Abstract
The Colorado potato beetle (CPB), Leptinotarsa decemlineata (Say), is widely considered as the most serious insect defoliator of potato plants (Solanum tuberosum L.). The CPB can completely destroy potato crops and cause tremendous yield losses if left uncontrolled. For decades, CPB populations have been suppressed mainly by chemical insecticides. However, this insect’s diverse and flexible life history, combined with its remarkable adaptability to a variety of stressors, makes the CPB a very challenging pest to control. Potato foliage, which contains high amounts of volatile and non-volatile chemicals, is the CPB’s main food source. Researchers indicated that variations in the feeding performance and abundance of this beetle are attributable to the quality and quantity of chemical components in the host plant foliage. This review investigated the effects of volatile chemicals, carbohydrates, amino acids, glycoalkaloids, and mineral elements in potato foliage on the feeding behaviour and performance of the CPB. In general, the chemical components in potato foliage could enhance or reduce the feeding of the CPB. Altering the chemical composition of potato foliage could be an interesting alternative to reduce the use of insecticides to manage CPB populations in potato crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Colorado potato beetle (CPB), Leptinotarsa decemlineata (Say), is one of the most destructive insect pests of potato crops (Solanum tuberosum L.) (de Ladurantaye et al. 2010). Approximately 40 cm2 of potato leaves can be consumed by a single beetle during the larval stage, and close to 10 cm2 of foliage is consumed per day during the adult stage (Ferro et al. 1985). In North America, potential potato yield losses have been estimated at 30 to 50% due to uncontrolled CPB populations (Boiteau 2010). Although the scientific community and commercial producers have paid a great deal of attention to the CPB, it remains a real threat to the potato industry in already colonized potato growing areas and continues to expand its geographic range into new regions of the world.
Colorado potato beetle populations are usually suppressed by means of chemical insecticides, which are likely to remain the predominant approach for the foreseeable future (Alyokhin 2009). Over the years, the CPB has rapidly developed resistance to most registered chemical insecticides. Many other control approaches have also been attempted in the past decades. For example, crop rotation is an effective and easily implemented cultural practice for controlling this pest (Wright 1984). However, the lack of annual rotation in North America has been the primary limiting factor for managing this beetle (Alyokhin et al. 2015). Moreover, Khelifi et al. (2007) have reviewed many physical control methods, including physical barriers and thermal, pneumatic, and electromagnetic control. However, none of these approaches can be applied alone to successfully manage the CPB on a large scale. In recent years, genetic technology was applied as an alternative to chemical pesticides. Zhang et al. (2015) used long double-stranded RNAs to trigger a lethal RNA interference and disorder the essential gene expression in the body of the CPB. The drawback of this technology is that it is difficult to implement under real-field conditions because the targeted RNAs are hampered by the presence of the endogenous plant RNAi pathway. Another frequently used method is to make host plants express toxic proteins in their leaves in order to reduce CPB population. Cingel et al. (2017) transformed three potato cultivars (Desiree, Dragacevka, and Jelica) through adding rice cystatin oryzacystatin I and II genes and observed that the co-expression of the proteinase inhibitors oryzacystatin I and II could decrease plant damage caused by CPB. So far, transgenic crops are not yet popular with customers because it is unknown how safe they are.
During its life cycle and development process, the CPB requires a huge quantity of nutrients for its growth from larva to adult. All the required nutrients come from host plants via the CPB’s feeding behaviours. Previous studies have determined that the feeding behaviour of the CPB is attributed to the nutrients’ quality and quantity although other factors, such as air temperature, are also important. Hsiao and Fraenkel (1968) carried out a lab test using agar media to evaluate the effects of different nutrients on the CPB’s feeding behaviour and observed that sucrose and several amino acids (alanine, γ-aminobutyric acid, and serine) elicited marked feeding behaviour in CPB larvae. Furthermore, the lack of specific food materials in the tissues of some resistant plants may explain why those plants have a detrimental effect on the CPB growth (Cibula et al. 1967). Except for foliar nutrients, there are also some toxic chemicals, such as glycoalkaloids and non-protein amino acids, that can inhibit CPB feeding. Therefore, the chemical composition (nutrients and toxic chemicals) of host plants can affect the CPB’s growth and feeding behaviour. The objective of this review was therefore to provide an in-depth look at the impact of foliar chemicals (volatiles and non-volatiles) on CPB growth and development.
Life Cycle of the Colorado Potato Beetle
The complete life cycle of the CPB consists of four stages (Fig. 1). At the end of summer, a considerable number of adults move outside potato fields and prepare for overwintering diapause (De Kort 1990). They burrow themselves into the soil to an average depth of 20 to 25 cm (Casagrande 2014), even reaching as far as 120 cm in severe climates in Canada (Khelifi et al. 2007). In the spring, the overwintered adults emerge from the ground when the air temperature exceeds 10 °C and begin searching for host plants in order to obtain the essential nutrients for their development (Jansson and Smilowitz 1985). After colonizing host vegetation, the overwintered adults begin feeding. Once well fed, the females lay orange eggs on the underside of potato leaves. The eggs are generally laid in clusters of 20 to 30 eggs each (Khelifi et al. 2007). The CPB is very prolific, and a single female could lay approximately 300 to 800 eggs over its lifespan (Harcourt 1971). The eggs normally hatch within 1 week, and red-coloured larvae with some blackish spots emerge. These larvae go through four stages during a 3-week period, reaching their full development at a length of 0.5 to 1.25 cm. When the fourth instar larva ceases feeding, it drops on the ground and burrows into the soil to pupate. Adult beetles emerge from below ground 1 to 2 weeks later to complete their life cycle. Depending on the climate conditions and food availability, one to four generations of CPB can occur during a single year.
Life cycle of the Colorado potato beetle: (1) eggs, (2) larvae, which go through four stages, (3) pupae, and (4) adults (Maharijaya and Vosman 2015)
Chemical Composition of Potato Foliage
After emerging from the ground in spring, CPBs begin searching for host plants in order to find the nutrients essential for their development. In this context, volatile chemicals from the host plants play an important role in host plant recognition and acceptance. Once they inhabit the plants, the CPBs begin feeding and require a huge quantity of nutrients for their growth and development. The feeding behaviours of the CPB are attributed to the quality and quantity of the chemical composition of the host plants (Hsiao and Fraenkel 1968). Therefore, it would be worthwhile to review the chemical composition of host plant foliage, including volatile and non-volatile chemicals. Characterizing and quantifying these chemicals is the starting point for the eventual repression of the CPB. Altering the chemical composition of potato leaves without affecting the quality and yield of tubers could therefore represent an interesting alternative to reduce the use of chemical insecticides to manage the CPB populations.
Some volatile chemicals of potato leaves have been identified as the main components of potato odour to attract the CPB by stimulating its olfactory receptors, namely (E)-2-hexen-1-ol, (Z)-3-hexen-1-ol, 1-hexano, (E)-2-hexenal, hexanal, and (Z)-3-hexenyl-acetate (Visser 1979; Dickens 2002). The concentrations of these volatile chemicals in potato leaves are closely related to many factors. Agelopoulos et al. (1999) observed that the variation in volatile chemicals was dependent on the mechanical damage of potato leaves. The volatiles of (Z)-3-hexenal, (E)-2-hexenal, and (Z)-3-hexen-1-ol were released in high amounts after potato leaf damage when compared to intact leaves. The emission of volatile compounds was also affected by the potato cultivar. Vancanneyt et al. (2001) reported that 187.4 nmol (E)-2-hexenal was released from 24 plants in 30 min for cultivar Desiree but no (E)-2-hexenal emission was detected from potato cultivar Granola (Schuetz et al. 1997). However, very little information related to the variation of these components in potato leaves during the entire growing season between different cultivars was found in the literature.
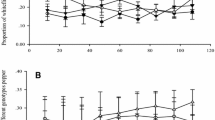
The carbohydrates in potato foliage vary significantly according to the leaf age. As a major carbohydrate, sucrose concentrations reach their highest point in the oldest potato foliage (Fig. 2). However, glucose and fructose concentrations peak at about 60 days after potato plant emergence in the fruit development stage (Kolbe and Stephan-Beckmann 1997). For each growing day, the sugar content reaches its peak value in the afternoon and drops to its lowest point before sunrise (Kolbe and Stephan-Beckmann 1997). This variation is mainly due to the sugar metabolism process, which depends on photosynthesis and respiration in higher plants. Additionally, the carbohydrates concentrations in potato leaves were affected by cultivation practice, such as nitrogen (N) fertilization and cultivar. Braun et al. (2016) reported that more sugars accumulated in potato leaves when N rate increased from 0 to 300 kg N ha−1. Potato cultivar is also an important factor to affect the carbohydrates concentrations in leaves. Lafta and Lorenzen (1995) found that potato cultivars Norchip and Up-to Date have different carbohydrates accumulation capacity because they have different sensitivity to climatic conditions, such as air temperature.
Variations in sucrose, glucose, and fructose in potato foliage as a function of days after emergence (Kolbe and Stephan-Beckmann 1997). DM, dry matter
The quantity of amino acids in potato foliage varies seasonally. According to Cibula et al. (1967), the content of amino acids is one to five times higher in young potato plant foliage than in senescent foliage. A similar result was obtained by Domek et al. (1995), who indicated that concentrations of amino acids are higher in young than in older foliage. However, Kolbe and Stephan-Beckmann (1997) showed that the contents of amino acids increase at the end of the growing season owing to the catabolism of protein in the leaves. To explore the variation in amino acid content, Karley et al. (2002) compared young and old potato leaves and indicated that young plants are mainly composed of non-essential amino acids (glutamine, asparagine, serine, and threonine), while old foliage is dominated by the essential amino acids (arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine). In practical terms, the metabolism of amino acids in potato foliage is a complex process that depends on various elements, such as weather conditions and mineral fertilizers (Miflin and Lea 1977; Foyer et al. 2003; Muttucumaru et al. 2013).
The major glycoalkaloids found in potato plants are a mixture of α-chaconine and α-solanine (Friedman 2004) and their concentration ratios range from 2:1 to 7:1 (Speijers 1998; Bejarano et al. 2000). In potato leaves, the glycoalkaloids reach their maximum concentration early in the growing season, and decrease markedly thereafter in older leaves (Peferoen et al. 1981). The highest levels of glycoalkaloids are found in the tissues of new leaves, fruits, flowers, and sprouts because they have the highest metabolic activity (Friedman et al. 1997). The concentration of glycoalkaloids in plants is partially sensitive to stress, such as mechanical wounding and light exposure (Petersson et al. 2013).
Inorganic salts are a crucial part of plant nutrition and their presence plays an important role in the healthy growth of plants. An excess or deficiency of inorganic salts in potato tissues may disturb the metabolism or favour plant pathogens. Taking potassium as an example, Subhani et al. (2015) investigated three potato cultivars (FD 8-1, N-22, and SH788) and found a decreasing tendency over time in all potato cultivars. Although other mineral salts are also important in potato plant resistance, there is not much related information from previous research.
Roles of Chemical Composition in the Management of the Colorado Potato Beetle
Leaf Surface Chemicals
Many kinds of volatile chemicals can be biosynthesized in plant leaves, releasing different odours. These odours play an important role for herbivorous pests searching for food sources. Regarding the CPB, this insect pest begins searching for food immediately after emergence from the ground in spring. It identifies the different odours through its olfactory system and then locates the preferred plants. It is well established that these odours come from volatile chemicals in the plants (Wilde et al. 1969).
It is well known that the CPB prefers potato crops, followed by eggplants and tomatoes (Hitchner et al. 2008). The main reason is probably that different plants contain different volatile chemicals and release different odours. Some odours can enhance the interest of the beetles, and others may be repulsive to them. Mitchell and McCashin (1994) studied the response of galeal sensilla to volatile chemicals (Fig. 3) and found that the primary alcohols (hexanol and heptanol) and other components, such as the monounsaturated (Z)- and (E)-isomers of hexen-1-ol and (E)-2-hexenal strongly appeal to the CPB. However, some other volatiles, such as β-caryophyllene and β-selinene, produced little response in the CPB (Visser 1979; Dickens 2002). On the other hand, the quantity of volatiles in plants is also important during host plant recognition. It is well known that the CPB shows different degrees of attraction depending on the age and damage conditions even in the same plants. Bolter et al. (1997) found that the CPB prefers potato plants of 5 to 6 weeks old over those of 2 to 3 weeks old, and the damaged plants become more attractive to CPB than undamaged plants (Schuetz et al. 1997). This could be explained by the larger quantity of volatile chemicals in injured plants, which is seven to ten times higher than in healthy plants (Bolter et al. 1997). Overall, the relationship between volatile chemicals and the CPB’s feeding behaviour is complex. Thiery and Visser (1995) indicated that starved beetles showed more sensitivity to potato volatile chemicals compared with fed beetles, and some starved CPBs were even attracted to undamaged potato plants (Visser 2011). This phenomenon suggests that hunger is also an important factor in determining the final food source selection for the CPB. Further studies should be conducted to consider all aspects of climate, plant cultivation, and the life habits of the CPB related to the search for food.
Scanning electron micrograph showing the front view of the head of an adult Colorado potato beetle, with mandibles and antennal flagella removed. ANT, antennal scape; LB, labrum; MD, base of mandible; MP, maxillary palp; LP, labial palp; GA, galea; E, compound eye (Mitchell and Harrison 1984)
After CPB arrives on the host plants, its feeding begins to be closely related to the cuticular waxes on the leaf surface. Szafranek et al. (2008) stated that cuticular waxes may be involved in host plant recognition by the CPB. Prüm et al. (2013) reported that cuticular waxes covering the plant leaf surface have strongly reduced the ability of insects to cling to them. The possible reasons are (1) the cuticular waxes may make the leaf surface smooth, preventing the beetles from gripping firmly; and (2) the waxes have anti-adhesive properties and they can interact with the insect’s adhesive fluid, resulting in a slippery leaf surface. However, Harrison (1987) indicated that some cuticular waxes, such as sterol of cholesterol, b-sitosterol, and stigmasterol, stimulate CPB feeding. Szafranek et al. (2008) studied the cuticular wax composition of potato leaves and found that alkanes, sesquiterpene hydrocarbons, wax esters, benzoic acid esters, fatty acid methyl, ethyl, isopropyl and phenylethyl esters, aldehydes, ketones, methyl ketones, fatty acids, primary alcohols, b-amyrin, and sterols did not affect adult CPB feeding. Also, alkanes, sesquiterpene hydrocarbons, wax esters, methyl ketones, sesquiterpene alcohols, and secondary alcohols had no effect on larval CPB feeding.
Overall, foliage chemicals, including volatiles and cuticular waxes, are the critical components in the host plant search and recognition process of the CPB. These chemicals are also influenced by many factors, such as the cultivar (Szafranek and Synak 2006), the circumstance conditions (temperatures, water stress, and photosynthetic radiation), and plant damage (Shepherd and Griffiths 2006).
Carbohydrates
The CPB adults begin feeding on the leaves immediately after successfully damaging the cuticular waxes on the leaf surface. During this process, the nutrients in host plant foliage are essential for CPB growth. Hsiao and Fraenkel (1968) showed that nutrients can elicit marked feeding responses in CPBs and that the absence of specific food elements in plant tissues can inhibit the beetles’ growth. Sugars, a series of carbohydrate nutrients in potato foliage, play a significant role in CPB development and growth (Weeda et al. 1979). This significant role was mainly referred to as (1) promoting feeding behaviours (Mitchell 1974); (2) serving as an energy source during flight (Arrese and Soulages 2010); (3) providing energy for CPB diapause (Lefevere et al. 1989); and (4) tolerating thermal stress, particularly in high latitudes (Storey 1997). The importance of sugars to CPB feeding was studied decades ago. A summary of the effects of sugars on the CPB feeding is presented in Table 1.
The quantity and quality of sugar-related compounds in potato foliage have a significant effect on the feeding behaviour of the CPB. Hsiao and Fraenkel (1968) conducted an agar-medium culture test to examine the response of CPB feeding performance to various sugars and related compounds. They reported that sucrose can stimulate and promote feeding behaviour in the CPB, while fructose and glucose have little effect on the development of the CPB. These results may be attributed to the sensitivity of the sensilla, present on the galeae of the CPB (Fig. 3). There are many sensilla and all of them are sensitive to sucrose (Mitchell and Harrison 1984). Therefore, some sugars can enhance the feeding of the CPB and provide energy for the beetle’s growth.
A previous study on the variation of sugars in potato foliage showed that the sucrose content increases throughout the potato growing season, with a sharp increase in the first 40 days after potato plant emergence (Kolbe and Stephan-Beckmann 1997); that increase could be related to the rapid development of CPB populations from the larval stage to adulthood (Hsiao and Fraenkel 1968). However, fructose and glucose were found to have little effect on CPB development (Hsiao and Fraenkel 1968).
For the CPB, flight is more important than walking for the colonization of new habitats and also for escaping from hostile environments (Weber and Ferro 1994). It is well established that flight muscles meet their energy requirements by carbohydrate consumption. Weeda et al. (1979) reported that carbohydrates such as glycogen and glucose are decreased in the flight muscles of the CPB, which indicates that these carbohydrates provide energy for flight. Furthermore, the glucose concentration in the flight muscles of the CPB was found to be reduced during starvation as well, which supports the idea that CPB flight muscles have the capacity to utilize carbohydrates when there is a lack of food (Weeda et al. 1979). Lack of necessary carbohydrates may incapacitate the CPB during flight for food searches (Arrese and Soulages 2010). Moreover, insufficient energy sources may shorten the diapause period and result in death under unfavourable conditions (Lefevere et al. 1989). Further studies should be conducted to clarify the impacts of potato leaf carbohydrates on CPB growth for the purpose of reducing its population.
Amino Acids
Protein Amino Acids
Protein amino acids refer to the basic 20 standard genetic code amino acids and are the building blocks of protein biosynthesis in organisms (Lu and Stephen 2006). It is well known that protein amino acids are essential for the CPB as well (Dortland and de Kort 1978). The actual concentration of amino acids reflected the steady state among protein synthesis, proteolysis, and transport processes to and from the organs involved. Tomlin and Sears (1992) reported that CPB populations vary greatly when they receive different categories and concentrations of amino acids. If amino acids are deficient, some problems could occur in terms of protein metabolism and some protein deficiency diseases in beetles may appear subsequently (Lee et al. 2008). The lack of essential amino acids in the tissues of potato leaves may have detrimental effects on CPBs growth, including lengthening the pupation period (Cibula et al. 1967).
Protein amino acids also affect the feeding behaviour of CPBs. Hsiao and Fraenkel (1968) reported that protein amino acids could serve as effective feeding stimulants for the CPB. Several aliphatic amino acids (glycine, alanine, serine, and valine), the sulfur-containing amino acid cysteine, and the heterocyclic amino acid proline can elicit marked feeding responses and promote CPB development (Hsiao and Fraenkel 1968). Some amino acids (leucine and isoleucine) could serve as stimulants to promote the beetle’s growth when their content in potato foliage reaches a relatively high level (Hsiao and Fraenkel 1968; Domek et al. 1995). In addition, protein amino acids are beneficial for the CPB during diapause and flight. For example, proline might have a cryoprotective function during diapause (Lefevere et al. 1989). It can also provide energy during the CPB’s flight in search of food. Brouwers and de Kort (1979) observed a significant decrease in the proline and glutamate content during flight with a concomitant accumulation of alanine. This indicates that proline and glutamate stored in the muscles may represent two energy amino acids for the CPB, and they form alanine subsequently through the transamination pathway. However, not all amino acids can induce such positive stimulation. The amino acids lysine, arginine, and histidine were found to have little or no effect on the feeding behaviour of the CPB (Hsiao and Fraenkel 1968). It was concluded that protein amino acids are essential for CPB growth, influencing feeding and flight behaviours. Although the CPB has been evolving and adapting to diverse climates, it also requires a considerable amount and high quality of protein amino acids for its health. A better way to control the CPB may be to find a cultivar that accumulates more protein amino acids in the tubers and less in the host plant leaves.
Non-protein Amino Acids
Generally, the non-protein amino acids are analogs or derivatives of genetic code amino acids. They are common in plants and are usually used to protect plants against insects (McSweeney et al. 2008). The toxicity of non-protein amino acids is related to their ability to replace a protein amino acid in a metabolic pathway or biological process after being absorbed by insects. They are easily misincorporated into proteins, making them nonfunctional or toxic, although the protein synthesizing machinery can discriminate between the protein and non-protein amino acids. Some non-protein amino acids can even block the synthesis or uptake of protein amino acids (Singh 2018).
Canavanine, an arginine analog, plays a pivotal role in plant chemical defence against herbivorous insects (Rosenthal 2001). Nakajima et al. (2001) stated that canavanine is highly toxic to a wide range of organisms including bacteria, fungi, algae, and insects. Incorporating canavanine instead of arginine is considered a major mode of action and would produce structurally aberrant proteins and then bring toxicity to insects, such as Manduca sexta and Heliothis virescens (Rosenthal and Dahlman 1986; Berge et al. 1986). However, little information was found in the literature about the toxicity of canavanine to the CPB. Due to its toxicity to many other insects, more studies should be carried out to investigate the relationship between canavanine and the CPB growth and feeding behaviours.
The GABA is another important non-protein amino acid. Huang et al. (2011) reported that the presence of GABA in plants could reduce the growth and survival of herbivorous insects. The deleterious effects of GABA on insects result from the inhibition of GABA-gated chloride channels that are important in the peripheral nervous system of insects (Hosie et al. 1997). However, this inhibition may not be applicable to the CPB. Mitchell (1974) compared the responses of fourth instar larva to GABA and found that it was slightly more stimulatory than other amino acids in the same concentrations. This stimulation was caused by the presence of amino acid-sensitive cells in the sensilla on the galea and palpal tips (Fig. 3) (Mitchell and Harrison 1984). Over time, the CPB had already succeeded in developing resistance to toxic proteins. Rivard et al. (2004) studied CPB resistance to proteinase inhibitors. These inhibitors can damage the digestive proteolytic functions, which can be recovered after several generations. The development of such a resistance suggests that using non-protein amino acids to control the CPB may be limited under natural conditions. The effects of protein and non-protein amino acids on the feeding of CPB are presented in Table 2.
Glycoalkaloids
The α-chaconine and α-solanine are two major components of glycoalkaloids, which are a series of secondary metabolites and are produced in every plant organ. Generally, glycoalkaloids serve as stress metabolites or phytoalexins for protection against insects (Singh 2018). Sablon et al. (2013) mentioned that glycoalkaloids in plant foliage can inhibit the feeding behaviour and performance of the CPB. A similar result was obtained by Jonasson and Olsson (1994), who indicated that the high levels of glycoalkaloids in host plant foliage are the key factor in preventing larval feeding, and leaves with lower glycoalkaloid levels are more susceptible to attack by beetles. However, Kowalski et al. (1999) reported that neither α-chaconine nor α-solanine at concentrations commonly found in potato foliage impaired CPB feeding performance. When they reach a high level (about 7 mg g−1 fresh weight), the glycoalkaloids can act as feeding deterrents for the CPB (Sinden et al. 1986; Sablon et al. 2013).
Compared with α-chaconine and α-solanine, another glycoalkaloid of leptine I is more toxic. When the concentration reaches 0.55 mg g−1 fresh weight, leptine I can reduce the CPB feeding to about 60%, and the fatal concentration was 1 mg g−1 fresh weight (Kowalski et al. 1999). Another alkaloid of α-tomatine reduced adult feeding to 50% at 2.0 mg g−1 fresh weight.
Glycoalkaloid-related compounds can affect insects at all levels of biological organization by disturbing cellular and physiological processes, such as by altering the redox balance, hormonal regulation, and neuronal signalization, or reproducing in exposed individuals (Chowński et al. 2016). Glycoalkaloids can act as a cell membrane-disrupting factor to inhibit the activity of beetles (Friedman et al. 1997). Furthermore, these glycoalkaloid-related compounds can disorder the neurons of the chemosensory hairs on the galeae of the CPB and result in the inhibition of the CPB’s feeding behaviour (Hollister et al. 2001). Sinden et al. (1986) believed that the toxicity of α-chaconine and α-solanine to CPB adults and larvae was due to the acetylation after being absorbed by the beetles. Leptine is transformed into leptinine through the loss of the acetyl group after entering the insects’ digestive system, reducing its feeding activity (Kowalski et al. 1999). The approach of using glycoalkaloids for CPB management is limited because the CPB would develop resistance to glycoalkaloids. Lyytinen et al. (2007) studied the glycoalkaloid concentrations in three potato cultivars and observed no significant difference in beetle performance related to the glycoalkaloid content of the potato. Moreover, Armer (2004) showed that fourth instar CPB larvae and adults neither sequester nor metabolize glycoalkaloids (solanine and chaconine). However, many attempts at increasing glycoalkaloid contents of potato foliage have been made in order to reduce CPB population. Lafta and Lorenzen (2000) found that high air temperature at 32 °C could enhance potato foliar glycoalkaloid concentrations up to 169% of that at 27 °C. Additionally, N fertilization also increased glycoalkaloid concentration in potato plant (Mondy and Munshi 1990). Thus, air temperature and mineral fertilization may influence the CPB behaviour through altering glycoalkaloid content of potato leaves. As with chemical insecticides, glycoalkaloids have to be carefully managed, and their activities against various target and non-target species should be further studied. Table 3 summarizes the effects of glycoalkaloids on the feeding of the CPB.
Mineral Elements
Mineral elements are a crucial part of plant nutrition and their presence plays an important role in plant growth. An excess or deficiency in potato tissues may disturb the metabolism or favour plant pathogens (Wang et al. 2013). Minerals also play an important role in protecting plants from pests. For example, potassium (KCl and KH2PO4) and sodium (NaCl) in potato leaves can act as co-factors of phagostimulants and enhance CPB feeding (Hsiao and Fraenkel 1968). Alyokhin et al. (2005) reported that boron has a strong negative effect on all beetle stages except for the overwintered adults, while zinc had a consistently positive effect. Mineral elements, such as phosphorus, iron, and manganese are also important in controlling the CPB and they can explain 40–57% of the variation in CPB populations because they are crucial factors to maintain an optimal nutrient balance in plants which can result in resistance to herbivory (Alyokhin et al. 2005). These minerals are inactive alone but act synergistically with other feeding stimulants. Furthermore, Ballan-Dufrançais (2002) mentioned that the insects possess mineral bioaccumulation structures in the cells of numerous organs. Currently, the mineral accumulation mechanism in insects is still unclear. Further studies should focus on the roles of mineral elements in controlling the CPB feeding behaviour.
Summary and Future Perspectives
This review investigated the responses of CPB to potato foliar chemical compositions. It showed that these chemical compositions can stimulate or inhibit the feeding behaviours of the CPB. Volatiles and cuticular waxes on the leaf surface affect the recognition of the host plants by the CPB after their emergence from the ground. Sugars and protein amino acids can stimulate feeding behaviours and increase the survival rate of the CPB. Non-protein amino acids are used to biosynthesize toxic proteins and then damage the digestive functions of the CPB. High concentrations of glycoalkaloids can disorder the CPB’s neural system and then reduce its feeding capability. Finally, mineral salts are co-factors in stimulating the feeding behaviours of the CPB while working with other stimulants.
Today, research is still being carried out to find effective alternatives to chemicals to control the CPB. Using nutrients in the host plants combined with naturally produced toxic chemicals to alter feeding behaviours and eventually reduce the beetles’ population is a promising alternative to the use of chemical insecticides to manage CPB populations. Managing the chemical compositions of potato leaves is important to increase the plant resistance to CPBs. When nutrients are deficient, such as amino acids and sugar, the CPB cannot biosynthesize enough protein and also lacks energy for its activity and diapause. Sufficient toxic chemicals (non-protein amino acids and glycoalkaloids) can damage the digestive function, which benefits CPB control. Therefore, CPB populations may be reduced significantly if a way to accumulate more toxic chemicals and fewer nutrients in host leaves is found. Many measures can be taken to alter the chemical composition of potato leaves. Using mineral fertilization, particularly N fertilizer, is a basic attempt because of the significant role of N in plant metabolism. Additionally, keep in mind that it is critical to balance the mineral fertilizers input which can dramatically enhance the host plants’ resistance to pests. Another important measure is potato cultivar selection. Differences in the chemical composition of different host plants are basically dependent on genotype. In the literature, it is reported that some wild Solanum species have much higher levels of resistance sources, such as glycoalkaloids, against CPB than potato cultivars (Friedman 2006). Therefore, intercrossing two potato cultivars may vary the levels of nutrients and toxic compounds and then improve the host plants’ resistance. An innovative potato cultivar will probably accumulate a large fraction of beneficial nutrients in the tubers and toxic metabolites in the leaves. Many other factors, such as air temperatures and rainfall, also remarkably influence the responses of CPB to host plants. In practice, the manipulation of potato foliar chemical compositions is a complicated process because the metabolism process in plants is really complex and is dependent on various climatic and cultivation conditions. More studies need to be carried out to investigate how the chemical composition of potato foliage can be varied without altering the quality and yield of tubers.
References
Agelopoulos NG, Hooper AM, Maniar SP, Pickett JA, Wadhams LJ (1999) A novel approach for isolation of volatile chemicals released by individual leaves of a plant in situ. J Chem Ecol 25(6):1411–1425. https://doi.org/10.1023/A:1020939112234
Alyokhin A (2009) Colorado potato beetle management on potatoes: current challenges and future prospects. Fruit Veg Cereal Sci Biotechnol 3:10–19
Alyokhin A, Mota-Sanchez D, Baker M, Snyder WE, Menasha S, Whalon M, Dively G, Moarsi WF (2015) The Red Queen in a potato field: integrated pest management versus chemical dependency in Colorado potato beetle control. Pest Manag Sci 71:343–356. https://doi.org/10.1002/ps.3826
Alyokhin A, Porter G, Groden E, Drummond F (2005) Colorado potato beetle response to soil amendments: a case in support of the mineral balance hypothesis? Agric Ecosyst Environ 109:234–244. https://doi.org/10.1016/j.agee.2005.03.005
Armer CA (2004) Colorado potato beetle toxins revisited: evidence the beetle does not sequester host plant glycoalkaloids. J Chem Ecol 30(4):883–888. https://doi.org/10.1023/B:JOEC.0000028495.26931.c7
Arrese EL, Soulages JL (2010) Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol 55:207–225. https://doi.org/10.1146/annurev-ento-112408-085356
Ballan-Dufrançais C (2002) Localization of metals in cells of pterygote insects. Microsc Res Tech 56:403–420. https://doi.org/10.1002/jemt.10041
Bejarano L, Mignolet E, Devaux A, Espinola N, Carrasco E, Larondelle Y (2000) Glycoalkaloids in potato tubers: the effect of variety and drought stress on the α-solanine and α-chaconine contents of potatoes. J Sci Food Agric 80:2096–2100. https://doi.org/10.1002/1097-0010(200011)80:14<2096::AID-JSFA757>3.0.CO;2-6
Berge MA, Rosenthal GA, Dahlman DL (1986) Tobacco budworm, Heliothis virescens [Noctuidae] resistance to L-canavanine, a protective allelochemical. Pestic Biochem Physiol 25:319–326. https://doi.org/10.1016/0048-3575(86)90005-2
Boiteau G (2010) Insect pest control on potato: harmonization of alternative and conventional control methods. Am J Potato Res 87:412–419. https://doi.org/10.1007/s12230-010-9158-z
Bolter CJ, Dicke M, Van Loon JJ, Visser JH, Posthumus MA (1997) Attraction of Colorado potato beetle to herbivore-damaged plants during herbivory and after its termination. J Chem Ecol 23:1003–1023. https://doi.org/10.1023/B:JOEC.0000006385.70652.5e
Braun H, Fontes PCR, Silva TPD, Finger FL, Cecon PR, Ferreira APS (2016) Carbohydrates concentration in leaves of potato plants affected by nitrogen fertilization rates. Revista Ceres 63(2):241–248. https://doi.org/10.1590/0034-737X201663020016
Brouwers EVM, de Kort CAD (1979) Amino acid metabolism during flight in the Colorado potato beetle, Leptinotarsa decemlineata. J Insect Physiol 25:411–414. https://doi.org/10.1016/0022-1910(79)90008-8
Casagrande RA (2014) The Colorado potato beetle: 125 years of mismanagement. Bull Entomol Soc Am 33:142–150. https://doi.org/10.1093/besa/33.3.142
Chowński S, Adamski Z, Marciniak P, Rosiński G, Büyükgüzel E, Büyükgüzel K, Falabella P, Scrano L, Ventrella E, Lelario F, Bufo SA (2016) A review of bioinsecticidal activity of Solanaceae alkaloids. Toxins 8:60. https://doi.org/10.3390/toxins8030060
Cibula AB, Davidson RH, Fisk FW, Lapidus JB (1967) Relationship of free amino acids of some solanaceous plants to growth and development of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Ann Entomol Soc Am 60:626–631. https://doi.org/10.1093/aesa/60.3.626
Cingel A, Savić J, Lazarević J, Ćosić T, Raspor M, Smigocki A, Ninković S (2017) Co-expression of the proteinase inhibitors oryzacystatin I and oryzacystatin II in transgenic potato alters Colorado potato beetle larval development. Insect Sci 24(5):768–780
de Kort CAD (1990) Thirty five years of diapause research with the Colorado potato beetle. Entomol Exp Appl 56:1–13. https://doi.org/10.1111/j.1570-7458.1990.tb01376.x
de Ladurantaye Y, Khelifi M, Cloutier C, Coudron TA (2010) Short-term storage conditions for transport and farm delivery of the stink bug Perillus bioculatus for the biological control of the Colorado potato beetle. Can Biosyst Eng Genie Biosyst Au Can 52:4.1–4.7
Dortland JF, de Kort CAD (1978) Protein synthesis and storage in the fat body of the Colorado potato beetle, Leptinotarsa decemlineata. Insect Biochem 8(2):93–98. https://doi.org/10.1016/0020-1790(78)90044-6
Dickens JC (2002) Behavioural responses of larvae of Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae), to host plant volatile blends attractive to adults. Agric For Entomol 4:309–314. https://doi.org/10.1046/j.1461-9563.2002.00153.x
Domek JM, Cantelo WW, Wagner RM, Li BW, Miller-Ihli NJ (1995) Nutritional composition of potato foliage. J Agric Food Chem 43:1512–1515. https://doi.org/10.1021/jf00054a018
Ferro DN, Logan JA, Voss RH, Elkinton JS (1985) Colorado potato beetle (Coleoptera: Chrysomelidae) temperature-dependent growth and feeding rates. Environ Entomol 14:343–348. https://doi.org/10.1093/ee/14.3.343
Friedman M (2004) Analysis of biologically active compounds in potatoes (Solanum tuberosum), tomatoes (Lycopersicon esculentum), and jimson weed (Datura stramonium) seeds. J Chromatogr A 1054:143–155. https://doi.org/10.1016/j.chroma.2004.04.049
Friedman M (2006) Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J Agric Food Chem 54(23):8655–8681. https://doi.org/10.1021/jf061471t
Friedman M, McDonald GM, Filadelfi-Keszi M (1997) Potato glycoalkaloids: chemistry, analysis, safety, and plant physiology. Crit Rev Plant Sci 16:55–132. https://doi.org/10.1080/07352689709701946
Foyer CH, Parry M, Noctor G (2003) Markers and signals associated with nitrogen assimilation in higher plants. J Exp Bot 54(382):585–593. https://doi.org/10.1093/jxb/erg053
González-Coloma A, Reina M, Medinaveitia A, Guadaño A, Santana O, Martínez-Díaz R, Ruiz-Mesía L, Alva A, Grandez M, Díaz R, Gavín JA (2004) Structural diversity and defensive properties of norditerpenoid alkaloids. J Chem Ecol 30:1393–1408. https://doi.org/10.1023/B:JOEC.0000037747.74665.0a
Harcourt DG (1971) Population dynamics of Leptinotarsa decemlineata (Say) in eastern Ontario. III. Major population processes. Can Entomol 103:1049–1061. https://doi.org/10.4039/Ent1031049-7
Harrison GD (1987) Host-plant discrimination and evolution of feeding preference in the Colorado potato beetle Leptinotarsa decemlineata. Physiol Entomol 12:407–415. https://doi.org/10.1111/j.1365-3032.1987.tb00767.x
Hitchner EM, Kuhar TP, Dickens JC, Youngman RR, Schultz PB, Pfeiffer DG (2008) Host plant choice experiments of Colorado potato beetle (Coleoptera: Chrysomelidae) in Virginia. J Econ Entomol 101:859–865. https://doi.org/10.1603/0022-0493(2008)101[859:HPCEOC]2.0.CO;2
Hollister B, Dickens JC, Perez F, Deahl KL (2001) Differential neurosensory responses of adult Colorado potato beetle, Leptinotarsa decemlineata, to glycoalkaloids. J Chem Ecol 27:1105–1118. https://doi.org/10.1023/A:1010307827348
Hosie AM, Aronstein K, Sattelle DB, ffrench-Constant RH (1997) Molecular biology of insect neuronal GABA receptors. Trends Neurosci 20:578–583. https://doi.org/10.1016/S0166-2236(97)01127-2
Hsiao TH, Fraenkel G (1968) The influence of nutrient chemicals on the feeding behavior of the Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Ann Entomol Soc Am 61:44–54. https://doi.org/10.1093/aesa/61.1.44
Huang T, Jander G, de Vos M (2011) Non-protein amino acids in plant defense against insect herbivores: representative cases and opportunities for further functional analysis. Phytochemistry 72:1531–1537. https://doi.org/10.1016/j.phytochem.2011.03.019
Jansson RK, Smilowitz Z (1985) Influence of nitrogen on population parameters of potato insects: abundance, development, and damage of the Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Environ Entomol 14:500–506. https://doi.org/10.1093/ee/14.4.500
Jonasson T, Olsson K (1994) The influence of glycoalkaloids, chlorogenic acid and sugars on the susceptibility of potato tubers to wireworm. Potato Res 37:205–216. https://doi.org/10.1007/BF02360510
Karley AJ, Douglas AE, Parker WE (2002) Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. J Exp Biol 205:3009–3018
Khelifi M, Laguë C, de Ladurantaye Y (2007) Physical control of Colorado potato beetle: a review. Appl Eng Agric 23:557–569. https://doi.org/10.13031/2013.23663
Kolbe H, Stephan-Beckmann S (1997) Development, growth and chemical composition of the potato crop (Solanum tuberosum L.). I. Leaf and stem. Potato Res 40:111–129. https://doi.org/10.1007/BF02407567
Kowalski SP, Domek JM, Deahl KL, Sanford LL (1999) Performance of Colorado potato beetle larvae, Leptinotarsa decemlineata (Say), reared on synthetic diets supplemented with Solanum glycoalkaloids. Am J Potato Res 76:305–312. https://doi.org/10.1007/BF02853629
Lafta AM, Lorenzen JH (1995) Effect of high temperature on plant growth and carbohydrate metabolism in potato. Plant Physiol 109(2):637–643. https://doi.org/10.1104/pp.109.2.637
Lafta AM, Lorenzen JH (2000) Influence of high temperature and reduced irradiance on glycoalkaloid levels in potato leaves. J Am Soc Hortic Sci 125(5):563–566
Lee KP, Simpson SJ, Wilson K (2008) Dietary protein–quality influences melanization and immune function in an insect. Funct Ecol 22(6):1052–1061. https://doi.org/10.1111/j.1365-2435.2008.01459.x
Lefevere KS, Koopmanschap AB, De K (1989) Changes in the concentrations of metabolites in haemolymph during and after diapause in female Colorado potato beetle, Leptinotarsa decemlineata. J Insect Physiol 35:121–128. https://doi.org/10.1016/0022-1910(89)90045-0
Lu Y, Stephen F (2006) On the evolution of the standard amino-acid alphabet. Genome Biol 7:102. https://doi.org/10.1186/gb-2006-7-1-102
Lyytinen A, Lindström L, Mappes J, Julkunen–Tiitto R, Fasulati SR, Tiilikkala K (2007) Variability in host plant chemistry: behavioural responses and life-history parameters of the Colorado potato beetle (Leptinotarsa decemlineata). Chemoecology 17(1):51–56. https://doi.org/10.1007/s00049-006-0361-9
Maharijaya A, Vosman B (2015) Managing the Colorado potato beetle; the need for resistance breeding. Euphytica 204:487–501. https://doi.org/10.1007/s10681-015-1467-3
McSweeney CS, Collins EMC, Blackall LL, Seawright AA (2008) A review of anti-nutritive factors limiting potential use of Acacia angustissima as a ruminant feed. Anim Feed Sci Technol 147:158–171. https://doi.org/10.1016/j.anifeedsci.2007.09.015
Miflin BJ, Lea PJ (1977) Amino acid metabolism. Annu Rev Plant Physiol 28:299–329
Mitchell BK (1974) Behavioural and electrophysiological investigations on the responses of larvae of the Colorado potato beetle (Leptinotarsa decemlineata) to amino acids. Entomol Exp Appl 17:255–264. https://doi.org/10.1111/j.1570-7458.1974.tb00343.x
Mitchell BK, Harrison GD (1984) Characterization of galeal chemosensilla in the adult Colorado beetle, Leptinotarsa decemlineata. Physiol Entomol 9:49–56. https://doi.org/10.1111/j.1365-3032.1984.tb00680.x
Mitchell BK, McCashin BG (1994) Tasting green leaf volatiles by larvae and adults of Colorado potato beetle, Leptinotarsa decemlineata. J Chem Ecol 20:753–769. https://doi.org/10.1007/BF02059611
Mondy NI, Munshi CB (1990) Effect of nitrogen fertilization on glycoalkaloid and nitrate content of potatoes. J Agric Food Chem 38(2):565–567. https://doi.org/10.1021/jf00092a050
Muttucumaru N, Powers SJ, Elmore JS, Mottram DS, Halford NG (2013) Effects of nitrogen and sulfur fertilization on free amino acids, sugars, and acrylamide-forming potential in potato. J Agric Food Chem 61(27):6734–6742. https://doi.org/10.1021/jf401570x
Nakajima N, Hiradate S, Fujii Y (2001) Plant growth inhibitory activity of L-canavanine and its mode of action. J Chem Ecol 27:19–31. https://doi.org/10.1023/A:1005659714947
Peferoen M, Huybrechts R, De Loof A (1981) Longevity and fecundity in the Colorado potato beetle, Leptinotarsa decemlineata. Entomol Exp Appl 29:321–329. https://doi.org/10.1111/j.1570-7458.1981.tb03075.x
Petersson EV, Arif U, Schulzova V, Krtková V, Hajšlová J, Meijer J, Andersson HC, Jonsson L, Sitbon F (2013) Glycoalkaloid and calystegine levels in table potato cultivars subjected to wounding, light, and heat treatments. J Agric Food Chem 61:5893–5902. https://doi.org/10.1021/jf400318p
Prüm B, Florian Bohn H, Seidel R, Rubach S, Speck T (2013) Plant surfaces with cuticular folds and their replicas: influence of microstructuring and surface chemistry on the attachment of a leaf beetle. Acta Biomater 9:6360–6368. https://doi.org/10.1016/j.actbio.2013.01.030
Rangarajan A, Miller AR, Veilleux RE (2000) Leptine glycoalkaloids reduce feeding by Colorado potato beetle in diploid Solanum sp. hybrids. J Am Soc Hortic Sci 125:689–693
Rivard D, Cloutier C, Michaud D (2004) Colorado potato beetles show differential digestive compensatory responses to host plants expressing distinct sets of defense proteins. Arch Insect Biochem Physiol 55:114–123. https://doi.org/10.1002/arch.10136
Rosenthal GA (2001) L-Canavanine: a higher plant insecticidal allelochemical. Amino Acids 21:319–330. https://doi.org/10.1007/s007260170017
Rosenthal GA, Dahlman DL (1986) L-Canavanine and protein synthesis in the tobacco hornworm Manduca sexta. Proc Natl Acad Sci 83:14–18
Sablon L, Dickens J, Haubruge É, Verheggen F (2013) Chemical ecology of the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: chrysomelidae), and potential for alternative control methods. Insects 4:31–54. https://doi.org/10.3390/insects4010031
Schuetz S, Weissbecker B, Klein A, Hummel H (1997) Host plant selection of the Colorado potato beetle as influenced by damage induced volatiles of the potato plant. Naturwissenschaften 84:212–217. https://doi.org/10.1007/s001140050381
Shepherd T, Griffiths DW (2006) The effects of stress on plant cuticular waxes. New Phytol 171:469–499. https://doi.org/10.1111/j.1469-8137.2006.01826.x
Sinden SL, Sanford LL, Cantelo WW, Deahl KL (1986) Leptine glycoalkaloids and resistance to the Colorado potato beetle (Coleoptera: Chrysomelidae) in Solanum chacoense. Environ Entomol 15:1057–1062. https://doi.org/10.1093/ee/15.5.1057
Singh SK (2018) Explorations of plant’s chemodiversity: role of nitrogen-containing secondary metabolites in plant defense. In: Molecular aspects of plant-pathogen interaction. Springer, Singapore, pp 309–332
Speijers GJA (1998) Risk assessment of potato-glycoalkaloids. In: AIR NETTOX project seminar report. pp 43–47
Storey KB (1997) Organic solutes in freezing tolerance. Comp Biochem Physiol A 117:319–326. https://doi.org/10.1016/S0300-9629(96)00270-8
Subhani MN, Sahi ST, Ali L, Rehman A, Wakil W (2015) Genotypic variations in potassium contents of potato leaves infested with late blight of potato incited by Phytophthora infestans (Mont.) de Bary. J Environ Agri Sci 2:2313–8629
Szafranek BM, Synak EE (2006) Cuticular waxes from potato (Solanum tuberosum) leaves. Phytochemistry 67(1):80–90. https://doi.org/10.1016/j.phytochem.2005.10.012
Szafranek BM, Synak EE, Waligóra D, Szafranek J, Nawrot J (2008) Leaf surface compounds of the potato (Solanum tuberosum) and their influence on Colorado potato beetle (Leptinotarsa decemlineata) feeding. Chemoecology 18:205–216. https://doi.org/10.1007/s00049-008-0407-2
Thiery D, Visser JH (1995) Satiation effects on olfactory orientation patterns of Colorado potato beetle females. C R Acad Sci III 318:105–111
Tomlin ES, Sears MK (1992) Indirect competition between the Colorado potato beetle (Coleoptera: Chrysomelidae) and the potato leafhopper (Homoptera: Cicadellidae) on potato: laboratory study. Environ Entomol 21:787–792. https://doi.org/10.1093/ee/21.4.787
Vancanneyt G, Sanz C, Farmaki T, Paneque M, Ortego F, Castañera P, Sánchez-Serrano JJ (2001) Hydroperoxide lyase depletion in transgenic potato plants leads to an increase in aphid performance. Proc Natl Acad Sci 98(14):8139–8144. https://doi.org/10.1073/pnas.141079498
Visser JH (2011) The design of a low-speed wind tunnel as an instrument for the study of olfactory orientation in the Colorado beetle (Leptinotarsa decemlineata). Entomol Exp Appl 20:275–288. https://doi.org/10.1111/j.1570-7458.1976.tb02644.x
Visser JH (1979) Electroantennogram responses of the Colorado beetle, Leptinotarsa decemlineata, to plant volatiles. Entomol Exp Appl 25:86–97. https://doi.org/10.1111/j.1570-7458.1979.tb02851.x
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14:7370–7390. https://doi.org/10.3390/ijms14047370
Weber DC, Ferro DN (1994) Colorado potato beetle: diverse life history poses challenge to management. Adv Potato Pest Biol Manag:54–70
Weeda E, de Kort CAD, Th Beenakkers AM (1979) Fuels for energy metabolism in the Colorado potato beetle, Leptinotarsa decemlineata Say. J Insect Physiol 25:951–955. https://doi.org/10.1016/0022-1910(79)90108-2
Wilde JD, Lambers-Suverkropp KHR, Tol AV (1969) Responses to air flow and airborne plant odour in the Colorado beetle. Neth J Plant Pathol 75:53–57. https://doi.org/10.1007/BF02137193
Wright RJ (1984) Evaluation of crop rotation for control of Colorado potato beetles (Coleoptera: Chrysomelidae) in commercial potato fields on Long Island. J Econ Entomol 77:1254–1259. https://doi.org/10.1093/jee/77.5.1254
Zhang J, Khan SA, Hasse C, Ruf S, Heckel DG, Bock R (2015) Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 347:991–994. https://doi.org/10.1126/science.1261680
Acknowledgments
The authors wish to thank Xiangru Zhang and Haixiao Li from Agriculture and Agri-Food Canada (AAFC) for their constructive comments on the manuscript. This work was supported by AAFC through the Growing Forward program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wen, G., Khelifi, M., Cambouris, A.N. et al. Responses of the Colorado Potato Beetle (Coleoptera: Chrysomelidae) to the Chemical Composition of Potato Plant Foliage. Potato Res. 62, 157–173 (2019). https://doi.org/10.1007/s11540-018-9405-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11540-018-9405-0