Abstract
Trastuzumab deruxtecan (Enhertu®) is a human epidermal growth factor receptor 2 (HER2)-targeted antibody-drug conjugate approved in several countries, including the USA and those of the EU, for adults with unresectable or metastatic HER2-positive breast cancer who have previously received at least one prior anti-HER2-based regimen. In a pivotal phase 3 trial in this setting, intravenous trastuzumab deruxtecan demonstrated prolonged progression-free survival compared with trastuzumab emtansine (previously the recommended second-line therapy in this indication). Trastuzumab deruxtecan had a generally manageable safety and tolerability profile. Common treatment-related adverse events included haematological and gastrointestinal disorders. Interstitial lung disease (ILD)/pneumonitis is associated with a regulatory warning and requires careful monitoring. In conclusion, trastuzumab deruxtecan is a valuable new treatment option for HER2-positive breast cancer, having been shown to be effective with a generally manageable safety and tolerability profile in adults with unresectable or metastatic disease who have received one or more prior anti-HER2-based regimens.
Plain Language Summary
Human epidermal growth factor receptor 2 (HER2)-targeted therapies have improved HER2-positive breast cancer outcomes in recent years. Despite this, almost all patients will eventually experience disease progression (cancer growth or spread). Trastuzumab deruxtecan (Enhertu®) is an intravenously administered treatment that combines a drug that is toxic to cells and an antibody that targets it to HER2-expressing cells. It has been approved in several countries for the treatment of adults with unresectable or metastatic HER2-positive breast cancer who have previously received one or more anti-HER2-based therapies. In a pivotal clinical trial, trastuzumab deruxtecan showed longer survival without disease progression than trastuzumab emtansine (the previously recommended treatment after first disease progression). Trastuzumab deruxtecan had a generally manageable safety and tolerability profile. The most common classes of adverse events were blood and gastrointestinal disorders. Fatal events of interstitial lung disease (ILD)/pneumonitis have occurred with trastuzumab deruxtecan and patient monitoring is required. Trastuzumab deruxtecan is a valuable new option for patients with unresectable or metastatic HER2-positive breast cancer who have received at least one prior anti-HER2-based regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.22186387 |
HER2-targeted monoclonal antibody conjugated to a topoisomerase I inhibitor |
Approved for treatment following one or more prior anti-HER2-based regimens |
Increases progression-free survival compared with trastuzumab emtansine |
Generally manageable safety and tolerability profile; ILD/pneumonitis is associated with a regulatory warning |
1 Introduction
Current therapies for metastatic breast cancer are non-curative; rather, they aim to prolong survival and manage symptoms [1]. Approximately 15–20% of primary breast cancers are human epidermal growth factor receptor 2 (HER2)-positive, defined as an immunohistochemistry result of 3+ or a positive in situ hybridisation test [2]. HER2-positive breast cancer has historically been associated with a poor prognosis; however, in recent years, anti-HER2 therapies have improved outcomes for patients with HER2-positive disease [3]. Despite this, almost all patients will eventually have disease progression following anti-HER2 therapy [4].
Clinical guidelines currently recommend a combination of trastuzumab (an anti-HER2 antibody), a taxane and pertuzumab as the first-line regimen for metastatic HER2-positive breast cancer [5,6,7]. Following disease progression on first-line treatment, trastuzumab emtansine, an antibody-drug conjugate (ADC) comprising an anti-HER2 antibody and a tubulin polymerisation inhibitor, was previously the recommended second-line therapy [5, 7]. However, the duration of efficacy with trastuzumab emtansine is limited and treatments that provide longer progression-free survival (PFS) and overall survival (OS) were needed [8].
Trastuzumab deruxtecan (Enhertu®), also known as fam-trastuzumab deruxtecan-nxki in the USA [9], is an intravenously administered ADC combining an anti-HER2 antibody and a novel topoisomerase I inhibitor [9, 10]. Previously approved for adults with unresectable or metastatic HER2-positive breast cancer who had received at least two prior anti-HER-2-based regimens, trastuzumab deruxtecan has now been approved for patients who have received one or more prior anti-HER2-based therapies. This article reviews the efficacy and tolerability of trastuzumab deruxtecan in this extended indication, and provides an overview of its pharmacological properties. Discussion of its use in other approved indications (i.e. HER2-low breast cancer, HER2-positive gastric cancer [9, 10] and HER2-mutant non-small cell lung cancer [9]) is beyond the scope of this review.
2 Pharmacodynamic Properties of Trastuzumab Deruxtecan
The anti-HER2 human monoclonal immunoglobulin (Ig) G1 antibody in trastuzumab deruxtecan has the same amino acid sequence as trastuzumab [11]. The novel linker-payload system (deruxtecan) consists of an enzymatically-cleavable peptide linker and a topoisomerase I inhibitor [DX-8951 derivative (DXd), a derivative of exatecan]. Trastuzumab deruxtecan has a high drug-to-antibody ratio of ≈ 8, which is greater than most other ADCs, but is still stable in plasma [11].
The antibody component of trastuzumab deruxtecan targets the overexpressed HER2 receptors in HER2-postive tumours [11]. Following trastuzumab deruxtecan internalisation, DXd is released when the peptide linker is cleaved by lysosomal enzymes that are highly expressed in tumour cells. DXd stabilises topoisomerase I inhibitor-DNA cleavable complexes, resulting in double-strand DNA breaks and apoptosis. This mechanism of action differs to that of tubulin polymerisation inhibitors, such as used in trastuzumab emtansine [11]. As DXd is membrane permeable, it also has a bystander killing effect on adjacent cells, even if they are HER2-negative [12]. Additionally, trastuzumab deruxtecan retains the ability of unconjugated trastuzumab to induce antibody-dependent cellular cytotoxicity and inhibit cell proliferation via downregulation of phosphorylated Akt [11].
The anti-tumour efficacy of trastuzumab deruxtecan was demonstrated in preclinical studies [11, 12]. In vitro, trastuzumab deruxtecan inhibited the cell growth of multiple HER2-expressing cancer cell lines, including human breast cancer cell lines, but not a HER2-negative cell line [11]. In mice, trastuzumab deruxtecan showed anti-tumour efficacy in multiple HER2-expressing tumour models, including patient-derived breast cancer xenograft tumours and trastuzumab emtansine-resistant tumours [11]. The bystander effect of trastuzumab deruxtecan was demonstrated in HER2-positive/HER2-negative cancer cell co-cultures and HER2-positive/HER2-negative mixed-tumour mouse models [12].
Like all therapeutic proteins, there is potential for immunogenicity with trastuzumab deruxtecan [10]. During the DESTINY-Breast03 trial (Sect. 4), the incidence of treatment-emergent anti-drug antibodies (ADAs) was 1.6% (4/256) among trastuzumab deruxtecan recipients [9]. The incidence of treatment-emergent neutralizing antibodies was 0.4% (1/256). It is unknown if treatment-emergent ADAs and neutralizing antibodies have any effect on the pharmacological properties of trastuzumab deruxtecan [9].
3 Pharmacokinetic Properties of Trastuzumab Deruxtecan
In patients with cancer, both trastuzumab deruxtecan and released DXd showed dose-proportional increases in maximum plasma concentration and area under the concentration-time curve after a single dose of intravenous trastuzumab deruxtecan over a range of 3.2–8.0 mg/kg (≈ 0.6–1.5 times the recommended dose for breast cancer) [9, 10]. Based on population pharmacokinetics, there is moderate accumulation of trastuzumab deruxtecan at steady state (≈ 35% during the third 21-day cycle compared with the first cycle) [10]. The volume of distribution of the central compartment is 2.68 L for trastuzumab deruxtecan and 27.0 L for DXd [10]. DXd is ≈ 97% plasma-protein bound in vitro; the blood-to-plasma ratio of DXd is ≈ 0.6 [9, 10].
As with endogenous IgG antibodies, the IgG antibody component of trastuzumab deruxtecan is expected to be degraded by catabolic pathways [9, 10]. In vitro, DXd is predominately metabolised by CYP3A4 and is a substrate of OATP1B1, OATP1B3, MATE2-K, P-gp, MRP1 and BCRP [9, 10]. Coadministration with itraconazole (a strong CYP3A4 and P-gp inhibitor) or ritonavir (a CYP3A, OATP1B and P-gp inhibitor) does not increase the exposure to trastuzumab deruxtecan or DXd to a clinically meaningful degree [10].
The apparent elimination half-life of both trastuzumab deruxtecan and DXd is ≈ 7 days in patients with metastatic HER2-positive or HER-2 low breast cancer during the third cycle of treatment, based on population pharmacokinetic analysis [10]. The clearance is 0.41 L/day for trastuzumab deruxtecan and 19.6 L/h for DXd. In animal models, intravenously administered trastuzumab deruxtecan was mostly excreted as unchanged, released DXd in urine and faeces. Intravenously administered DXd was primarily excreted in in faeces via the biliary route and was the major component in urine, faeces and bile; the excretion of DXd has not been studied in humans [10].
There are no clinically significant effects of age, race, sex, body weight, mildly impaired liver function or mild to moderately impaired kidney function on the pharmacokinetics of either trastuzumab deruxtecan or DXd [9, 10]. Patients with moderate to severe impairment of liver or kidney function should be monitored closely due to insufficient data and/or increased risk of adverse events (AEs) [Sect. 5]. The potential need for dose reduction has not been determined in patients with severely impaired kidney function [9, 10], or moderate to severely [10] or severely [9] impaired liver function.
4 Therapeutic Efficacy of Trastuzumab Deruxtecan
The efficacy of trastuzumab deruxtecan for unresectable or metastatic HER2-positive breast cancer after progression on trastuzumab emtansine was previously established in the single-arm phase 2 DESTINY-Breast01 trial [4] and confirmed in the randomized phase 3 DESTINY-Breast02 trial [13], which are not discussed further. This section focuses on the results of the pivotal DESTINY-Breast03 trial, a multinational, randomized, open-label, active-controlled phase 3 trial evaluating the efficacy of trastuzumab deruxtecan compared with trastuzumab emtansine in patients who had previously received trastuzumab and taxane therapy [14].
DESTINY-Breast03 enrolled patients aged ≥ 18 years with unresectable or metastatic HER2-positive breast cancer confirmed by immunohistochemical analysis at a central laboratory and who had disease progression during or after treatment with trastuzumab and a taxane for advanced or metastatic breast cancer, or had progressed within 6 months of receiving trastuzumab or a taxane as a neoadjuvant or adjuvant therapy [14]. Patients with clinically stable brain metastases were eligible for enrolment. Exclusion criteria included brain metastases that were symptomatic or required treatment, previous treatment with an anti-HER2 ADC (e.g. trastuzumab emtansine) in the metastatic setting, Eastern Cooperative Oncology Group (ECOG) performance status > 1, history of noninfectious interstitial lung disease (ILD) treated with glucocorticoids or suspected ILD that could not be ruled out during screening and uncontrolled or significant cardiovascular disease [14].
A total of 524 patients were randomized to receive intravenous treatment once every 3 weeks with either trastuzumab deruxtecan 5.4 mg/kg or trastuzumab emtansine 3.6 mg/kg [14]. Randomization was stratified by previous treatment with pertuzumab (61%), hormone receptor status (52% positive) and history of visceral disease (73%) [10]. Other baseline characteristics were generally similar between the two groups, including sex (99.6% female), age (median 54 years), race (60% Asian, 27% white), ECOG performance status of 0 (63%) or 1 (37%), number of previous lines of therapy in the metastatic setting (48% had one prior line of therapy, not including endocrine therapy; 9.5% had none), and stable brain metastases (22% with reported history) [10].
4.1 First Interim Analysis
A prespecified interim analysis was conducted after 245 events of disease progression or death (≈ 70% information fraction) [14]. Trastuzumab deruxtecan was associated with significantly longer median PFS as assessed by blinded independent central review (BICR) compared with trastuzumab emtansine (primary endpoint; Table 1), at a median follow-up of 16.2 and 15.3 months, respectively. At 12 months, 75.8% of trastuzumab deruxtecan recipients were alive and progression free compared with 34.1% of trastuzumab emtansine recipients. Investigator-assessed median PFS was also significantly longer with trastuzumab deruxtecan versus trastuzumab emtansine (secondary endpoint; Table 1) [14].
PFS results were supported by secondary efficacy outcomes (Table 1) [14]. Median OS (key secondary endpoint) data were not mature; there was a trend for benefit with trastuzumab deruxtecan compared with trastuzumab emtansine. The OS rate at 12 months was 94.1% versus 85.9% in the respective groups. Trastuzumab deruxtecan was associated with an objective response rate (ORR) of 79.7% [complete responses (CR) in 16.1% and partial responses (PR) in 63.6% of patients] versus 34.2% with trastuzumab emtansine (CR in 8.7% and PR in 25.5%). The median time to response was rapid in both groups (1.6 months with trastuzumab deruxtecan and 1.4 months with trastuzumab emtansine) [14]. The median duration of response (DoR) was not reached with either treatment [10]. The disease control rate (CR, PR or stable disease) was 96.6% and 76.8% in the respective groups [14].
Prespecified and further exploratory analyses also demonstrated the efficacy of trastuzumab deruxtecan [14,15,16,17]. In subgroup analyses, PFS benefits with trastuzumab deruxtecan were seen regardless of number of lines of previous therapy in the metastatic setting (0–1 or ≥ 2), hormone receptor status (positive/negative), previous pertuzumab treatment (yes/no), presence of visceral disease (yes/no), history of brain metastases (yes/no), ethnicity (Asian/overall population), metastatic disease (de novo/recurrent), early progression after prior neoadjuvant or adjuvant therapy and the number of lines of prior anti-HER2 therapy (1 or ≥ 2) [14,15,16]. The benefit of trastuzumab deruxtecan on ORR in subgroups were also demonstrated in exploratory analyses [17].
In a subgroup of patients with stable brain metastases as baseline (n = 82), trastuzumab deruxtecan was associated with significantly longer median PFS versus trastuzumab emtansine [15.0 vs 3.0 months; hazard ratio (HR) 0.25, 95% CI 0.13–0.45] [18]. The PFS rate at 12 months was 72.0% versus 20.9% in the respective groups (HR 0.27, 95% CI 0.13–0.45). The ORR achieved in trastuzumab deruxtecan and trastuzumab emtansine recipients was 67.4% versus 20.5% and the median DoR was 12.9 months versus 7.2 months. The intracranial ORR was 63.8% (CR in 27.8% of patients) versus 33.3% (CR in 2.8%) in the respective groups [18].
4.2 Second Interim Analysis
A second prespecified interim analysis was conducted at a median follow-up duration of 28.4 and 26.5 months in the trastuzumab deruxtecan and trastuzumab emtansine groups [19]. Median OS was significantly prolonged with trastuzumab deruxtecan versus trastuzumab emtansine (key secondary endpoint; Table 1). OS rates in the trastuzumab deruxtecan and trastuzumab emtansine groups were 94.1% (95% CI 90.4–96.4%) versus 86.0% (81.1–89.8%) at 12 months and 77.4% (71.7–82.1%) versus 69.9% (63.7–75.2%) at 24 months. The benefit of trastuzumab deruxtecan on OS was consistent across the subgroups analysed, including those based on hormone receptor status, previous pertuzumab treatment, baseline visceral disease, previous lines of systemic therapy (< 3 or ≥ 3) and baseline brain metastases [19].
Other efficacy results, including median PFS and ORR rates, remained consistent with the first interim analysis (Table 1) [19]. PFS rates with trastuzumab deruxtecan and trastuzumab emtansine were 75.2% (95% CI 69.3–80.2%) versus 33.9% (27.7–40.2%) at 12 months and 53.7% (46.8–60.1%) versus 26.4% (20.5–32.6%) at 24 months. In trastuzumab deruxtecan recipients, the ORR was 79% (CR in 21% and PR in 57% of patients) versus 35% (CR in 10% and PR in 25%) in trastuzumab emtansine recipients. The median DoR was 36.6 months versus 23.8 months in the respective groups. In an exploratory analysis, the clinical benefit rate (CR, PR or > 6 months stable disease) was 89% with trastuzumab deruxtecan and 46% with trastuzumab emtansine [19].
5 Tolerability of Trastuzumab Deruxtecan
Trastuzumab deruxtecan had a generally manageable safety and tolerability profile in the DESTINY-Breast03 trial (Sect. 4) [14]. The incidence of AEs with trastuzumab deruxtecan in the DESTINY-Breast03 trial was generally similar to that in the earlier DESTINY-Breast01 trial [14].
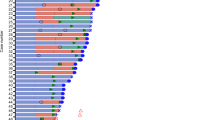
In the first interim analysis (Sect. 4.1), the median duration of treatment was 14.3 months in 257 patients who received ≥ 1 dose of trastuzumab deruxtecan and 6.9 months in 261 patients who received ≥ 1 dose of trastuzumab emtansine [14]. The incidences of all-grade and grade 3–4 treatment-related AEs (TRAEs) were 98.1% and 45.1% with trastuzumab deruxtecan and 86.6% and 39.8% with trastuzumab emtansine. TRAEs occurring at an incidence of ≥ 25% with trastuzumab deruxtecan included haematological disorders [neutropenia (42.8%), anaemia (30.4%), leukopenia (30.0%)], gastrointestinal disorders [nausea (72.8%), vomiting (44.0%)], fatigue (44.7%), alopecia (36.2%) and decreased appetite (26.1%). The most common grade ≥ 3 TRAEs occurring in the trastuzumab deruxtecan group are presented in Fig. 1. AEs that resulted in discontinuation of trastuzumab deruxtecan or trastuzumab emtansine occurred in 13.6% and 7.3% of recipients. No treatment-related deaths occurred during the trial [14].
Most common (occurring at ≥ 5% incidence) grade ≥ 3 treatment-related adverse events in the trastuzumab deruxtecan group in the phase 3 DESTINY-Breast03 trial [14]
The safety and tolerability profile of trastuzumab deruxtecan in the second interim analysis (Sect. 4.2) was consistent with the profile in the first interim analysis, where the same number of patients from the first interim analysis were treated for a median duration of 18.2 months with trastuzumab deruxtecan and 6.9 months with trastuzumab emtansine [19]. The exposure-adjusted incidence rate of grade ≥ 3 treatment-emergent AEs was 0.36 incidences per patient-year (PPY) with trastuzumab deruxtecan versus 0.65 incidences PPY with trastuzumab emtansine; the rate for serious AEs was 0.16 versus 0.28 incidences PPY. TRAEs led to treatment discontinuation in 20% of trastuzumab deruxtecan recipients (vs 7% with trastuzumab emtansine), dose reduction in 25% (vs 15%) and treatment interruption in 42% (vs 17%) [19].
5.1 Adverse Events of Special Interest
ILD/pneumonitis is an AE of special interest with trastuzumab deruxtecan [9, 10, 20]. All potential cases of ILD or pneumonitis in DESTINY-Breast03 were reviewed by an independent adjudication committee [14, 19]. Grade 1–3 TRAEs of ILD/pneumonitis were reported, with no grade 4–5 ILD/pneumonitis events reported in either treatment group [14, 19]. In the second interim analysis, 39 (15%) trastuzumab deruxtecan recipients and eight (3%) trastuzumab emtansine recipients had a TRAE of ILD/pneumonitis [19]. Per protocol, patients with grade ≥ 2 ILD/pneumonitis were required to discontinue trastuzumab deruxtecan [14, 19]. Pneumonitis [15 (6%) patients] and ILD [13 (5%) patients] were the most common TRAEs leading to discontinuation of trastuzumab deruxtecan [19].
In a pooled analysis of phase 1 and 2 clinical trials, the incidence of adjudicated treatment-related grade ≥ 3 ILD/pneumonitis in patients with HER2-positive breast cancer treated with trastuzumab deruxtecan 5.4 mg/kg every 3 weeks was 3.7%, including fatal events in 2.9% of patients [20]. Across multiple tumour types, most (87.0%) patients who experienced an ILD/pneumonitis event did so within 12 months of initiating trastuzumab deruxtecan therapy [20]. A higher incidence of grade 1–2 ILD/pneumonitis has been observed in patients with moderately impaired kidney function [9, 10].
Anti-HER2 therapies, including trastuzumab deruxtecan, have been associated with decreased left ventricular ejection fraction (LVEF) [9, 10]. Decreased LVEF was reported in six (2.3%) trastuzumab deruxtecan recipients in the first interim analysis of DESTINY-BREAST03 compared with one (0.4%) trastuzumab emtansine recipient; all events were grade 2 in severity [14]. All events resolved without action, except in one patient receiving trastuzumab deruxtecan who had a persistent decrease in LVEF. An additional patient in the trastuzumab deruxtecan group had grade 1 LV dysfunction, which resolved without action. All patients with decreased LVEF or LV dysfunction were asymptomatic and none experienced cardiac failure [14].
Severe neutropenia, including febrile neutropenia, has been reported in patients treated with trastuzumab deruxtecan [9, 10]. In the second interim analysis of DESTINY-Breast03, neutropenia occurred in 31% of trastuzumab deruxtecan recipients, including 16% of patients with grade 3–4 neutropenia, and 11% of trastuzumab emtansine recipients, including 3% with grade 3–4 neutropenia [19]. Febrile neutropenia was reported in 1.3% of patients treated with trastuzumab deruxtecan across multiple tumour types, including 0.1% of patients who had a grade 5 event [10].
6 Dosage and Administration of Trastuzumab Deruxtecan
In the EU, trastuzumab deruxtecan is approved for the treatment of adults with unresectable or metastatic HER2-positive breast cancer who have previously received one or more anti-HER2-based regimens [10]. In the USA, trastuzumab deruxtecan is approved for the treatment of adults with unresectable or metastatic HER2-positive breast cancer who have received prior anti-HER2-based therapy in the metastatic setting, or in the neoadjuvant or adjuvant setting and have developed disease recurrence during or within 6 months of completing therapy [9].
Trastuzumab deruxtecan is administered as an intravenous infusion, at a dose of 5.4 mg/kg body weight once every 3 weeks until disease progression or unacceptable toxicity [9, 10]. To prevent medication errors, care should be taken to ensure that the administered product is trastuzumab deruxtecan and not trastuzumab or trastuzumab emtansine. The first infusion is administered over 90 min; if well tolerated, subsequent infusions may be administered over 30 min. Patients should be premedicated with antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting [9, 10].
Trastuzumab deruxtecan carries regulatory warnings, including boxed warnings in the USA [9], regarding the potential for ILD/pneumonitis (Sect. 5.1) and embryo-foetal toxicity, as well as warnings regarding LV dysfunction, neutropenia (Sect. 5.1) [9, 10], and its use in patients with moderately or severely impaired liver function (in the EU) [10]. As exposure to trastuzumab deruxtecan during pregnancy may cause embryo-foetal harm, effective contraception should be used during and after treatment (i.e. 7 months after treatment in female patients of reproductive potential and 4 months after treatment in male patients with female partners of reproductive potential). Additionally, women should not breastfeed during treatment and for 7 months after the last dose of trastuzumab deruxtecan [9, 10].
Consult local prescribing information for further information on dosage and administration, use in special populations, warnings and precautions, drug interactions, and guidelines on the management of selected AEs, which may include monitoring strategies, dose interruption, dose reduction and/or treatment discontinuation [9, 10].
7 Place of Trastuzumab Deruxtecan in the Management of Unresectable or Metastatic HER2-Positive Breast Cancer
The treatment goals for metastatic breast cancer are to prolong survival, control symptoms, and maintain or improve quality of life [1]. Recommendations for the treatment of metastatic HER2-positive breast cancer have evolved rapidly since the approval of trastuzumab deruxtecan following one or more prior anti-HER2 therapies [5,6,7]. Current clinical practice guidelines recommend trastuzumab deruxtecan as the preferred second-line regimen for advanced [5], recurrent unresectable [6] or metastatic [6, 7] HER2-positive breast cancer following progression during or after first-line anti-HER2 targeted therapy. Trastuzumab deruxtecan may also be considered as first-line therapy in patients with early disease progression within 6 months of neoadjuvant or adjuvant therapy (12 months for regimens containing pertuzumab) [6], or in patients with metastatic recurrence within 12 months of receiving adjuvant trastuzumab and pertuzumab therapy [7]. In light of these recommendations, trastuzumab deruxtecan has the potential to become the new therapeutic standard of care in the second-line setting after prior anti-HER2 therapy [4, 7]. The UK National Institute for Health and Care Excellence (NICE) guidance recommends trastuzumab deruxtecan as an option for patients with HER2-positive unresectable or metastatic breast cancer who have received at least one anti-HER2 therapy and are eligible under a managed access agreement [21].
The efficacy of trastuzumab deruxtecan in patients with unresectable or metastatic HER2-positive breast cancer who had previously been treated with trastuzumab and a taxane was established in DESTINY-Breast03 (Sect. 4). Trastuzumab deruxtecan demonstrated longer PFS versus trastuzumab emtansine, which had previously been the recommended second-line therapy in this indication. Although OS data were immature, there was a significant extension in OS with trastuzumab deruxtecan versus trastuzumab emtansine at the second interim analysis (Sect. 4.2). Trastuzumab deruxtecan was also associated with benefits in ORR versus trastuzumab emtansine (Sect. 4). Further data, including mature OS data, from updated analyses of this trial will be useful to confirm these findings.
Trastuzumab deruxtecan has a generally manageable safety and tolerability profile (Sect. 5). In DESTINY-Breast03, the most common classes of TRAEs with trastuzumab deruxtecan were haematological and gastrointestinal disorders and the most common grade ≥ 3 TRAE was neutropenia (Sect. 5). Trastuzumab deruxtecan was associated with higher incidence rates of TRAEs, grade 3–4 TRAEs and AEs that led to treatment discontinuation than trastuzumab emtansine; however, the duration of treatment was longer with trastuzumab deruxtecan. The exposure-adjusted incidence rate of grade ≥ 3 treatment-emergent AEs was 0.36 and 0.65 incidences PPY with trastuzumab deruxtecan and trastuzumab emtansine; the rate for serious AEs was 0.16 and 0.28 incidences PPY. ILD/pneumonitis was the most common AE that led to discontinuation of trastuzumab deruxtecan (Sect. 5.1). Although no grade 4–5 ILD/pneumonitis events occurred in DESTINY-Breast03 (Sect. 5.1), fatal events of ILD/pneumonitis have been reported with trastuzumab deruxtecan and careful monitoring of patients for relevant signs and symptoms is required [20]. The potential differences in the incidence of grade ≥ 3 ILD/pneumonitis with trastuzumab deruxtecan in DESTINY-Breast03 compared with previous studies may have been due to the implementation of ILD/pneumonitis monitoring and management guidelines, or the use of trastuzumab deruxtecan as an earlier line of therapy [19, 20]. Real-world data evaluating the incidence of ILD/pneumonitis in patients receiving trastuzumab deruxtecan would be of interest.
Approximately 30–50% of patients with advanced HER2-postive breast cancer develop brain metastases [22]. These patients have a high unmet need, with a limited number of clinical trials including brain metastases-related outcomes as a primary endpoint. Furthermore, patients with clinically active (untreated or progressing) or symptomatic brain metastases are often excluded from clinical trials [23]. Trastuzumab deruxtecan demonstrated intracranial efficacy in patients with clinically stable or asymptomatic brain metastases in DESTINY-Breast03 (Sect 4); however, more data are needed, including data on the efficacy of trastuzumab deruxtecan in patients with active brain metastases. Results from a multinational, open-label phase 3b/4 study (DESTINY-Breast12) examining the use of trastuzumab deruxtecan in patients with previously-treated unresectable or metastatic HER2-positive breast cancer with or without baseline brain metastases (untreated or previously-treated stable or progressing) are awaited with interest [24]. A systematic review and meta-analysis found that trastuzumab deruxtecan and tucatinib (in combination with trastuzumab and capecitabine) may be the most effective systemic therapies for patients with brain metastases, based on intracranial ORR [25]. However, the results of this meta-analysis should be interpreted cautiously, owing to the heterogeneity of included studies, many of which had a moderate/serious risk of bias [25].
The routine use of trastuzumab deruxtecan in the real world will depend on its cost-effectiveness [21, 26,27,28]. Two US studies found that trastuzumab deruxtecan was cost-effective compared with trastuzumab emtansine, with an incremental cost-effectiveness ratio (ICER) of $13,342/quality-adjusted life years (QALY) [26] or $82,112/QALY [27], at a willingness-to-pay threshold of $150,000/QALY. However, another US study found that trastuzumab deruxtecan had an ICER of $220,533/QALY compared with trastuzumab emtansine and was unlikely to be cost-effective [28]. The NICE guidance concluded that evidence was still immature and the ICER of trastuzumab deruxtecan was uncertain but may be higher than the range usually considered to be cost-effective in the UK [21].
In conclusion, trastuzumab deruxtecan is an effective treatment with a generally manageable safety and tolerability profile for HER2-positive breast cancer, in adults with unresectable or metastatic disease who have received one or more prior anti-HER2-based regimens. Current evidence indicates that it provides longer PFS and OS than trastuzumab emtansine and is thus a valuable new treatment option for this indication.
Data Selection Trastuzumab Deruxtecan: 132 records identified
Duplicates removed | 1 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 82 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 21 |
Cited efficacy/tolerability articles | 9 |
Cited articles not efficacy/tolerability | 19 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were trastuzumab deruxtecan, fam-trastuzumab deruxtecan-nxki, Enhertu, T-DXd, DS-8201, DS-8201a. Records were limited to those in English language. Searches last updated 11 Apr 2023 | |
References
Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers. 2019;5(1):66.
Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013.
Bardia A, Harnden K, Mauro L, et al. Clinical practices and institutional protocols on prophylaxis, monitoring, and management of selected adverse events associated with trastuzumab deruxtecan. Oncologist. 2022;27(8):637–45.
Gupta R, Gupta S, Antonios B, et al. Therapeutic landscape of advanced HER2-positive breast cancer in 2022. Med Oncol. 2022;39(12):258.
Giordano SH, Franzoi MAB, Temin S, et al. Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO guideline update. J Clin Oncol. 2022;40(23):2612–35.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer, version 4.2023. 2023. https://www.nccn.org/. Accessed 11 Apr 2023.
Gennari A, André F, Barrios CH, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–95.
European Medicines Agency. Enhertu: public assessment report. 2022. https://www.ema.europa.eu/. Accessed 11 Apr 2023.
Daiichi Sankyo Inc. ENHERTU (fam-trastuzumab deruxtecan-nxki) for injection, for intravenous use: US prescribing information. 2023. https://dailymed.nlm.nih.gov/. Accessed 11 Apr 2023.
Daiichi Sankyo Europe GmbH. Enhertu 100 mg powder for concentrate for solution for infusion: EU summary of product characteristics. 2023. https://www.ema.europa.eu/. Accessed 11 Apr 2023.
Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–108.
Ogitani Y, Hagihara K, Oitate M, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46.
Krop I, Park YH, Kim S-B, et al. Trastuzumab deruxtecan vs physician’s choice in patients with HER2+ unresectable and/or metastatic breast cancer previously treated with trastuzumab emtansine: primary results of the randomized phase 3 study DESTINY‑Breast02 [abstract no. GS2-01 plus oral presentation]. In: SABCS. 2022.
Cortés J, Kim SB, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–54.
Im S-A, Xu B, Kim S-B, et al. Trastuzumab deruxtecan vs T-DM1 in HER2+ mBC in Asian subgroup: results of the randomized phase 3 study DESTINY-Breast03 [abstract no. PS2-1]. Ann Oncol. 2022;33(Suppl 6):S464–546.
Cortés J, Im SA, Iwata H, et al. Subgroup analysis by disease history and prior treatments of patients (pts) with HER2-positive (HER2+) metastatic breast cancer (MBC) from DESTINY-Breast03, a randomized phase III study of trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) [abstract no. 236P]. Ann Oncol. 2022;33(Suppl 7):S645–6.
Hurvitz S, Kim SB, Chung WP, et al. Trastuzumab deruxtecan (T-DXd; DS-8201a) vs trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03 [abstract no GS3-01]. Cancer Res. 2022;82(Suppl 4).
Jacobson A. Trastuzumab deruxtecan improves progression-free survival and intracranial response in patients with HER2-positive metastatic breast cancer and brain metastases. Oncologist. 2022;27(Suppl 1):S3-4.
Hurvitz SA, Hegg R, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105–17.
Powell CA, Modi S, Iwata H, et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open. 2022;7(4):1–11.
National Institute for Health and Care Excellence. Trastuzumab deruxtecan for treating HER2-positive unresectable or metastatic breast cancer after 1 or more anti-HER2 therapies: technology appraisal guidance. 2023. https://www.nice.org.uk/. Accessed 11 Apr 2023.
Perez-Garcia JM, Batista MV, Cortez P, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro Oncol. 2023;25(1):157–66.
Tan AC, Boggs DH, Lee EQ, et al. Clinical trial eligibility criteria and recently approved cancer therapies for patients with brain metastases. Front Oncol. 2022;11: 780379.
US National Institutes of Health. ClinicalTrials.gov identifier: NCT04739761. 2023. https://clinicaltrials.gov/. Accessed 11 Apr 2023.
Werter IM, Remmelzwaal S, Burchell GL, et al. Systemic therapy for patients with HER2-positive breast cancer and brain metastases: a systematic review and meta-analysis. Cancers (Basel). 2022;14(22):5612.
Zhu Y, Liu K, Wang M, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: a cost-effectiveness analysis. Breast. 2022;66:191–8.
Yang J, Han J, Zhang Y, et al. Cost-effectiveness analysis of trastuzumab deruxtecan versus trastuzumab emtansine for HER2-positive breast cancer. Front Pharmacol. 2022;13: 924126.
Wang J, Yi Y, Wan X, et al. Cost-effectiveness analysis of trastuzumab deruxtecan versus trastuzumab emtansine in human epidermal growth factor receptor 2-positive metastatic breast cancer in the USA. Adv Ther. 2022;39(10):4583–93.
Acknowledgments
During the peer review process, the manufacturer of trastuzumab deruxtecan was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
T. Nie and H. A. Blair are salaried employees of Adis International Ltd/Springer Nature, and declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: M. D. P. E. Diz, Radiology and Oncology, Institute of Cancer of São Paulo, São Paulo, Brazil; M. Mita, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nie, T., Blair, H.A. Trastuzumab Deruxtecan: A Review in Unresectable or Metastatic HER2-Positive Breast Cancer. Targ Oncol 18, 463–470 (2023). https://doi.org/10.1007/s11523-023-00971-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-00971-9