Abstract
Objective
To perform a systematic review of the contrast behaviour of HCC on Gd-EOB-DTPA hepato-biliary phase MRI.
Materials and methods
This review was completed in accordance with the recommendations outlined in the preferred reporting items for systematic reviews statement. In all reports, qualitative analysis of signal intensity (SI) of HCC on hepato-biliary phase was performed: the relative SI of HCC. When available, a quantitative analysis of tumour enhancement was evaluated.
Results
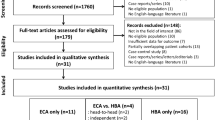
A total of 106 studies were retrieved, of which 41 met the inclusion criteria. The total number of patients was 2550, with 3132 HCC. MRI showed 3110 HCC (22 non-detected). 2692/3110 (87 %) HCC were hypointense on Gd-EOB-DTPA-enhanced hepatocyte-phase MRI, 134 (4 %) isointense; 106 (3 %) hyperintense and 178 (6 %) iso-hyperintense. In 26 articles, 1653 HCCs were classified as follows: 519 well-differentiated, 883 moderately differentiated, 251 poorly differentiated. Among well-differentiated HCC, 445 (86 %) were hypointense, 12 isointense (2 %), 9 hyperintense (2 %), 53 iso/hyperintense (10 %). Among moderately differentiated HCC, 774 (88 %) were hypointense, 8 isointense (1 %), 27 hyperintense (3 %), 74 iso/hyperintense (8 %). Among poorly differentiated HCCs, 245 (98 %) were hypointense, one isointense, one hyperintense and four iso-hyperintense (2 %). We found a Chi-square (χ 2) equivalent to 25,082 (p < 0.001).
Conclusion
The percentage of lesions iso/hyper/iso-hyper is the same when considering well-differentiated and moderately differentiated HCC; when considering poorly differentiated HCC, the percentage of lesions iso/hyper/iso-hyper is significantly lower. Conversely, the percentage of lesions hypointense is significantly more represented in poorly differentiated HCC compared to well-differentiated and moderately differentiated HCC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common tumour worldwide and the third most common cause of cancer-related death, after lung and stomach cancer [1]. HCC is the main cause of death among cirrhotic patients and the incidence is predicted to increase in the next two decades [2]. According to the American Association for the Study of Liver Diseases (AASLD) [3], contrast-enhanced multidetector computed tomography (CE-MDCT) and magnetic resonance imaging (MRI) are the best imaging modalities currently available in the diagnosis and staging of HCC [4]. Historically, extracellular gadolinium-based contrast agents have played a critical role in MRI of the cirrhotic liver. Two combined extracellular and hepato-biliary gadolinium-based contrast agents are currently available with the aim to assess hepatocellular function, in addition to vascularity, the gadobenate dimeglumine (Multihance, Bracco, Italy) and the gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid (Gd-EOB-DTPA), also known as gadoxetate disodium/gadoxetic acid (Primovist, Eovist, Bayer Schering Pharma, Germany). In relation to its more favourable pharmacokinetic and pharmacodynamic properties [5] and to the reported higher sensitivity in identifying hepatocellular carcinoma [6], Gd-EOB-DTPA seems to be the most helpful diagnostic tool in predicting stepwise carcinogenesis in cirrhotic liver. In particular, current literature demonstrated that the hepatocyte-specific properties of Gd-EOB-DTPA could give some important additional information, especially when dynamic MRI or CT imaging shows atypical vascular features [7, 8]. In the hepatocyte phase, typical HCCs are well described as areas of low signal intensity relative to the surrounding liver parenchyma because they do not have the ability to take up Gd-EOB-DTPA [9]. Otherwise, it has also been shown that some HCCs exhibit iso/hyperintensity on hepato-biliary phase imaging compared to the normal parenchyma. In particular, Cruite et al. [5] demonstrated that from 2.5 to 8.5 % of HCCs might show paradoxical uptake of Gd-EOB-DTPA in the hepato-biliary phase. Until now, the meaning of this atypical signal intensity as well as its clinical and prognostic value remains controversial. To our knowledge, there is no systematic report or review about qualitative and/or quantitative analysis of enhancement patterns of HCCs on Gd-EOB-DTPA hepato-biliary phase and only limited data regarding the association with the hepatocyte function are currently available. Therefore, the aim of the present study was to perform a review of previous articles, about the contrast behaviour of HCCs on Gd-EOB-DTPA hepato-biliary phase MR imaging, to elucidate whether there is a correlation with histological tumour grading.
Materials and methods
This systematic review was completed in accordance with the recommendations outlined in the preferred reporting items for systematic reviews statement [10]. PUBMED, EMBASE, Web of Science, Cochrane Library and the Chinese Biomedical Literature Database were searched using the terms “hepatocellular carcinoma or HCC”, “gadoxetic-acid or Gd-EOB-DTPA or gadoxetate-disodium or primovist/eovist”, “magnetic resonance imaging or MRI or contrast-enhanced dynamic MRI”, “hepatobiliary phase or hepato specific phase”, “focal liver lesions”, “cirrhotic liver or cirrhosis”, “liver specific contrast agents” (last search update September 2013). The search involved the use of free text words and MESH (medical subject headings) terms for increased sensitivity of the search strategy. The search was without restriction to the language and on studies conducted on human subjects. Review articles, abstracts, case reports, letters, comments and unpublished articles were excluded. Two reviewers independently searched the databases for eligible studies. They independently studied full text copies to make a decision as to which studies met the inclusion criteria.
Inclusion and exclusion criteria
Inclusion criteria were: (1) patients suspected of having HCC, undergoing hepatic gadoxetic acid disodium-enhanced MR imaging including the triple dynamic post-contrast (arterial, portal, venous) phase and hepato-biliary phase; (2) patients receiving a diagnosis of HCC based on surgical findings (pathological examination or intra-operative ultrasound), findings at percutaneous/ultrasound core-needle biopsy, or on a follow-up period including a typical clinical history with tumour marker levels in combination with Lipiodol uptake after transhepatic arterial chemoembolization (TACE), or the progression of the disease as depicted at follow-up diagnostic (CT/MR) imaging performed at least 3–6 months after the initial imaging; (3) where available hepatocellular carcinoma was histologically classified, according to the definition of the World Health Organization [11] on the basis of Edmondson and Steiner criteria [12] as follows: well-differentiated, moderately differentiated, poorly differentiated; (4) MR image analysis performed in consensus by at least two radiologists with at least 5 years of abdominal radiological experience. Studies were excluded if (a) any one of the inclusion criteria was not met; (b) multiple reports were published for the same study population (in this case, the publication with the most details and/or most recently published was chosen); and (c) the study included patients who had previously undergone treatment for liver tumours. All MRI examinations were performed using either a 1.5 or 3 T imaging system. For dynamic imaging, in all patients a dose of 0.1 mL/kg of body weight of Primovist (0.025 mmol/mL of Gd-EOB-DTPA, Bayer Schering Pharma, Berlin, Germany) was injected intravenously at a flow rate of 2 mL/s, followed by 20–30 mL saline flush. In all patients the hepato-biliary phase was imaged 20 min after the contrast agent administration. In all the reports included in our work, qualitative analysis of signal intensity (SI) of HCCs on hepato-biliary phase was performed: the relative SI of HCCs compared with that of the surrounding liver parenchyma was graded and recorded as low, iso, and high SI. Articles that did not clearly describe the SI of hepatocarcinoma nodules on hepato-biliary phase were excluded. According to SI characteristics, on hepato-biliary phase imaging, lesions were classified into two major groups: (a) hypointense lesions; (b) iso/hyper/iso-hyperintense lesions. When available, a quantitative analysis of tumour enhancement was evaluated. Image analysis was performed by two abdominal imaging radiologists, expert in hepato-biliary MRI, who were blinded to the final histopathological results. Signal intensities of the liver parenchyma and hepatic tumour lesion were calculated and measured by placing regions of interest (ROIs). The ROI of the tumour was determined as the maximum oval/round area at the level of the largest diameter of the tumour; a similar-sized ROI was set over the adjacent liver parenchyma. The following quantitative parameters were evaluated: (1) the relative intensity ratio (RIR) either on pre-contrast (RIRpre) and post-contrast (RIRpost) MR images equivalent to SInod/SIpar, where SInod is the SI of the nodule, and SIpar is the SI of the liver parenchyma; (2) the relative enhancement ratio (RER) equivalent to RIRpost/RIRpre; (3) the contrast “enhancement ratio” (ER) of hepatocarcinoma nodule equivalent to (post-contrast SI–pre-contrast SI/pre-contrast SI) × 100.
Statistical analysis
Statistical differences in percentage of tumour uptake of Gd-EOB-DTPA on hepato-biliary MR imaging among the tumour differentiation degree were determined using a c 2 test (SPSS statistical analysis). Post hoc analysis was performed using analysis of residuals that allows to find differences among the percentages. A p value of less than 0.05 was considered statistically significant.
Results
A total of 106 studies were retrieved, of which 56 articles potentially met the inclusion criteria. Individual study characteristics are summarized in Table 1. Fifteen articles were excluded because they studied the added value of gadoxetic acid MRI imaging without clarifying the SI of hepatocarcinoma on hepato-biliary phase Gd-EOB-DTPA MRI. In 20 out of 41 studies included [6, 8, 13–16, 18, 20, 21, 26–28, 30, 32, 34, 35, 37, 53, 55, 57], MRI was performed with a 1.5 T scanner; a 3 T whole-body system was employed in 14 out of 41 studies [9, 17, 19, 22, 29, 31, 45–49, 52, 54, 56], both 1.5 and 3 T scanners in the remnant 7 out of 41 studies [23–25, 33, 36, 50, 51]. The number of patients ranged from 11 to 192 patients per study, with a total of 2550 patients evaluated in all included studies (1908 male patients). A total number of 3132 HCCs were diagnosed. The diagnosis was achieved based on pathological specimens in 2528 malignant nodules, respectively, through surgical resection (n = 2182) and core-needle biopsy, percutaneous or ultrasound guided (n = 346). The other 604 lesions were diagnosed on radiological features as HCCs on the basis of definite characteristics revealed on a follow-up period, as mentioned in “Materials and methods” section. MRI showed 3110 nodular lesions consistent with HCC (22 non-detected). 2692 out of 3110 (87 %) of HCCs were hypointense on Gd-EOB-DTPA-enhanced hepatocyte-phase MR imaging, whereas 134 out of 3110 (4 %) lesions were isointense; 106 out of 3110 (3 %) lesions were hyperintense and 178 out of 3110 (6 %) were iso-hyperintense.
In 31 out of 41 articles, the final histological classification of HCCs was systematically reported, but only in 26 out of 41 of them [15–18, 21–23, 25, 28, 31–37, 45–47, 51–57] a specific correlation between histological tumour grading and signal intensity on Gd-EOB-DTPA hepato-biliary phase was expressed. In these 26 articles, the total amount of HCCs was 1653, classified as follows, according to Edmondson and Steiner criteria [12]: 519 well-differentiated, 883 moderately differentiated, 251 poorly differentiated (Fig. 1: summary of study design and final histological classification of HCC). Among well-differentiated HCCs, 445 (86 %) were hypointense on Gd-EOB-DTPA hepato-biliary phase, 12 isointense (2 %), 9 hyperintense (2 %), 53 iso/hyperintense (10 %). Among moderately differentiated HCCs, 774 (88 %) were hypointense on hepato-biliary phase MR imaging, 8 isointense (1 %), 27 hyperintense (3 %), 74 iso/hyperintense (8 %). Among poorly differentiated HCCs, 245 (98 %) were hypointense on hepato-specific phase, one isointense, one hyperintense and four iso-hyperintense (2 %). Grouping the HCCs lesions in hypointense or isointense (isointense, hyperintense or iso-hyperintense) we found a statistical significant difference in the percentage of isointense lesions among the lesion classification (p < 0.001). In particular, the percentage of lesions classified as iso/hyper/iso-hyper was the same when considering well-differentiated and moderately differentiated HCCs; when considering poorly differentiated HCCs, the percentage of lesions iso/hyper/iso-hyper was significantly lower. Conversely, the percentage of lesions classified as hypointense was significantly more represented in poorly differentiated HCCs compared to well-differentiated and moderately differentiated HCCs (Fig. 2).
In 9 out of 41 articles [9, 15, 32–34, 36, 47, 51, 56] a quantitative analysis for tumour enhancement at hepato-biliary phase imaging was performed and related to the histological grade of HCCs, but it was expressed through different quantitative parameters (Table 2). In particular, Kogita [15] and Okada [32], in a total of, respectively, 83 and 37 HCCs, showed that the “relative intensity ratio” (RIRpost) on hepato-biliary phase images of well-differentiated HCCs were significantly higher than those of moderately and poorly differentiated HCCs. In agreement with these data, Kim et al. [47] demonstrated that the degree of tumour enhancement, which included the RIRpre, the RIRpost, and the RER for well-differentiated HCCs was significantly higher than the degree of tumour enhancement for moderately differentiated and poorly differentiated HCCs. The contrast enhancement ratio (ER) compared with background liver has also been used as a quantitative parameter of tumour enhancement on Gd-EOB-DTPA hepato-biliary phase imaging, showing a correlation with the differentiation degree of HCCs. In particular, Kitao et al. [33] showed that after excluding those atypical HCCs which were iso-hyperintense on hepato-biliary phase imaging, the mean values of contrast ER, significantly decreased as the tumour differentiation declined, varying from 1.33 ± 0.53 for well-differentiated HCCs, to 0.76 ± 0.36 and 0.54 ± 0.23, respectively, for moderately HCCs and poorly HCCs. In agreement with these data, Inoue et al. [51] demonstrated that the contrast ERs decreased in parallel with the degree of tumour differentiation. On the other hand, Frericks et al. [9] showed that, when considering all the tumours, hypo and iso-hyperintense within the same quantitative analysis, the ERs did not differ significantly for the different tumour grades.
Discussion
We reviewed the signal intensity of HCCs on Gd-EOB-DTPA hepato-biliary phase MR imaging, in 41 published articles, focusing either on qualitative or quantitative analysis. Accurate and early detection of HCC is crucial in cirrhotic patients and investigating about non-invasive diagnostic imaging modalities has a noteworthy impact in terms of prognosis and therapy.
Our review demonstrates that 87 % of 3110 HCCs were hypointense on Gd-EOB-DTPA hepato-biliary phase.
It has been well established that the hypointensity of HCCs is due to diminished normal function of hepatocytes in the tumour [9, 16], whereas the uptake of hepatocyte-selective agents occurs in normal liver parenchyma and in focal liver lesions of hepato-cellular origin. In addition, it has also been demonstrated that the hepatocyte-specific properties of Gd-EOB-DTPA could contribute to early HCC detection and characterization [8, 57] with reported increasing sensitivities when hepato-biliary images are obtained [14].
According to our systematic review, 418 out of 3110 (13 %) of HCCs show uptake of Gd-EOB-DTPA in the hepatocyte phase; appearing as iso-hyperintense lesions relative to the surrounding parenchyma on qualitative analysis. Our results emphasize that relevant uptake of “liver-specific” contrast agents does not always exclude malignancy, as already revealed by Huppertz et al. [16]; in particular, an iso-hyperintense nodule on Gd-EOB-DTPA hepato-biliary phase images may not be a benign nodule, especially in patients with evidence of risk of HCCs. However, the critical issue in cirrhotic patients is that an iso-hyperintense nodule might be either an HCC or a regenerative/dysplastic nodule. Up to now, there are no definite and accurate guidelines for differentiating between these two entities. Suh et al. [29] attempted to define imaging features that may help to characterize hyperintense lesions seen in the hepato-biliary phase of Gd-EOB-DTPA-enhanced MR examination and concluded that hyperintense HCCs more commonly present focal defects in uptake, nodule-in-nodule appearance, absence of a central scar, internal septation and a hypointense rim in comparison to benign lesions. Further studies should be performed to clarify this issue; however, it is clear that pre-contrast and vascular post-contrast MR sequences are needed for the final differential diagnosis.
In our systematic review, we observed that on qualitative analysis of hepato-biliary phase images, well-differentiated and moderately differentiated HCCs showed a similar percentage of hypointensity (respectively, 86 and 88 %) and iso-hyperintensity (14 and 12 %); only poorly differentiated HCCs showed higher incidence (98 %) of hypointensity on delayed phase images, compared with 2 % of iso-hyperintense nodules. We have not found any review in the literature concerning the correlation between the enhancement pattern of HCCs on hepato-biliary phase and histological classification. Early studies performed on experimental liver tumours and on induced HCCs in rats demonstrated that the hepatocyte-selective uptake of Gd-EOB-DTPA reflects tumour differentiation grade [38, 40, 41]. In agreement with these data, Huppertz et al. [16] demonstrated that two out of four well-differentiated HCCs, in patients with liver cirrhosis exhibited an exceeding or equal uptake in comparison to the surrounding parenchyma, whereas no uptake was depicted in four moderately or poorly differentiated HCC. Later additional experimental and clinical studies have not confirmed a correlation between HCC grade and Gd-EOB-DTPA uptake [21, 39, 42, 43]. As a result, it must be supposed that other molecular mechanisms might be involved in the paradoxical contrast uptake Gd-EOB-DTPA by HCCs. Several human studies have performed a correlation between diagnostic imaging and molecular mechanisms, to compare different enhancement patterns of HCCs. It has been suggested that in human hepatocytes organic anion-transporting polypeptide 8 (OATP8) is the most probable uptake transporter for Gd-EOB-DTPA, which is subsequently excreted into bile secretions by MRP3, a multidrug resistance protein [25, 44]. Narita et al. [23] investigated the enhancement ratios (ERs) and expression levels of the organic anion transporter OATP1B3 (that is a synonymous of OATP8) in 22 confirmed HCCs, six of which, all moderately differentiated, accumulated Gd-EOB-DTPA in the hepato-biliary phase and showed high ER. They showed that HCCs with Gd-EOB-DTPA uptake overexpressed OATP1B3 compared with HCCs without Gd-EOB-DTPA uptake, consequently concluding that expression of OATP1B3 determines the hyperintensity of HCCs in hepato-biliary phase, rather than tumour differentiation or bile production. These results were confirmed by Tsuboyama et al. [36] and by Kitao in two later works including a wider patient population, respectively, 32 HCCs [25] and 70 HCCs [33], in which it was clearly underlined the correlation between the uptake of Gd-EOB-DTPA of focal lesions in hepato-biliary phase images and the OATP8 expression. In addition, these authors also showed that the immunohistochemical expression of OATP8 significantly decreased, from well-differentiated HCCs to poorly differentiated HCCs and so they suggested OATP8 might be considered as a marker of the multi-step hepato-carcinogenesis.
Our results are in agreement with previous articles based on smaller patient population about the higher incidence of moderately differentiated HCCs among iso-hyperintense HCCs. The reason why this happens is still not clear; in particular, Kitao et al. [25] showed that moderately differentiated HCCs might have a different cellular origin from the ordinary type of HCCs or that they might undergo to a genetic reversion to their original hepatocyte nature during hepato-carcinogenesis. On the basis of our results, we can state that the percentage of lesions classified as iso/hyper/iso-hyper is the same if we consider well-differentiated and moderately differentiated HCCs. When we take into account poorly differentiated HCCs, the percentage of lesions iso/hyper/iso-hyper is significantly lower. Conversely, the percentage of lesions classified as hypointense is significantly more represented in poorly differentiated HCCs compared to well-differentiated and moderately differentiated HCCs. Hence, a quantitative approach and analysis of tumour enhancement might be considered as a useful tool for estimating malignancy grade. There are few articles in the literature considering the correlation between the histological tumour grade and the quantitative analysis and, in addition, they employ different quantitative parameters; as a consequence, it is hard to evaluate their statistical significance. On the basis of our literature review, in a small subset of nine articles, we have found discordant results regarding the correlation between either the relative intensity ratio (RIRpost) on hepato-biliary phase or the contrast enhancement ratio (ER) with tumour differentiation grade [15, 32, 33, 36, 47, 51, 56]. In particular, Frericks et al. [9] in a small group of 25 patients with HCCs, showed that, when considering all the tumours, hypo and iso-hyperintense within the same quantitative analysis, the ERs did not differ significantly for the different tumour grades. Conversely, in a more recent paper, Kitao et al. [33] used the static T1 value for measurement of the contrast enhancement ratio, because it has linearity with contrast agent concentration and is more reliable for quantitative evaluation. The variable flip angle method used in their study has been proven to be useful for calculating the enhancement ratio in Gd-EOB-DTPA contrast-enhanced MR imaging. They found that, when excluding those atypical HCCs showing iso-hyperintensity on hepato-biliary phase imaging, the ER significantly decreased in comparison to the background liver, as the tumour differentiation declined. They also confirmed a significative positive correlation between the ER and the grade of immunohistochemical OATP8 expression, showing a decrease from well-differentiated HCCs to poorly differentiated HCCs. Therefore, according to these results, the contrast enhancement ratio might be considered a useful tool to evaluate multi-step hepato-carcinogenesis, if we consider as exception 10 % of HCCs which are iso-hyperintense on hepato-biliary phase images and show a higher contrast enhancement ratio. Although these hypotheses need to be confirmed in further studies and in larger patient population; it is possible that standardizing the quantitative measurement of the enhancement ratio could help to obtain a more homogeneous and accurate analysis of Gd-EOB-DTPA hepato-biliary phase on MRI.
Our study has several limitations. First of all, this is a review of published articles and, therefore, heterogeneity among studies is present. Not all lesions were confirmed by final histopathologic examination. In addition, we included patients with different grades of liver cirrhosis, varying from early to advanced stages. This factor could affect our final results because it is well known that in patients with impaired liver function the contrast agent uptake by liver parenchyma is reduced and lesion-to-liver contrast may result lower; as a consequence, the signal intensity on hepato-biliary phase may be conditioned by these circumstances. It should also be noted that the majority of patients included in this review were affected by chronic hepatitis/cirrhosis due to hepatitis C/B virus infection; other types of chronic hepatitis or cirrhosis, may show different enhancement pattern with gadoxetate disodium. Moreover, the acquisition imaging sequences for the hepato-biliary phase are not characterized by the same parameters TE and/or TR for all the studies as well as there may be potential for criticism regarding the use of either a 1.5 or a 3.0 T MR system. Furthermore, although dynamic images and T2-weighted images are essential part of Gd-EOB-DTPA-enhanced MRI, these image traits were not included for the analysis. For example, hypervascular HCCs not showing washout may have the similar results with the hyperenhancing tumours on hepato-biliary phase of Gd-EOB-DTPA-enhanced MRI. Therefore, adding the results of incidence of washout on dynamic phase imaging to the manuscript would increase the clinical value of this systematic review. Finally, the results of the quantitative analysis are based on a small and not significant subset of nine articles; therefore, more quantitative studies are necessary, to better clarify the potential role of a quantitative analysis, in addition to a qualitative assessment for prediction of the degree of malignancy, in patients with HCC.
Conclusion
The results of this systematic review confirm the value of gadoxetic acid-enhanced hepato-biliary phase MR imaging in the evaluation of hepato-cellular carcinoma. The percentage of lesions classified as iso/hyper/iso-hyper is the same when considering well-differentiated and moderately differentiated HCCs; when considering poorly differentiated HCCs, the percentage of lesions iso/hyper/iso-hyper is significantly lower. Conversely, the percentage of lesions classified as hypointense is significantly more represented in poorly differentiated HCCs compared to well-differentiated and moderately differentiated HCCs.
Further studies are necessary to clarify the potential role of a quantitative analysis, as a possible adjunct to the standard qualitative evaluation to accurately predict the degree of malignancy, in patients with HCC.
References
Clark HP, Carson WF, Kavanagh PV, Ho CP, Shen P, Zagoria RJ (2005) Staging and current treatment of hepatocellular carcinoma. Radiographics 25(Suppl 1):S3–S23
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Bruix J, Practice Sherman M, Committee Guidelines (2005) American Association for the Study of liver diseases. Management of hepatocellular carcinoma. Hepatology 42:1208–1236
Colli A, Fraquelli M, Casazza G et al (2006) Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol 101:513–523
Cruite I, Schroeder M, Merkle EM, Sirlin CB (2010) Gadoxetate disodium-enhanced MRI of the liver: part 2, protocol optimization and lesion appearance in the cirrhotic liver. AJR 195:29–41
Park G, Kim YK, Kim CS, Yu HC, Hwang SB (2010) Diagnostic efficacy of gadoxetic acid-enhanced MRI in the detection of hepatocellular carcinoma: comparison with gadopentetate dimeglumine. Br J Radiol 83:1010–1016
Lee JM, Zech CJ, Bolondi L et al (2011) Consensus report of the 4th International Forum for gadolinium–ethoxybenzyl–diethylenetriamine pentaacetic acid magnetic resonance imaging. Korean J Radiol 12:403–415
Haradome H, Grazioli L, Tinti R et al (2011) Additional value of gadoxetic acid-DTPA-enhanced hepatobiliary phase MR imaging in the diagnosis of early-stage hepatocellular carcinoma: comparison with dynamic triple-phase multidetector CT imaging. J Magn Reson Imaging 34:69–78
Frericks BB, Loddenkemper C, Huppertz A et al (2009) Qualitative and quantitative evaluation of hepatocellular carcinoma and cirrhotic liver enhancement using Gd-EOB-DTPA. AJR 193:1053–1060
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341
Ishak KG, Anthony PP, Solin LH (1994) Primary carcinoma of the liver. 2nd ed. Berlin, Germany: Springer 20
Edmondson HA, Steiner PE (1954) Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 7:462–503
Golfieri R, Renzulli M, Lucidi V, Corcioni B, Trevisani F, Bolondi L (2011) Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to dynamic MRI in the detection of hypovascular small (≤ 2 cm) HCC in cirrhosis. Eur Rad 21:1233–1242
Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JYSS (2010) Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology 255:459–466
Kogita S, Imai Y, Okada M et al (2010) Gd-EOB-DTPA-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading and portal blood flow. Eur Radiol 20:2405–2413
Huppertz A, Haraida S, Kraus A et al (2005) Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT: initial observations. Radiology 234:468–478
Kim SH, Kim SH, Lee J et al (2009) Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR 192:1675–1681
Reimer P, Rummeny EJ, Daldrup HE et al (1997) Enhancement characteristics of liver metastases, hepatocellular carcinomas, and hemangiomas with Gd-EOB-DTPA: preliminary results with dynamic MR imaging. Eur Rad 7:275–280
Kim JE, Kim SH, Lee SJ, Rhim H (2011) Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. AJR 196:W758–W765
Motosugi U, Ichikawa T, Sou HU et al (2010) Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology 256:151–158
Saito K, Kotake F, Ito N et al (2005) Gd-EOB-DTPA enhanced MRI for hepatocellular carcinoma: quantitative evaluation of tumor enhancement in hepatobiliary phase. Magn Reson Med Sci 4:1–9
Park Y, Kim SH, Kim SH et al (2010) Gadoxetic acid (Gd-EOB-DTPA)-enhanced MR versus gadobenate dimeglumine (Gd-BOPTA)-enhanced MRI for preoperatively detecting hepatocellular carcinoma: an initial experience. Korean J Radiol 11:433–440
Narita M, Hatano E, Arizono S et al (2009) Expression of AOTP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol 44:793–798
Sun HY, Lee JM, Shin CI et al (2010) Gadoxetic acid-enhanced magnetic resonance imaging for differentiating small hepatocellular carcinomas (< or = 2 cm in diameter) from arterial enhancing pseudolesions: special emphasis on hepatobiliary phase imaging. Invest Radiol 45:96–103
Kitao A, Zen Y, Matsui O et al (2010) Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR imaging correlation with molecular transporters and histopathologic features. Radiology 256:817–826
Akai H, Kiryu S, Matsuda I et al (2011) Detection of hepatocellular carcinoma by Gd-EOB-DTPA-enhanced liver MRI: comparison with triple phase 64 detector row helical CT. Eur J Radiol 80:310–315
Di Martino M, Marin D, Guerrisi A et al (2010) Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology 256:806–816
Saito K, Moriyasu F, Sugimoto K et al (2011) Diagnostic efficacy of gadoxetic acid-enhanced MRI for hepatocellular carcinoma and dysplastic nodule. World J Gastroenterol 17:3503–3509
Suh YJ, Kim MJ, Choi JY, Park YN, Park MS, Kim KWYJ (2011) Differentiation of hepatic hyperintense lesions seen on gadoxetic acid-enhanced hepatobiliary phase MRI. AJR 197:W44–W452
Kim YK, Kim CS, Han YM, Park G, Hwang SB, Yu HC (2010) Comparison of gadoxetic acid-enhanced MRI and superparamagnetic iron oxide-enhanced MRI for the detection of hepatocellular carcinoma. Clin Radiol 65:358–365
Choi JY, Kim MJ, Park YN et al (2011) Gadoxetate disodium-enhanced hepatobiliary phase MRI of hepatocellular carcinoma: correlation with histological characteristics. AJR 197:399–405
Okada M, Imai Y, Kim T et al (2010) Comparison of enhancement patterns of histologically confirmed hepatocellular carcinoma between gadoxetate- and ferucarbotran-enhanced magnetic resonance imaging. J Magn Reson Imaging 32:903–913
Kitao A, Matsui O, Yoneda N et al (2011) The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Rad 21:2056–2066
Chou CT, Chen YL, Su WW, Wu HK, Chen RC (2010) Characterization of cirrhotic nodules with gadoxetic acid-enhanced magnetic resonance imaging: the efficacy of hepatocyte-phase imaging. J Magn Reson Imaging 32:895–902
Sano K, Ichikawa T, Motosugi U et al (2011) Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology 261:834–844
Tsuboyama T, Onishi H, Kim T et al (2010) Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging—correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology 255:824–833
Asayama Y, Tajima T, Nishie A et al (2011) Uptake of Gd-EOB-DTPA by hepatocellular carcinoma: radiologic-pathologic correlation with special reference to bile production. Eur J Radiol 80:e243–e248
Ni Y, Marchal G, Yu J, Mühler A, Lukito G, Baert AL (1994) Prolonged positive contrast enhancement with Gd-EOB-DTPA in experimental liver tumours: potential value in tissue characterization. J Magn Reson Imaging 4:355–363
Fujita M, Yamamoto R, Fritz-Zieroth B et al (1996) Contrast enhancement with Gd-EOB-DTPA in MR imaging of hepatocellular carcinoma in mice: a comparison with superparamagnetic iron oxide. J Magn Reson Imaging 6:472–477
Vogl TJ, Kümmel S, Hammerstingl R et al (1996) Liver tumours: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology 200:59–67
Marchal G, Zhang X, Ni Y, Van Hecke P, Yu J, Baert AL (1993) Comparison between Gd-DTPA, Gd-EOB-DTPA, and Mn-DPDP in induced HCC in rats: a correlation study of MR imaging, microangiography, and histology. Magn Reson Imaging 11:665–674
Jung G, Breuer J, Poll LW et al (2006) Imaging characteristics of hepatocellular carcinoma using the hepatobiliary contrast agent Gd-EOB-DTPA. Acta Radiol 47:15–23
Fujita M, Yamamoto R, Takahashi M et al (1997) Paradoxic uptake of Gd-EOB-DTPA by hepatocellular carcinoma in mice: quantitative image analysis. J Magn Reson Imaging 7:768–770
Tsuda N, Matsui O (2010) Cirrhotic rat liver: reference to transporter activity and morphologic changes in bile canaliculi-gadoxetic acid-enhanced MR imaging. Radiology 256:76
Choi JW, Lee JM, Kim SJ, Yoon JH, Baek JH, Han JK, Choi BI (2013) Hepatocellular carcinoma: imaging patterns on gadoxetic acid-enhanced MR Images and their value as an imaging biomarker. Radiology 267:776–786
Rhee H, Kim MJ, Park MS, Kim KA (2012) Differentiation of early hepatocellular carcinoma from benign hepatocellular nodules on gadoxetic acid-enhanced MRI. Br J Radiol 85:e837–844
Kim HY, Choi JY, Kim CW et al (2012) Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging predicts the histological grade of hepatocellular carcinoma only in patients with Child–Pugh class A cirrhosis. Liver Transpl 18:850–857
Kim JY, Kim MJ, Kim KA, Jeong HT, Park YN (2012) Hyperintense HCC on hepatobiliary phase images of gadoxetic acid-enhanced MRI: correlation with clinical and pathological features. Eur J Radiol 81:3877–3882
Park MJ, Kim YK, Lee MW et al (2012) Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology 264:761–770
Kobayashi S, Matsui O, Gabata T et al (2012) Relationship between signal intensity on hepatobiliary phase of gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)-enhanced MR imaging and prognosis of borderline lesions of hepatocellular carcinoma. Eur J Radiol 81:3002–3009
Inoue T, Kudo M, Komuta M et al (2012) Assessment of Gd-EOB-DTPA-enhanced MRI for HCC and dysplastic nodules and comparison of detection sensitivity versus MDCT. J Gastroenterol 47:1036–1047
Rhee H, Kim MJ, Park YN, Choi JS, Kim KS (2012) Gadoxetic acid-enhanced MRI findings of early hepatocellular carcinoma as defined by new histologic criteria. J Magn Reson Imaging 35:393–398
Akai H, Matsuda I, Kiryu S et al (2012) Fate of hypointense lesions on Gd-EOB-DTPA- enhanced magnetic resonance imaging. Eur J Radiol 81:2973–2977
An C, Park MS, Jeon HM et al (2012) Related citations prediction of the histopathological grade of hepatocellular carcinoma using qualitative diffusion-weighted, dynamic, and hepatobiliary phase MRI. Eur Radiol 22:1701–1708
Nakamura Y, Tashiro H, Nambu J, Ohdan H, Kakizawa H, Date S, Awai K (2013) Detectability of hepatocellular carcinoma by gadoxetate disodium-enhanced hepatic MRI: tumor-by-tumor analysis in explant livers. J Magn Reson Imaging 37:684–691
Lee MH, Kim SH, Park MJ, Park CK, Rhim H (2011) Gadoxetic acid-enhanced hepatobiliary phase MRI and high b-value diffusion-weighted imaging to distinguish well-differentiated hepatocellular carcinomas from benign nodules in patients with chronic liver disease. Am J Roentgenol 197:W868–W875
Takahashi M, Maruyama H, Shimada T et al (2013) Characterization of hepatic lesions (≤30 mm) with liver-specific contrast agents: a comparison between ultrasound and magnetic resonance imaging. Eur J Radiol 82:75–84
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erra, P., Puglia, M., Ragozzino, A. et al. Appearance of hepatocellular carcinoma on gadoxetic acid-enhanced hepato-biliary phase MR imaging: a systematic review. Radiol med 120, 1002–1011 (2015). https://doi.org/10.1007/s11547-015-0539-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-015-0539-8