [22]. The meta-analysis published by Auperin et al. did not show an effect on OS according to different fractionation schemes. A second meta-analysis with nearly identical results confirmed that PCI reduced the incidence of brain metastases and provided survival benefit [23]. Most recently, a phase III randomized trial was conducted to define the standard of care of PCI for LS-SCLC [24]. Patients in complete remission after first-line chemotherapy and thoracic radiotherapy were randomized to 25 Gy in 10 fractions or 36 Gy delivered with either conventional fractionation or an accelerated hyperfractionated regimen in 24 fractions (twice-daily 1.5 Gy per fraction). This study showed a non-significant reduction in the occurrence of brain metastasis, but a worsening of overall survival in the high dose group at 2 years (42% versus 37%, P = 0.05). A more recent retrospective study of a large number of patients with LS-SCLC suggests that elderly patients with large tumors may not benefit from PCI in terms of OS, even after a complete response to definitive chemoradiation therapy [25].
Most Italian radiation oncologists (97,96%) would offer PCI not only in patients in complete remission, but even in patients with partial or stable response after chemotherapy and dose and fractionation schedule was almost unanimously 25 Gy in 10 daily fractions.
The use of PCI has been evaluated in patients with ES-SCLC who responded to initial chemotherapy. The earliest meta-analysis [22] included 14% of patients with stage IV disease and showed a benefit that was similar to that of limited-stage disease, but only in patients with complete response to systemic treatment.
As clearly reported by Kim, to date, there have been two clinical trials with opposite results regarding PCI for patients with ES-SCLC [26]. A phase III study from European Organization for Research and Treatment of Cancer (EORTC) randomized 286 patients to either PCI or no radiation after any response to chemotherapy [9]. This trial showed a statistically significant improvement in median OS in the PCI group, but it was recently debated, because routine pre-PCI brain imaging was not performed, and concerns about long-term neurocognitive dysfunction arose [27]. A Japanese trial, presented to the 2016 ASCO annual meeting, randomized patients with ES-SCLC and a negative magnetic resonance of the brain to PCI or no radiation after any response to chemotherapy [28]. The interim analysis of this study found no superiority for the PCI group and the study was terminated. The investigators reported inferior survival rates for the patient group undergoing PCI. A recent propensity score matched analysis showed that treatment with PCI (n¼ 473) was associated with improved survival (hazard ratio, 0.66; 95% confidence interval, 0.60–0.74; P < 0.0001), in terms of median OS (13.9 vs. 11.1 months; P < 0.0001), as well as 1- and 2-year OS (61.2 vs. 44.0 and 19.8 vs. 11.5%, respectively; P < 0.0001) [29]. Despite the conflicting studies, according to our survey, most of respondents would offer PCI with even a partial or minimal response to initial therapy. Also, a recent analysis of practice pattern among US radiation oncologists revealed that 98% offers PCI to patients with ES-SCLC after a response to systemic chemotherapy, according to current National Comprehensive Cancer Network guidelines [30, NCCN].
The use of thoracic consolidation RT for patients with ES-SCLC who achieved clinical response to chemotherapy is supported by current NCCN guidelines, but patient selection and RT doses and volumes are not well-defined in literature. Past studies showed mixed results with no definite support for consolidation thoracic RT in this set of patients [NCCN 31,32,33,34]. More recently, Jeremic et al. published the first trial to address the use of radical thoracic RT given with modern platinum–etoposide chemotherapy in ES-SCLC [35]. In this study, thoracic RT achieved significantly better results than CT alone (patients in both arms underwent PCI): the median survival was 17 months versus 11 months, and 5-year OS rate was 9.1% versus 3.7%, respectively. More than 15 years after this study, the Duch Chest Radiotherapy in Extensive Stage (CREST) trial was published [8]. Patients with ES-SCLC who achieved any response to 4–6 cycles of platinum–etoposide chemotherapy were randomized to receive either thoracic RT (30 Gy in 10 fractions) and PCI or PCI alone. This trial showed an increase in PFS and OS with thoracic RT, with nearly a 50% reduction of intrathoracic recurrences. A more recent SEER (Surveillance, Epidemiology, and End Results Program) database analysis showed an improvement in 2-year OS from 2.5 to 6% and median OS from 4 to 7 months with thoracic RT in patients with ES-SCLC [36].
Additional analysis on the CREST data demonstrated that only patients with residual intrathoracic disease following chemotherapy had a benefit from thoracic RT [37]. In our survey, 51% of respondents would offer thoracic RT in all ES-SCLC patients with good response (complete or partial) after first-line chemotherapy, despite the CREST trial results.
The major limit of the present study is the low response rate (6%), although this is similar to other oncology surveys (10%) [38, 39]. One possible reason is that usually only clinicians who are interested in the topic or who deal with it in their daily practice tend to complete the survey. This would definitely lower the response rate. As a matter of fact, when we asked if in the RT Center, clinicians were divided into anatomical district-based groups, > 55% answered they were dedicated (also) to lung cancer, 36% that they were not dedicated to any disease, and only 9% of respondents was not involved in lung tumor treatment. Moreover, “young” specialists or in training clinicians might be hesitant to answer questionnaires about such a specific topic: only 6% of respondents have less than 5 years of experience in RT. Furthermore, as for all surveys, wording of questions and limited available information or response options provided may have influenced the results.
Our paper represents the first survey on SCLC open questions in Italian radiation oncology scenario and its results can help to optimize the treatment of SCLC.
References
Govindan R, Page N, Morgensztern D et al (2006) Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24:4539–4544
Jett JR, Schild SE, Kesler KA et al (2013) Treatment of small cell lung cancer: diagnosis and management of lung cancer, 3rd edn: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e400S-19
Früh M, De Ruysscher D, Popat S et al (2013) Small-cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24:99–105
Pignon JP, Arriagada R, Ihde DC et al (1992) A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 327:1618–1624
Warde P, Payne D (1992) Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 10:890–895
Lally BE, Urbanic JJ, Blackstock AW et al (2009) Small cell lung cancer: have we made any progress over the last 25 years? Oncologist 12:1096–1104
Riess JW, Lara PM (2014) Left behind? Drug discovery in extensive stage small cell lung cancer. Clin Lung Cancer 15:93–95
Slotman BJ, van Tinteren H, Praag JO et al (2015) Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 385:36–42
Slotman B, Faivre-Finn C, Kramer G et al (2007) Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 357:664–672
Fonseca R, O’Neill BP, Foote RL et al (1999) Cerebral toxicity in patients treated for small cell carcinoma of the lung. Mayo Clin Proc 74:461–465
Greenspoon JN, Evans WK, Cai W et al (2011) Selecting patients with extensive-stage small cell lung cancer for prophylactic cranial irradiation by predicting brain metastases. J Thorac Oncol 6:808–812
Lad T, Piantadosi S, Thomas P et al (1994) A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 106(suppl):320S–323S
Murray N, Coy P, Pater JL et al (1993) Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 11:336–344
Spiro SG, James LE, Rudd RM, on behalf of the London Lung Cancer Group et al (2006) Early compared with late radiotherapy in combined modality treatment for limited disease small cell lung cancer: a London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J Clin Oncol 24:3823–3830
De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J et al (2006) Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol 17:543–552
Salama JK, Hodgson L, Pang H, on behalf of Cancer and Leukemia Group B et al (2013) A pooled analysis of limited-stage small cell lung cancer patients treated with induction chemotherapy followed by concurrent platinum-based chemotherapy and 70 Gy daily radiotherapy: calgb 30904. J Thorac Oncol 8:1043–1049
Komaki R, Paulus R, Ettinger DS et al (2012) Phase II study of accelerated high-dose radiotherapy with concurrent chemotherapy for patients with limited small-cell lung cancer: Radiation Therapy Oncology Group protocol 0239. Int J Radiat Oncol Biol Phys 83:e531–e536
Jeremic B, Shibamoto Y, Acimovic L et al (1997) Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: a randomized study. J Clin Oncol 15:893–900
Takada M (2002) Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 20:3054–3060
Turrisi AT, Kim K, Blum R et al (1999) Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 340:265–271
Faivre-Finn C, Snee M, Ashcroft L et al (2016) CONVERT: an international randomised trial of concurrent chemo-radiotherapy comparing twice-daily and once-daily radiotherapy schedules in patients with limited stage small cell lung cancer and good performance status. In: 2016 ASCO annual meeting. Abstract 8504
Auperin A, Arriagada R, Pignon JP et al (1999) Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 341:476–484
Meert AP, Paesmans M, Berghmans T et al (2001) Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer 1:5
Le Pèchoux C, Dunant A, Senan S et al (2009) Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 10:467–474
Damhuis RAM, Senan S, Belderbos JS (2018) Usage of prophylactic cranial irradiation in elderly patients with small-cell lung cancer. Clin Lung Cancer 19(2):e263–e267
Kim YH (2016) Prophylactic cranial irradiation for extensive-stage small cell lung cancer. J Thorac Oncol. 11(12):e151–e152
Gondi V, Paulus R, Bruner DW et al (2013) Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys 86:656–664
Seto T, Takahashi T, Yamanaka T et al (2014) Prophylactic cranial irradiation (PCI) has a detrimental effect on the overall survival (OS) of patients (pts) with extensive disease small cell lung cancer (ED-SCLC): results of a Japanese randomized phase III trial [abstract 7503]. J Clin Oncol 32:5s
Sharma S, McMillan MT, Doucette A et al (2017) Effect of prophylactic cranial irradiation on overall survival in metastatic small-cell lung cancer: a propensity score-matched analysis. Clin Lung Cancer. https://doi.org/10.1016/j.cllc.2017.12.003
Jain A, Luo J, Chen Y et al (2016) Current patterns of care for patients with extensive-stage SCLC: survey of U.S. radiation oncologists on their recommendations regarding prophylactic cranial irradiation. J Thorac Oncol 11:1305–1310
Livingston RB, Moore TN, Heilbrun L et al (1978) Small-cell carcinoma of the lung. Combined chemotherapy and radiation. A Southwest Oncology Group study. Ann Intern Med 88:194–199
Dillman RO, Taetle R, Seagren S et al (1982) Extensive disease small cell carcinoma of the lung. Trial of non-cross resistant chemotherapy and consolidation radiotherapy. Cancer 49:2003–2008
Nou E, Brodin O, Bergh J (1988) A randomized study of radiation treatment in small cell bronchial carcinoma treated with two types of four-drug chemotherapy regimens. Cancer 62:1079–1090
Beith JM, Clarke SJ, Woods RL et al (1996) Long-term follow-up of a randomized trial of combined chemoradiotherapy induction treatment with and without maintenance chemotherapy in patients with small cell carcinoma of the lung. Eur J Cancer 32A:438–443
Jeremic B, Shibamoto Y, Nikolic N et al (1999) Role of radiation therapy in the combined modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol 17:2092–2099
Mahmoud O, Kwon D, Greenfield B et al (2016) Intrathoracic extensive-stage small cell lung cancer: assessment of the benefit of thoracic and brain radiotherapy using the SEER database. Int J Clin Oncol. 21(6):1062–1070
Palma DA, Warner A, Louie AV et al (2015) Thoracic radiotherapy for extensive stage small-cell lung cancer: a meta-analysis. Clin Lung Cancer 17:239–244
Shahi J, Wright JR, Gabos Z et al (2016) Management of small-cell lung cancer with radiotherapy—a pan-Canadian survey of radiation oncologists. Curr Oncol 23:184–195
Nabivizadeh N, Elliott DA, Chen Y et al (2016) Image guided radiation therapy (IGRT) practice patterns and IGRT’s impact on workflow and treatment planning: results from a national survey of American Society for Radiation Oncology members. Int J Radiat Oncol Biol Phys 94:850–857
Funding
No specific fundings to be declared.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
All authors disclose no actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations that could inappropriately influence their work.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Ciammella, P., Timon, G., Bruni, A. et al. Radiation therapy in small cell lung cancer: a national Italian survey. Radiol med 123, 554–560 (2018). https://doi.org/10.1007/s11547-018-0868-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-018-0868-5