Abstract
Purpose
To investigate the role of orbital color Doppler ultrasound (OCDUS) in the diagnosis of carotid-cavernous fistula (CCF) with anterior drainage and particularly whether a negative OCDUS could avoid an invasive diagnostic cerebral angiography (DSA).
Materials and methods
Twenty-two consecutive patients with ophthalmic signs suspecting CCF were submitted to ophthalmologic examination, OCDUS and DSA. CCF diagnosis with OCDUS was based on the finding of a reversed, arterialized and low-resistive-index (RI <0.5) blood flow in the superior ophthalmic vein (SOV). Sensibility, specificity, PPV, NPV, and accuracy of OCDUS were calculated considering both patients and eyes, using DSA as gold standard.
Results
DSA demonstrated 20 CCFs in 18 patients. Considering the patients, in 18/22 CCF diagnosis was positive at OCDUS and DSA while 4/22 were negative at both. Considering the eyes, in 24/43 CCF diagnosis was positive at both DSA and OCDUS (total eyes = 43, due to one case of SOV thrombosis). In 19/43 eyes diagnosis was negative at both OCDUS and DSA. So sensitivity, specificity, PPV, NPV, and accuracy of OCDUS in the patients and eyes analysis were all 100 %.
Conclusions
OCDUS is a reliable, noninvasive tool in the diagnosis of CCF; a negative OCDUS could avoid an invasive DSA in patients suspected for anterior-draining CCF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotid-cavernous fistulas (CCFs) represent a relatively rare, abnormal communication between the carotid arterial system and the surrounding cavernous sinus. They can be divided into direct and indirect fistulas based on their flow rate and source of feeder vessels [1]. In direct fistulas, the intra-cavernous portion of the internal carotid artery develops a direct shunt with the cavernous sinus. They are usually post-traumatic and characterized by a high flow [2]. Indirect fistulas, also called dural arteriovenous fistulas, derive from communications between the dural branches of the internal or external carotid artery and the intracranial venous sinuses. They are usually spontaneous, and their flow rates may be lower [3]. The clinical symptoms are highly dependent on the fistula’s venous drainage pattern. The arterial blood entering the cavernous sinus can usually exit anteriorly through the ophthalmic veins, causing ophthalmologic symptoms due to the reversed blood flow. Congestion of the venous structures determines ocular symptoms such as exophthalmos, chemosis, conjunctival congestion, and glaucoma [3]. These clinical signs are more evident in high-flow fistulas, such as in direct fistulas, while dural fistulas frequently have delayed diagnosis due to more subtle manifestations [1]. Congestion of the cavernous sinus may also lead to diplopia and sometimes to intracranial hemorrhage when a cortical venous drainage is found [4]. When CCFs drain posteriorly into the superior and inferior petrosal sinuses, they are usually asymptomatic [3, 5]. Cerebral digital subtraction angiography (DSA) still remains the gold-standard technique to detect and define the abnormal vascular pattern of a fistula [6, 7]; angiography represents also an important therapeutic option to treat the fistula with transarterial (or transvenous) embolization of the cavernous sinus [8–11]. Considering the high frequency of mild and aspecific ocular symptoms (e.g., in patients with dural fistulas) and the invasiveness of diagnostic angiography, requiring hospitalization, a first-line noninvasive diagnostic approach for the evaluation of a suspected fistula would avoid unnecessary invasive examinations. Many techniques can show dilatation of the cavernous sinus and ophthalmic veins induced by fistulas, such as CT Angiography [12–14] and MR angiography [15–17]. By the way, they are less sensitive in diagnosing subtle alterations produced by indirect fistulas, so that a negative finding is still not conclusive. Orbital color Doppler ultrasound (OCDUS) is a simple and noninvasive technique useful in many ophthalmological [18, 19], vascular [20, 21], and systemic diseases [22, 23]. Also orbital tumors can be studied with OCDUS [24, 25] and contrast-enhanced US [26]. OCDUS was previously used, despite the lack of large series, for the diagnosis of high-flow CCF, based on the typical finding of a dilated superior ophthalmic vein (SOV) with reversed, arterialized, low-resistance blood flow [27–29]. In the present study, our purpose was to investigate the role of OCDUS as a noninvasive screening technique in the diagnosis of CCF using DSA as gold standard, particularly to assess its negative predictive value and to verify whether DSA could be avoided in patients with clinically suspected CCF but negative OCDUS.
Materials and methods
Subjects
We retrospectively evaluated data from all the subjects with clinically suspected CCF who underwent both OCDUS and DSA at our institution from February 2000 to July 2014. Subjects who did not undergo either ultrasound or angiographic examination, or whose angiography preceded OCDUS, were excluded, while eyes with SOV thrombosis were not considered. All patients were evaluated by experienced ophthalmologists and enrolled according to the presence of one or more of the following ophthalmologic symptoms: exophthalmos, chemosis, conjunctival congestion, elevated intraocular pressure (>20 mmHg), and cranial nerves deficit.
OCDUS technique
All the OCDUS examinations were performed by the same 25-year-experienced radiologist and sonographer (MV), blinded to the final diagnosis, before cerebral angiography. Written informed consent was obtained from all patients. OCDUS examinations (ATL-Philips IU22/HDI-5000, Bothell, WA, USA) were performed with the patient in supine position with closed eyelids, limiting as much as possible eyeball movements. B-Mode and color Doppler transversal and longitudinal scans of the orbital regions were obtained with a linear high-frequency probe (5–12 MHz), with adequate setting for low blood flow velocity (acquisition parameters were optimized with a 1200-Hz pulse repetition frequency). After identification of the optic nerve as a hypoechoic tubular structure behind the eyeball, color Doppler US imaging of the superior ophthalmic vein (SOV) was performed, both in resting state and during Valsalva maneuver. Size, flow direction, and spectral waveform of the SOV were analyzed with OCDUS using a gate width of 1 mm. In each examined SOV, a physiological continuous venous flow, regularly depicted as blue at OCDUS (Fig. 1) moving away from the orbit, with maximum and minimum velocities, was distinguished from a pathological signal, typical of CCF, arterialized blood flow, depicted as red at OCDUS coming toward the ocular globe, characterized by a peak systolic and end diastolic velocity such as the ophthalmic artery. A further distinction between a normal arterial flow in the ophthalmic artery and a pathological arterialized flow in the SOV was based on the resistive index [RI, defined as (peak systolic velocity—end diastolic velocity)/peak systolic velocity], higher than 0.5 in the ophthalmic arteries, lower than 0.5 in SOV involved by CCF with anterior drainage. The finding at OCDUS of a dilated SOV (diameter >2 mm), with reversed, arterialized, low-resistance blood flow (RI < 0.5), was considered diagnostic for the presence of a CCF with anterior drainage, as previously reported by other groups [27–29]. OCDUS was considered pathological when at least three out of four mentioned criteria were satisfied. Definitive diagnosis was based on cerebral DSA used as gold standard. Six out of 22 patients underwent also MR angiography.
Cerebral DSA
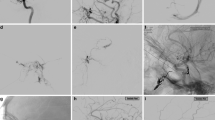
Cerebral DSA (Philips, Allura Xper biplane system) was performed in all cases with selective catheterization of the internal and external carotid and vertebral arteries on both sides; frontal, lateral, and oblique views were acquired. The presence of intracranial CCFs in the cavernous sinuses was evaluated. In positive cases, the angiographic study defined precisely the site of the fistula, its arterial supply, the angioarchitecture of the shunt, and the pattern of venous drainage. The identification of an anterior retrograde venous flow in the orbital venous system either monolateral, bilateral, or contralateral to the fistula was assessed. Presence or absence of SOV’s involvement was accurately considered in each eye (Fig. 2).
Cerebral DSA (left internal a and external b carotid artery selective injections in lateral view) shows a CCF of the left cavernous sinus with anterior drainage in the left superior ophthalmic vein. Dural branches of both internal and external carotid artery feed the fistula. The left superior ophthalmic vein is early opacified, abnormally dilated with reversed blood flow
Statistical analysis
Positive/negative diagnosis of CCF with OCDUS was based on the findings of the cerebral DSA, and sensitivity, specificity, positive (PPV), negative predictive value (NPV), and accuracy of OCDUS for the diagnosis of CCF were calculated separately considering the number of patients and the number of eyes, respectively. Statistical analyses were conducted using SPSS version 20.0 package (SPSS, Chicago, IL, USA). Continuous variables are presented as mean ±SD for data with a normal distribution. The distribution of variables was evaluated using the one-sample Kolmogorov–Smirnov test.
Results
Twenty-two consecutive patients [mean age 61.0 ± 14.4 years; 17 (77.3 %) females, 5 (22.7 %) males] were referred to our department because of ophthalmologically suspected CCF.
Symptoms prevalence (at least in one of the two sides) in the study population was as follows: exophthalmos 16/22 patients (72.7 %), chemosis 11/22 (50 %), conjunctival congestion 18/22 (81.8 %), elevated intraocular pressure 10/22 (45.4 %), and cranial nerves deficit 7/22 (31.8 %). SOV were identified in 100 % of the cases.
Among the total 22 patients, OCDUS was positive in 18 (81.8 %) and negative in four (18.2 %) patients. OCDUS findings based on SOV alterations were as follows: SOV dilatation in 14/18 (77.7 %) cases, reversed blood flow in 17/18 (94.4 %) cases, arterialized blood flow in 18/18 (100 %) cases, and low-resistance blood flow in 16/18 (88.9 %) cases. At least three of the above-mentioned SOV findings were observed in all patients; moreover, all findings were present in 13/18 (72.2 %) patients (Figs. 3, 4). In the subgroup of patients who had a positive diagnosis at OCDUS, CCF was confirmed at DSA in 18/18 (100 %) cases, while in those with a negative diagnosis at OCDUS, CCF was found in none. Hence, regarding the ability of OCDUS in identifying patients with CCF, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of OCDUS were all of 100 % (Table 1).
DSA demonstrated 20 CCFs (18 dural and 2 direct carotid-cavernous sinus fistulas) in 18 patients; 16 patients presented monolateral and two patients bilateral CCF.
Among the 16 patients with monolateral CCF, four patients presented bilateral SOV involvement, while the remaining 12 patients demonstrated a monolateral SOV involvement.
Considering a separate analysis of the eyes, one eye was not evaluable due to SOV thrombosis (for a total amount of 43 eyes); 24/43 eyes (55.8 %) were positive, whereas 19/43 eyes (44.2 %) were negative at OCDUS. In all the 24 OCDUS-positive eyes, a CCF was confirmed by DSA; in particular in 20/24 (75 %) cases, the fistula was homolateral to the OCDUS finding, whereas in the remaining four eyes, it was contralateral (monolateral fistula causing bilateral SOV involvement and, consequently, bilateral OCDUS positivity).
In none of the 19 OCDUS-negative eyes, CCF was detected at DSA (none false negative).
Therefore, sensitivity, specificity, PPV, NPV, and accuracy of OCDUS in the eyes sub-analysis were all 100 %.
In two out of six patients who underwent also MR angiography, MR provided a false-negative result, and both cases were low-flow dural fistulas not determining SOV dilation.
Discussion
CCFs represent 10–15 % of all intracranial arteriovenous shunts [30]. DSA is considered the gold standard for the diagnosis and characterization of the venous drainage, anterior vs posterior [31, 32]. Moreover, it is crucial in the choice of CCF treatment: endovascular vs surgical [8–11]. However, DSA remains an invasive tool with potential risk of peri-procedural complications [33], requires hospitalization, and exposes patients to ionizing radiations. In case of clinical suspect of CCF, usually CT [12–14] or MR angiography [15–17] is the first technique employed to confirm the diagnosis and to decide for subsequent DSA. Limits of both techniques are represented by the potential risk of contrast administration, impossibility to perform both in case of severe nephropathy, and lack of hemodynamic information. Moreover, CT angiography provides a significant radiation dose, and MR angiography cannot be performed in patients with pacemakers. From 1991, on the basis of SOV blood flow arterialization, OCDUS was used as noninvasive diagnostic technique to diagnose and monitor carotid-cavernous and dural fistulas, in a very small number of patients [27, 28]. The SOV findings reflect the intracranial venous hemodynamics because the SOV reaches the cavernous sinus without a valve through the sphenoidal fissure, and no valve is present in the intracranial venous system [29]. In 2002, Kawaguchi et al. [29] concluded their study with OCDUS, based on a larger cohort of patients (n = 20) affected by dural fistulas previously diagnosed with DSA, with the following sentence: “these findings were useful to evaluate the intracranial venous hemodynamics in dural fistulas.” Differently from CT and MR angiography, the ability of OCDUS technique to give hemodynamic information in CCFs through SOV blood flow changes is known. but actually nowadays. there is a lack of studies regarding the diagnostic accuracy of OCDUS in CCFs. The aim of the present study, based on 22 patients, was not only to assess the sensitivity and specificity of OCDUS using DSA as gold standard in CCF diagnosis, but also extended to emphasize the negative predictive value. Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were all 100 %, considering either 22 patients or 43 eyes (one excluded due to SOV thrombosis). Therefore, in our experience, OCDUS was significantly accurate in the diagnosis of CCF with anterior drainage conditioning an ocular symptomatology mainly based on exophthalmos and conjunctival congestion. DSA demonstrated 20 CCFs in 18 patients with significant prevalence of dural fistulas (n = 18) on carotid-cavernous sinus fistulas (n = 2). We have evaluated the true negatives (100 %), using DSA to accurately define either the involved or the non-involved SOVs, to verify whether, in a patient with a clinically suspected CCF, a negative OCDUS could avoid DSA. As the crucial point of a positive diagnosis of CCF with OCDUS is represented by the finding of arterialized, pulsatile flow of SOV, at the same way, the finding of a normal continuous venous flow of SOV is essential for a negative diagnosis. SOVs usually course in the superior-medial part of the orbit, and their blood flow characterization is not always easy. In some cases, Valsalva maneuver may be necessary to identify the SOV: In the present study, all the SOVs were identified and their blood flow characterized. In physiological conditions, SOV usually shows a caliber less than 1–1.5 mm at US, a flow direction moving away from the orbit depicted as blue at color, and a spectral waveform with a typical continuous venous flow with minimal differences between maximum and a minimum velocities at Doppler [34, 35]. In particular cases, significant differences between maximum and minimum velocities with cyclical, transient reversed blood flow due to the cardiorespiratory kinetic may be also recorded, as previously described [34, 35]. In case of high-flow CCF, the typical involved SOV shows a dilated caliber more than 2 mm at US, a reversed blood flow directed toward the ocular globe depicted as red at color, and a spectral waveform characterized by a pulsatile, arterialized flow with a peak systolic and an end diastolic velocity at Doppler. Usually, the arterialized blood flow is characterized by a low resistive index less than 0.5, typical signal of the arteriovenous communications [36]: This aspect allows a differential diagnosis with ophthalmic artery, also characterized by a flow directed toward the globe with a peak systolic and an end diastolic velocity, but with an higher resistive index usually more than 0.7. Furthermore, ophthalmic artery has a more curvilinear course than a dilated SOV with arterialized blood flow. The diagnosis of CCF with anterior drainage at OCDUS is obviously easier in cases of high-flow fistulas compared with low-flow fistulas, where SOV may be un-dilated, as in four of our cases: The identification of the un-dilated SOV and the distinction between a reversed, arterialized flow and a regular, continuous venous flow is essential for a correct diagnosis. In case of low-flow CCFs, also ocular symptoms may be mild differently from high-flow CCFs characterized by pulsating exophthalmos and ocular congestion. The differential diagnosis is with specific ocular diseases or with thyroid ophthalmopathy, the latter more suspected in case of bilateral ocular involvement. Our study has some major limitations. It is a single-center study, and as for the other previous reported experiences, the population number is limited, also because CCFs represent a relatively rare disease. A multicenter larger experience would be desirable to provide further insights into this specific field. Furthermore, OCDUS is an operator-dependent technology. Our study was performed by single operator to avoid the inter-observer bias, which would have been even more deleterious given the small number of patients.
Anyway, visualization of orbital vessels at retrobulbar level with OCDUS through ocular globe, a “water ball” representing a natural acoustic window, is easy. Experienced sonographers using adequate color Doppler ultrasound technologies significantly improved in the last years (harmonic imaging etc.) can always be able to identify SOVs, accurately setting their OCDUS units for low blood flow velocities, sometimes during Valsalva maneuver. Moreover, we also believe that they can always characterize SOV blood flow in physiological and pathological conditions, as for example CCFs with anterior drainage, and distinguish a normal venous from an arterialized flow, allowing an accurate noninvasive diagnosis. Differently from CT and MR angiography, OCDUS is a low-cost, simple, quick technique able in real time to provide hemodynamic information about SOV blood flow and probably to diagnose CCFs, also in case of low-flow fistulas not determining SOV dilation. As a matter of fact, in two out of six patients who underwent MR angiography, it provided a false-negative result due to the lack of SOV dilation.
Summarizing, our study has several limitations (single center, retrospective, small numbers even though CCF is a rare disease), thus we acknowledge that no definitive conclusion can be drawn. Nevertheless, we believe that given the high agreement between OCDUS and DSA, important insights can still be provided. Of note, it must be remembered all the previous similar experiences also reported very small patient populations, and none of them assessed the negative predictive value of OCDUS. Therefore, in our opinion, the present study adds a small advancement in knowledge to the current scenario. In conclusion, in case of clinically suspected anterior-draining CCF, OCDUS can be considered a noninvasive, reliable screening tool to detect the presence of CCF; on the other hand, further studies involving a larger cohort of patients will be necessary to establish whether a negative finding at OCDUS could avoid an invasive DSA, as our findings seem to suggest.
Abbreviations
- CCF:
-
Carotid-cavernous fistula
- OCDUS:
-
Orbital color Doppler ultrasound
- DSA:
-
Digital subtraction angiography
- SOV:
-
Superior ophthalmic vein
- RI:
-
Resistive index
References
Feiner L, Bennett J, Volpe NJ (2003) Cavernous sinus fistulas: carotid cavernous fistulas and dural arteriovenous malformations. Curr Neurol Neurosci Rep 3:415–420
Gobin YP, Duckwiler GR, Viñuela F (1998) Direct arteriovenous fistulas (carotid-cavernous and vertebral-venous). Diagnosis and intervention. Neuroimaging Clin N Am 8:425–443
Miller NR (2012) Dural carotid-cavernous fistulas: epidemiology, clinical presentation, and management. Neurosurg Clin N Am 23:179–192
Liu HM, Wang YH, Chen YF, Cheng JS, Yip PK, Tu YK (2001) Long-term clinical outcome of spontaneous carotid cavernous sinus fistulae supplied by dural branches of the internal carotid artery. Neuroradiology 43:1007–1014
Wu H, Ro L, Chen C, Chen S, Lee T, Chen Y, Chen C (2006) Isolated ocular motor nerve palsy in dural carotid-cavernous sinus fistula. Eur J Neurol 13:1221–1225
Choi JH, Mohr JP (2005) Brain arteriovenous malformations in adults. Lancet Neurol 4:299–308
Signorelli F, Della Pepa GM, Sabatino G, Marchese E, Maira G, Puca A, Albanese A (2015) Diagnosis and management of dural arteriovenous fistulas: a 10 years single-center experience. Clin Neurol Neurosurg 128:123–129
Deng JP, Zhang T, Li J, Yu J, Zhao ZW, Gao GD (2013) Treatment of dural arteriovenous fistula by balloon-assisted transarterial embolization with Onyx. Clin Neurol Neurosurg 115:1992–1997
Yu Y, Li Q, Huang Q, Zhang Y, Fang Y, Xu Y, Hong B, Zhao W, Liu J (2014) Embolization of direct carotid cavernous fistula with Onyx and coils under transarterial balloon protection. Cardiovasc Intervent Radiol 37:679–685
Lu X, Hussain M, Ni L, Huang Q, Zhou F, Gu Z, Chen J, Ding Y, Xu F (2014) A comparison of different transarterial embolization techniques for direct carotid cavernous fistulas: a single center experience in 32 patients. J Vasc Interv Neurol 7:35–47
Aixut Lorenzo S, TomaselloWeitz A, BlascoAndaluz J, Sanroman Manzanera L, Macho Fernández JM (2011) Transvenous approach to intracranial dural arteriovenous fistula (Cognard v): a treatment option. A case report. Interv Neuroradiol 17:108–114
Lee CW, Huang A, Wang YH, Yang CY, Chen YF, Liu HM (2010) Intracranial dural arteriovenous fistulas: diagnosis and evaluation with 64-detector row CT Angiography. Radiology 256:219–228
Willems PW, Brouwer PA, Barfett JJ, Terbrugge KG, Krings T (2011) Detection and classification of cranial dural arteriovenous fistulas using 4D-CT Angiography: initial experience. AJNR Am J Neuroradiol 32:49–53
Narvid J, Do HM, Blevnis NH, Fishbein NJ (2011) CT Angiography as a screening tool for dural arteriovenous fistula in patients with pulsatile tinnitus: feasibility and test characteristics. AJNR Am J Neuroradiol 32:446–453
Mossa-Basha M, Chen J, Gandhi D (2012) Imaging of cerebral arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am 23:27–42
Schanker BD, Walcott BP, Nahed BV, Ogilvy CS, Kiruluta AJ, Rabinov JD, Copen WA (2011) Time-resolved contrast-enhanced magnetic resonance angiography in the investigation of suspected intracranial dural arteriovenous fistula. J Clin Neurosci 18:837–839
Meckel S, Maier M, Ruiz DSM, Yilmaz H, Scheffler K, Radue EW, Wetzel SG (2007) MR Angiography of dural arteriovenous fistulas: diagnosis and follow-up after treatment using a time-resolved 3D contrast-enhanced technique. AJNR Am J Neuroradiol 28:877–884
Arséne S, Giraudeau B, Le Lez ML, Pisella PJ, Pourcelot L, Tranquart F (2002) Follow up by colour Doppler imaging of 102 patients with retinal vein occlusion over 1 year. Br J Ophthalmol 86:1243–1247
Suprasanna K, Shetty CM, Charudutt S, Kadavigere R (2014) Doppler evaluation of ocular vessels in patients with primary open angle glaucoma. J Clin Ultrasound 42:486–491
Cohn EJ Jr, Sandager GP, Benjamin ME, Lilly MP, Hanna DJ, Flinn WR (1999) Assessment of ocular perfusion after carotid endarterectomy with color-flow duplex scanning. J Vasc Surg 29:665–671
Venturini M, Zambon M, Cristel G, Agostini G, Querques G, Colombo M, Benussi S, Landoni G, Zangrillo A, Del Maschio A (2014) Monitoring of central retinal artery and vein with color Doppler ultrasound during heart surgery as an alternative to transcranial Doppler ultrasonography: a case report. J Clin Ultrasound 42:112–115
Meng N, Liu J, Zhang Y, Ma J, Li H, Qu Y (2014) Color Doppler imaging analysis of retrobulbar blood flow velocities in diabetic patients without or with retinopathy: a meta-analysis. J Ultrasound Med 33:1381–1389
Venturini M, Fiorina P, Maffi P, Losio C, Vergani A, Secchi A, Del Maschio A (2006) Early increase of retinal arterial and venous blood flow velocities at color Doppler imaging in brittle type 1 diabetes after islet transplant alone. Transplantation 81:1274–1277
Spierer O, Neudorfer M, Leibovitch I, Stolovitch C, Kessler A (2012) Colour Doppler ultrasound imaging findings in paediatric periocular and orbital haemangiomas. Acta Ophthalmol 90:727–732
Regan S, Egan KM, Hart L, Gragoudas ES (2001) Color Doppler imaging of untreated and irradiated choroidal melanomas. Eur J Ophthalmol 11:150–155
Venturini M, Colantoni C, Modorati G, Di Nicola M, Colucci A, Agostini G, Picozzi P, De Cobelli F, Parmiani G, Mortini P, Bandello F, Del Maschio A (2015) Preliminary results of contrast-enhanced sonography in the evaluation of the response of uveal melanoma to gamma-knife radiosurgery. J Clin Ultrasound. doi:10.1002/jcu.22262
Flaharty P, Lieb WE, Sergott RC, Bosley TM, Savino PJ (1991) Color Doppler imaging: a new non-invasive technique to diagnose and monitor carotid-cavernous sinus fistulas. Arch Ophthalmol 109:522–526
Costa VP, Molnar LJ, Cerri G (1997) Diagnosing and monitoring carotid cavernous fistulas with color Doppler imaging. J Clin Ultrasound 25:448–452
Kawaguchi S, Sakaki T, Uranishi R (2002) Color Doppler flow imaging of the superior ophtalmic vein in dural arteriovenous fistulas. Stroke 33:2009–2013
Newton TH (1969) Cronqvist. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology 93:1071–1078
Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, Chiras J, Merland JJ (1995) Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 194:671–680
Borden JA, Wu JK, Shucart WA (1995) A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 82:166–179
Cloft HJ, Joseph GJ, Dion JE (1999) Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke 30:317–320
Berges O (1992) Color Doppler flow imaging of the orbital veins. Acta Ophthalmol 204:55–58
Venturini M, Zaganelli E, Angeli E, Castrucci M, Pierro L, Salvioni M, Brancato R, Del Maschio A (1996) Ocular color Doppler echography: the examination technique, identification and flow of the orbital vessels. Radiol Med 91:60–65
Polat P, Suma S, Kantarcý M, Alper F, Levent A (2002) Color Doppler US in the evaluation of uterine vascular abnormalities. Radiographics 22:47–53
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Venturini, M., Cristel, G., Marzoli, S.B. et al. Orbital color Doppler ultrasound as noninvasive tool in the diagnosis of anterior-draining carotid-cavernous fistula. Radiol med 121, 301–307 (2016). https://doi.org/10.1007/s11547-015-0607-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-015-0607-0