Abstract
Introduction
Blood perfusion of liver metastases can be non-invasively assessed by dynamic contrast enhanced magnetic resonance imaging (DCE-MRI). The aim of this study was to explore whether the ratio of hepatic arterial to total liver blood flow (Hepatic Perfusion Index—HPI) and the area under the enhancement curve (AUC) of selected liver areas in patients with hepatic metastases from colorectal cancer treated with first-line chemotherapy could predict response and/or be a prognostic variable.
Patients and methods
Sequential liver DCE-MRI studies with morphological imaging reconstruction were performed in 43 consecutive patients at baseline and every 3 months during oxaliplatin-based first-line chemotherapy. Data about HPI of the whole liver, and AUC of metastatic and healthy areas were calculated at each time-point and compared both at baseline and sequentially during the treatment.
Results
Baseline HPI and AUC values did not discriminate patients responsive to chemotherapy, nor those with better survival outcomes. HPI and AUC values at 3 months decreased significantly more in responders than non-responders. AUCs calculated from areas of the liver with or without neoplastic lesions varied consistently, being increased in progressing patients and decreased in responding patients.
Discussion
Our results did not support the hypothesis of a predictive or prognostic role of HPI and AUCs calculated by DCE-MRI in liver metastatic CRC patients, thus the primary endpoint of the study was not reached. However, reduced arterial blood flow in metastatic liver can be obtained by chemotherapy alone, without any anti-angiogenic agent; interestingly, HPI and AUC data suggest a possible relationship between tumor metabolism and entire liver perfusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) accounted for more than one million new cases and nearly 700,000 deaths worldwide in 2012 [1] and continues to be a relevant health and social problem. Hepatic metastases are a secondary site in approximately one third of the advanced disease patients [2]. Curative resection in selected patients has been proposed since early ‘60s and became largely accepted in the ‘80s [3], because a higher proportion of long-term survivors was observed with the surgical approach than in unselected series of patients treated with chemotherapy alone. Subsequently, in patients with unresectable hepatic metastases who obtained a response to chemotherapy, the subsequent hepatic surgery was associated with improved outcomes [4]. Consequently, medical oncologists are looking for active chemotherapy regimens that may give the patients the highest probability of tumor shrinkage. In this context, any predictive and/or prognostic variable that may help to identify the best therapeutic strategy would be clinically useful.
The efficacy of chemotherapy depends not only on drug pharmacodynamics, but also on several other factors, such as the delivery of cytotoxic drugs through the tumor vasculature, drug uptake and retention in tumor cells, metabolic activation of pro-drugs, intrinsic chemosensitivity of tumor cells, catabolism and excretion of drugs, and by the total amount of drugs reaching tumor cells. Tumor blood flow in liver metastases and its changes following therapy is easily detectable by imaging techniques based on dynamic evaluation. Portal vein perfusion accounts for 60–80 % of the total physiological liver blood supply, because only a limited proportion of blood supply to the normal liver comes through the arterial vessels, whereas in liver metastases the vascular supply derives predominantly from the hepatic artery. Consequently, liver with metastases has a higher arterial blood flow than normal hepatic tissue. The ratio of hepatic arterial to total liver blood flow (hepatic perfusion index, HPI) was first investigated using dynamic scintigraphy and was found to be abnormal in 88 and 58 % of colorectal cancer patients with and without liver metastases, respectively [5]. Subsequently, HPI measurement methodology was adapted to dynamic CT, Doppler ultrasound, and finally to dynamic contrast-enhanced MR Imaging (DCE-MRI). HPI has been demonstrated to be a prognostic indicator of early liver recurrence, both in colorectal and in esophageal cancer patients [6–8]. In these studies, HPI was determined preoperatively in patients without metastases who underwent curative resection of the primary tumor, and patients with higher HPI presented shorter disease free and overall survival. In the metastatic setting hepatic basal HPI, measured by DCE-MRI, was increased in metastatic patients [9] but no data are available about HPI changes following chemotherapy.
Using DCE-MRI, the area under the enhancement curve (AUC) is another parameter that can be used to assess the blood flow of selected areas of the liver. Decreases in AUC calculated from a region-of-interest (ROI) including the whole liver were demonstrated to correlate with tumor shrinkage and with a better time to progression in patients treated with standard chemotherapy plus bevacizumab [10, 11].
However, in all the above-mentioned studies, imaging acquisition and reconstruction protocols did not allow a morphological evaluation of the liver, preventing any measurement of the metastases. Thus, to assess chemotherapy activity, additional CT or FDG-PET scan should be performed, with time consumption and additional costs. The aim of this study was to prospectively evaluate the correlation between HPI and activity of first-line chemotherapy in terms of response rate and survival, and to assess the potential role of AUC computation in normal and neoplastic hepatic areas by DCE-MRI, based on a protocol of image acquisition and reconstruction that in addition allows the morphological evaluation of the liver.
Patients and methods
Study design
Patients with liver metastases from colorectal cancer, without contraindications for first-line chemotherapy, received an abdominal DCE-MRI at baseline, at 3 months, and eventually at 6 months after the initiation of chemotherapy. Chemotherapy consisted in 5-fluorouracil (5-FU) or capecitabine associated to oxaliplatin, started within 1 month from baseline DCE-MRI. The study aimed to demonstrate an increase in overall response rate (ORR) in patients with HPI values >0.3 (HPI high group) with respect to those with HPI ≤0.3 (HPI low group). Hypothesizing an ORR of 50 % in HPI high group and of 25 % in HPI low group, with α error of 0.5 and β error of 0.2, the total number of patients to be enrolled was 106 (53 per arm). Patients gave their written consent and protocol was approved by our Local Ethical Committee. All the applied procedures followed the Helsinki Declaration.
Treatment response was assessed repeating the same MRI technique and the best tumor response was classified according to the RECIST criteria version 1.1 [12].
DCE-MRI method
DCE-MRI was performed by the mean of a Philips Achieva 1.5 T scanner, administering intravenously an extracellular contrast agent (gadobutrol 1 mmol/mL—Gadovist®) at a total dose of 0.1 ml/kg. Images were acquired through T1 weighted sequences and interpreted both morphologically and dynamically through a specific perfusion sequence. In particular, during the administration of gadobutrol at an injection rate of 4 mL/s, 16 dynamic phases were acquired. HPI and AUC were calculated using Philips ViewForum Perfusion T1 Software.
HPI
HPI represents the ratio of hepatic arterial to total liver blood flow and it is calculated from a time intensity curves derived from regions of interest (ROI) drawn manually in the aorta, liver and spleen. The ROIs in the liver were drawn to encompass the parenchyma and metastases but no major vessels. To estimate HPI, the “combined method” by White et al. [13] was used. Arterial perfusion (P art) is calculated by dividing the peak gradient in the liver during arterial phase (g art) by the peak enhancement of the aorta (I aorta), while portal perfusion (P port) is derived from gradient after subtraction of the arterial component from the liver curve (g *port ), normalized by the enhancement of the aorta (I aorta). On the basis of previous studies assessing HPI values in healthy subjects and in patients with clinically detected hepatic metastases in patients with CRC, values above 0.3 were considered as abnormal [8, 14].
AUC
While HPI is calculated considering the entire hepatic parenchyma, tumour metastases AUC was evaluated on a single metastatic nodule, followed throughout the entire study as a target lesion, drawing manually a ROI of 20 pixel in the hyperintense zone of the metastasis to exclude necrotic and not vascularized areas. Normal liver AUC was calculated drawing a 20 pixel ROI in apparently non-metastatic liver parenchyma. The AUC was calculated as the area under the time-intensity curve of the selected ROI over the entire procedure, normalized by the time of imaging acquisition expressed in seconds. Patients were then divided into two groups according whether their AUC values were greater than or less than or equal to 1000. This cut-off threshold was chosen as it represents the median of baseline normal AUC values of our patients.
Survival evaluation
Progression free survival and overall survival were estimated from the start of systemic treatment until disease progression or death or date of the last follow-up. The cut-off date for statistics analyses was March 15th, 2015. Patients not progressing or alive at the time of data analyses were censored at the time of the last follow-up examination.
Statistical analyses
HPI
To explore the relationship between HPI and response to chemotherapy, patients were divided into two groups: responders vs non-responders (including progressive or stable disease). HPI values at baseline, 3 and 6 months of the two groups were then compared using the Mann–Whitney U non-parametric test for unpaired variables. The same test was used to compare HPI variations along time of the two patient groups. Patients were then grouped according to HPI values at baseline (HPI <0.3 vs HPI ≥0.3). Proportions of responding patients in each group were compared using the Chi-square test with Yates correction, if appropriate. Progression free survival and overall survival for each group were calculated and plotted using the Kaplan–Meier method and compared using the log-rank test.
AUC
Similarly to HPI, patients were grouped according to tumor response and AUC values and their variations along time were compared using the Mann–Whitney U test. Correlation coefficients (r) between tumor and normal AUC were calculated and validated according to the Spearman-Rank method. Progression free survival and overall survival for patients stratified according the cut-off value of 1000 were calculated and plotted using the Kaplan–Meier method and compared using the log-rank test. Finally, patients were grouped according whether their tumor progressed at 3 months, responded or remained stable at 3 months and then progressed at 6 months, or responded or remained stable at 6 months. Differences in AUC values between groups were compared and validated using the Kruskal–Wallis analysis of variance.
These statistical computations were performed using the SPSS for Windows Ver 22.0 and STATISTICA for Windows Ver 8.0 softwares.
Results
Patients’ characteristics
From March 2008 to September 2012, a total of 43 consecutive patients entered the study. Recruitment was prematurely stopped due to low enrollment rate. Patients’ characteristics are summarized in Table 1. All the patients but one had synchronous metastases. Males were predominant (25/43, 58.1 %) and more than two-third of the primary tumors were located in the colon (30/43, 69.8 %). Finally, the most frequent site of extra-liver metastases was the lung (20/43 patients, 46.5 %).
All patients received oxaliplatin combined with a fluoropyrimidine: 5-FU (FOLFOX scheme) in 38 patients, or capecitabine (XELOX scheme) in 5 patients. Globally after the first 3 months of therapy, 29 patients had objective response (67.4 %), 7 stable disease (16.3 %), whereas 7 progressed (16.3 %). Among the 29 responding patients, 8 subsequently were treated with surgical resection (n = 6) or radiofrequency ablation (n = 2) for liver metastases. For the subsequent analyses, patients were grouped into two subgroups according to the clinical response: 29 responding patients vs 14 non-responding patients (7 with stable and 7 with progressive disease).
At the data cut-off of March 15th, 2015, a total of 41 patients (95.3 %) had experienced disease progression, with a median time to progression (TTP) of 9.8 months. At the same time point, after a median follow-up period of 35.5 months, 38 patients (88.4 %) died, with a median overall survival (OS) of 20.8 months.
HPI
Data on HPI were obtained from 42 patients at baseline (in one patient it was not obtained, due to insufficient apnea time); from 41 patients at 3 months (one patient progressed and one was submitted to liver surgery before 3 months of therapy); and from 26 patients at 6 months (seven patients progressed at 3 months, eight was submitted to liver surgery or local ablation of the metastases, and two failed to obtain HPI data due to technical reasons).
Median (range) HPIs were: 0.249 (0.139–0.881) at baseline, 0.294 (0.127–0.590) at 3 months, and 0.241 (0.142–0.676) at 6 months. According to response to chemotherapy, median (range) HPIs at baseline were: 0.224 (0.147–0.881) for responders, and 0.253 (0.139–0.563) for non-responders (p = 0.78). According to the chosen HPI cut-off of 0.3, 18/26 (69.2 %) patients with low HPI values and 10/15 (66.6 %) patients with high HPI values responded to chemotherapy (p = 0.85).
Median (range) HPIs at 3 months for patients according to clinical response were: 0.222 (0.127–0.590) and 0.433 (0.182–0.527) for responders and non-responders, respectively (Fig. 1; p = 0.001). Overall HPI change between the two time points (baseline and 3 months) varied by a 10.3 % (range −74.8 to +212.4 %). The same figure was −5.9 % (−74.8 to +173.6 %) and 60.6 % (−7.6 to +212.4 %) in responders and non-responders, respectively (p = 0.003).
Median (range) HPI at 6 months for responders was 0.215 (0.142–0.458) vs 0.290 (0.157–0.676) for non-responders (p = ns). No statistically significant differences neither in HPI values nor in their relative variations was demonstrated when patients were stratified according to tumor response between 3 and 6 months time points.
Differences between groups in median TTP and OS did not reach statistical significance (TTP: 11.0 vs 8.7 months; OS: 24.7 vs 14.9 months, for patients with basal HPI <0.3 and >0.3, respectively).
AUC
Data on liver metastasis AUC were obtained in 39 patients at baseline, in 36 patients at 3 months, and in 24 patients at 6 months. The same figures for normal liver AUC were: 39, 37, and 24 patients, respectively. Median image acquisition time was 285 s (range 180–590 s).
Median (range) lesioned AUCs were: 973.1 (275.2–2036.6) at baseline, 894.0 (449.6–2237.0) at 3 months, and 727.3 (373.7–1741.7) at 6 months. The same figures for healthy liver AUCs were: 688.4 (182.0–1441.7), 633.6 (355.4–2163.5), and 724.2 (418.4–1188.9), respectively. A direct correlation between tumor and normal liver AUCs was evident at each time point. The relative correlation coefficients (r) were: 0.67 at baseline (p < 0.05), 0.65 at 3 months (p < 0.05), and 0.47 at 6 months (p < 0.05).
Median (range) AUC change between baseline and 3 months were: −15.6 % (−57.4 to 127.3 %) in tumor areas and −14.8 % (−59.7 to 261.1 %) in normal liver (correlation r = 0.61, p < 0.05). The same figures between 3 and 6 months were: −17.1 % (−53.0 to 85.9 %) and 22.6 % (−36.9 to 139.3 %), respectively (correlation r = 0.32, p = 0.05).
Tumor and normal liver tissue AUCs and their changes according to time points and response to chemotherapy are shown in Table 2. A difference in normal liver AUCs at 3 months was shown in responding vs non-responding patients (absolute median values: 591.9 vs 740.1, p = 0.05; change: −17.9 vs −6.9 %, p = 0.05).
When patients were grouped according to baseline AUC values using the cut-off value of 1000 (<1000 AUC low; >1000 AUC high), no difference in median TTP or in median OS was demonstrated (Table 3). Similar results were obtained when patients were divided using an arbitrary cut-off of 30 % in AUC variation at 3 months compared to baseline.
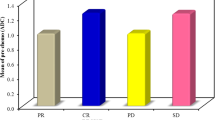
As an exploratory unplanned analysis, patients were further divided into three groups: patients progressing at 3 months (G1; 7 patients), patients not progressing at 3 months and progressing at 6 months (G2; 7 patients), and patients who were progression-free at 6 months (G3; 29 patients). As far as normal liver AUC was concerned, in G1 median values increased at 3 months compared to baseline (551.2 vs 873.4, p = ns); in G2 decreased at 3 months and remained stable at 6 months (917.8 vs 704.5 vs 654.5; p = 0.03); in G3 decreased at 3 months and then slightly increased at 6 months (688.4 vs 591.6 vs 727.2; p = ns) (Fig. 2a). When considering tumor tissue AUCs, median values increased in G1 (728.7 vs 1091.5, p = 0.04); remained stable in G2 (895.2 vs 928.0 vs 999.3; p = ns); and decreased in G3 (991.3 vs 833.7 vs 709.8; p = ns) (Fig. 2b).
Healthy (a) and lesioned (b) liver AUCs at baseline, 3 and 6 months (when the case) for patients stratified according whether they progressed at 3 months (P), the did not progressed at 3 months and progressed at 6 months (No P → P), or they never progressed (no P). Squares are median values, boxes are quartiles, lines are extremes
Discussion
Analysis of DCE-MRI data is based on continuous image acquisition lasting several minutes and thus also during patient free-breathing. This condition, however, does not allow obtaining good anatomical image reconstructions due to motion artifacts, preventing size measurement. In our study, we captured images under breath-hold conditions during 16 dynamic phases. While this procedure did not affect HPI algorithm, the graph of signal intensity over time from which AUC is calculated was dependent from the total time of image acquisition, different for any single scan. In fact, scan total time ranged from 180 up to 590 s, with a variability of more than 220 %. Then, to standardize data we normalized results dividing AUC for the total duration of the procedure, thus obtaining a median intensity value per second. It is worth to remember that the plasmatic half-life of the contrast agent is sufficiently long (1.8 h) [15] to prevent significant decrease of signal intensity in the time range of our acquisitions.
Another possible bias that may lead to discordant results could be the difference in magnetic induction power of the scanners. As an example, Hirashima et al. [10] used a 3-T whole-body magnet, giving a higher signal performance than our scanner of 1.5 T. Again, this difference is supposed to influence more AUC computations rather than HPI, as the latter is a proportion of data obtained from the same scanner. Finally, while HPI was calculated drawing the ROI including the entire liver, AUC data derived from an area chosen by the operator and this may contribute to jeopardize results.
Considering the clinical impact of our study, ORR in the two HPI arms (HPI >0.3 vs HPI <0.3) could be calculated and was similar (66.2 vs 69.2 %). Thus, we can conclude that the DCE-MRI assessment in our limited series of patients did not support the hypothesis of a difference in ORR according to HPI at baseline, primary endpoint of the study. Moreover, the data of our study in metastatic colorectal cancer patients did not support the hypothesis of any correlation between the HPI changes during first line systemic chemotherapy, or AUCs of metastases or normal liver, and chemotherapy activity in terms of tumor response, TTP and OS. Nevertheless, interesting findings were reported including the reduction of arterial blood flow in metastatic liver generated by chemotherapy alone without adding any anti-angiogenic agent. Moreover, HPI and AUC data suggest a possible control of the entire liver vasculature by substances directly produced and released into the bloodstream by tumor cells such as inflammatory cytokines.
HPI data of our study agree with others already published [16], with median baseline HPI value of 0.249, suggesting a reproducibility of this variable. Unfortunately, we failed to demonstrate a predictive role of baseline HPI because chemotherapy activity was not superior in those patients with higher HPI. Even though not statistically significant, a longer TTP and OS was observed in patients with HPI <0.3. A lower tumor aggressiveness expressed as a lower level of neo-angiogenesis could explain this observation. However, it would worth to verify this finding in a higher number of patients. HPI values decreased in responding patients whereas it increased in those non-responding. This observation is in line with the hypothesis that active chemotherapy impact on the arterial vasculature of the metastases, showing an anti-angiogenic activity. Furthermore, even though generally reduced, HPI did not significantly vary between 3- and 6-month time points in responding and non-responding patients. This could be explained by the fact that progressive patients withdrawn the study at 3 months, and thus only those patients with clinical response or disease stabilization (i.e. those with at least a minimal antitumoral response to chemotherapy) continued up to 6 months of therapy, reducing sample size and smoothing differences between groups.
Tumor and normal tissue AUCs were not correlated to chemotherapy activity or to survival. The above reported limitations and biases could have also accounted for these negative results. Our data do not support the use of this parameter in a routine clinical setting outside of an experimental trial. Both tumor and normal liver area AUCs increased in progressing patients, a slightly different trend was shown in those patients not progressing at 3 months and progressing at 6 months. In this patients tumor AUC remained stable throughout the observation time with an increasing trend, whereas normal liver AUC initially decreased and then remained stable. We believe this preliminary observation should be tested in a larger number of patients to verify whether variations of tumor AUC could be an early predictor of tumor progression, as this might be useful in the early switch to other active chemotherapeutic regimens.
Despite these negative results, some interesting findings are worth of discussion. First, it should be pointed out that in our study HPI reduction was observed following chemotherapy administration alone without the addition of an anti-neoangiogenetic agent as published elsewhere [17]. This finding raises several doubts about the interpretation of those studies in which reduction of perfusion parameters after administration of chemotherapy combined with anti-angiogenetics have been indicated as an efficacy index of this latter class of agents.
Second, we have shown a direct correlation between tumor and normal liver AUCs and their variations at each time point. This could be easily explained by external factors such as the total amount of contrast agent administered at each time. What was surprising is the correlation between both AUCs and tumor response. In progressing patients both tumor and normal tissue AUCs increased, whereas an inverse pattern was demonstrated in responding patients. This was unexpected as metastases have a higher arterial blood flow than normal liver due to the tumor neo-angiogenesis and thus only lesioned AUCs were supposed to be influenced by tumor response. This might suggest that growth factors, cytokines and inflammatory mediators produced by tumor cells or by tumor environment are able to influence the microcirculation of the liver. Furthermore, the correlation coefficient of 0.6 between changes in tumor vs normal AUCs accounts for the observation of a decrease in HPI in responding patients, as in these patients arterial blood reduced more than portal blood flow.
Conclusion
HPI could be easily assessed by routine DCE-MRI. While its putative prognostic role should be analyzed in a larger number of patients, its baseline values did not support the idea of a correlation between response to first line chemotherapy and patient outcomes. AUC assessed by an image acquisition protocol which permits morphological evaluation of the liver metastases did not demonstrate to be useful in predicting response rate and survival and it should be eventually reserved to the experimental setting.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics. CA Cancer J Clin 65:87–108
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM (2006) Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 244(2):254–259
Fong Y, Blumgart LH, Cohen AM (1995) Surgical treatment of colorectal metastases to the liver. CA Cancer J Clin 45(1):50–62
Adam R, Wicherts DA, de Haas RJ, Ciacio O, Lévi F, Paule B et al (2009) Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 27(11):1829–1835
Warren HW, Gallagher H, Hemingway DM, Angerson WJ, Bessent RG, Wotherspoon H et al (1998) Prospective assessment of the hepatic perfusion index in patients with colorectal cancer. Br J Surg 85(12):1708–1712
Fujishiro T, Shuto K, Hayano K, Satoh A, Kono T, Ohira G et al (2014) Preoperative hepatic CT perfusion as an early predictor for the recurrence of esophageal squamous cell carcinoma: initial clinical results. Oncol Rep 31(3):1083–1088
Sarela AI, Gallagher HJ, Macadam RC, O’Riordain DS, Parkin A, Guillou PJ (2000) Abnormal hepatic perfusion index predicts recurrence of colorectal carcinoma. Colorectal Dis 2(6):346–350
Leen E, Goldberg JA, Angerson WJ, McArdle CS (2000) Potential role of doppler perfusion index in selection of patients with colorectal cancer for adjuvant chemotherapy. Lancet 355(9197):34–37
White MJ, O’Gorman RL, Charles-Edwards EM, Kane PA, Karani JB, Leach MO et al (2007) Parametric mapping of the hepatic perfusion index with gadolinium-enhanced volumetric MRI. Br J Radiol 80(950):113–120
Hirashima Y, Yamada Y, Tateishi U, Kato K, Miyake M, Horita Y (2012) Pharmacokinetic parameters from 3-Tesla DCE-MRI as surrogate biomarkers of antitumor effects of bevacizumab plus FOLFIRI in colorectal cancer with liver metastasis. Int J Cancer 130(10):2359–2365
De Bruyne S, Van Damme N, Smeets P, Ferdinande L, Ceelen W, Mertens J et al (2012) Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br J Cancer 106(12):1926–1933
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
White MJ, O’Gorman RL, Charles-Edwards EM, Kane PA, Karani JB, Leach MO et al (2007) Parametric mapping of the hepatic perfusion index with gadolinium-enhanced volumetric MRI. Br J Radiol 80(950):113–120
Leen E, Angerson WJ, Wotherspoon H, Moule B, Cooke TG, McArdle CS (1995) Comparison of laparotomy, CT, US and DPI in the detection of colorectal liver metastases. Radiology 195:113–116
Kunze C, Mentzel HJ, Krishnamurthy R, Fleck R, Stenzel M, Bhargava R et al (2016) Pharmacokinetics and safety of macrocyclic gadobutrol in children aged younger than 2 years including term newborns in comparison to older populations. Invest Radiol 51(1):50–57
Totman JJ, O’gorman RL, Kane PA, Karani JB (2005) Comparison of the hepatic perfusion index measured with gadolinium-enhanced volumetric MRI in controls and in patients with colorectal cancer. Br J Radiol 78(926):105–109
Miyazaki K, Collins DJ, Walker-Samuel S, Taylor JN, Padhani AR, Leach MO et al (2008) Quantitative mapping of hepatic perfusion index using MR imaging: a potential reproducible tool for assessing tumour response to treatment with the antiangiogenic compound BIBF 1120, a potent triple angiokinase inhibitor. Eur Radiol 18:1414–1421
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Tampellini, M., Gned, D., Baratelli, C. et al. Changes in hepatic perfusion assessed by dynamic contrast enhanced MRI, associated with morphologic evaluation, in patients with liver metastases from colorectal cancer treated with first-line chemotherapy. Radiol med 121, 950–957 (2016). https://doi.org/10.1007/s11547-016-0685-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-016-0685-7