Abstract
Identifying the presence or absence of a BRAFV600E mutation is paramount for the management of patients with metastatic colorectal cancer (mCRC) as there are distinct predictive and prognostic implications, as well as unique therapeutic approaches for this molecular subtype. Traditional cytotoxic doublet chemotherapy has historically been ineffective for this poor prognostic group, thereby highlighting the critical need for novel targeted therapies to drive management. Unlike the early success achieved with BRAF-inhibitor monotherapy for patients with BRAFV600E-mutated metastatic melanoma, response rates were found to only be 5% in early-phase clinical trials for patients with BRAFV600E mCRC. A deeper understanding of predominant resistance mechanisms in BRAFV600E mCRC after exposure to BRAF inhibition has resulted in innovative combinatorial approaches targeting the mitogen-activated protein kinase (MAPK) pathway, revitalizing the treatment portfolio for these patients. Of note, in recent years non-V600 BRAF mutations have been appreciated as a distinct molecular subset in mCRC, representing 2–4% of patients with a unique clinical presentation and complex signaling biology. These mutations, referred to as “atypical” BRAF mutations, warrant individual clinical investigation and demand innovative drug development that leverages known signaling class biology. Here, we summarize the current molecular and clinicopathologic understanding of BRAFV600E mCRC, as well as the landmark clinical trials that have led to successful targeted therapy for this historically aggressive subtype of colorectal cancer. Additionally, we briefly describe the current understanding of patients with atypical BRAF mutations, highlighting the importance of continued research efforts to appropriately treat this evolving subset of BRAF mutations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Identification of the BRAF mutation status in patients with newly diagnosed metastatic colorectal cancer is critical for the optimal management and appropriate sequencing of chemotherapy and biologic treatment options. |
Doublet biologic therapy with a BRAF inhibitor in combination with an EGFR antibody is now standard of care for patients with previously treated BRAFV600E-mutant metastatic colorectal cancer based on results of the phase III BEACON CRC study. |
Patients with atypical BRAF (non-V600) mutations have a distinct subset of metastatic colorectal cancer reflecting an unmet need that warrants continued research efforts to discover novel therapies. |
1 Unique Clinical, Pathologic, and Molecular Features of the BRAF Mutation
Colorectal cancer (CRC) remains the fourth most common cancer diagnosis and the second most common cause of cancer-related deaths in the USA [1] Due to the limitations of early detection, variability of clinical presentation and an increased incidence of young-onset disease, many patients present with metastatic disease not amenable to curative resection. Underlying innate chemotherapy resistance and rate of progression are intimately related to the unique molecular subtype of CRC, therefore a biomarker-driven clinical (prognostic, predictive) and research (novel targets) focus represents the best path forward to enhance patient outcomes [2]. CRC develops from normal colon tissue after numerous epigenetic and genetic aberrations occur [3]. In metastatic colorectal cancer (mCRC), approximately 7–10% of patients carry a BRAFV600E mutation, and this molecular subset is associated with a poor prognosis [4,5,6]. Of note, recent population-based data suggests an increased prevalence with worse outcomes than previously reported for patients with BRAFV600E when compared to the academic series [7].
BRAF is a serine threonine kinase downstream of RAS that results in RAS-independent mitogen-activated protein kinase (MAPK) pathway activation, promoting tumor cell proliferation, colorectal cancer metastases, and survival [8]. The most common BRAF mutation occurs at codon 600 with a valine to glutamic acid change (c.1799T>A or p.V600E), resulting in downstream phosphorylation of MEK and ERK kinase with resultant constitutive activation of MAPK signaling, mutually exclusive from RAS [9, 10]. Atypical, non-V600 BRAF (aBRAF) mutations that occur outside of codon 600 are less common and represent approximately 2–4% of mCRCs [11]. These aBRAF mutations can be further characterized into class II or class III designations based on their underlying signaling biology [12] (Fig. 1). Class II aBRAF mutants signal via constitutive dimers and are RAS-independent, suggesting resistance to traditional BRAF monomer inhibitors such as vemurafenib with possible sensitivity to novel RAF dimer or MEK inhibitors [12]. Class III aBRAF mutants are kinase-dead and associated with RAS activation via receptor tyrosine kinase signaling, suggesting potential sensitivity to epidermal growth factor receptor inhibition (EGFR) in some cases, although recent data have suggested limitations to this approach [12,13,14].
Patients with BRAFV600E mCRC have a characteristic clinicopathologic profile of female preponderance; elderly age at diagnosis; right-sided primary tumors; high-grade mucinous histology; T4 tumors; increased incidence of peritoneal, brain, lymph node, and bone metastases; and an estimated 20–30% of patients with an associated sporadic microsatellite instability high (MSI-H) phenotype [15,16,17,18,19]. Of note, the latter is distinct from Lynch syndrome and reflects somatic hypermethylation of the MLH1 promoter yielding a non-hereditary MSI-H [16]. Additionally, BRAFV600E mutations are associated with high mutation burden and a CpG island methylator phenotype (CIMP) [20]. CRC has been more recently described by gene expression profiles as consensus molecular subtypes [21]. BRAFV600E mCRC are associated with consensus molecular subtype 1 (CMS1), with noted overlay between characteristics of this subtype and BRAFV600E mutations [21]. Similar to MSI-H tumors, CMS1 CRC are associated with significant immune-cell infiltration and activated immune-response pathways manifested by increased cytotoxic T-cells, natural killer cells, and type 1 helper T cells [21, 22]. Furthermore, BRAFV600E tumors arise from a distinct biologic pathway known as the sessile serrated adenomatous pathway, characterized by the BRAF mutation, MLH1 hypermethylation, MAPK pathway mutations, and MSI-H features [23, 24].
In contrast to BRAF wild-type and aBRAF mCRC, patients with BRAFV600E mCRC have an extremely poor prognosis and innate resistance to standard chemotherapy. While acknowledging ascertainment bias, our MDACC experience has been in line with previously published trends with the median overall survival (OS) for patients with BRAFV600E mCRC being significantly shorter compared to patients with aBRAF and BRAF wild-type mCRC (21 months vs. 36.1 and 42.3 months, respectively) [14, 19, 25, 26]. Although BRAFV600E is associated with wild-type RAS, numerous studies have suggested BRAFV600E mutations likely represent a negative predictive biomarker to EGFR inhibition alone or in combination with chemotherapy [6, 27]. Additionally, quality-of-life metrics for BRAFV600E patients have been shown to be worse when compared to those with BRAF wild-type disease, highlighting the need for uniquely tailored therapeutic approaches for this subset of mCRC [28]. Considering the prognostic, predictive, and clinical implications of BRAFV600E mutations in CRC, the National Comprehensive Cancer Network (NCCN) recommends screening be performed for all patients with mCRC upon initial diagnosis [29, 30].
2 Treatment Challenges in the Management of Patients with BRAFV600E mCRC
Due to the inherent chemotherapy-refractory nature of BRAFV600E mCRC, patients are unable to benefit from exposure to subsequent lines of doublet chemotherapy as routinely offered to those with less aggressive disease, such as RAS/BRAF wild-type mCRC patients. In fact, the 5-year OS for all patients with stages I–IV BRAFV600E is noted to be 47.5% compared to 60.7% for patients with BRAF wild-type cancers [31]. In the stage III adjuvant arena, patients with BRAFV600E CRC experience a truncated progression-free survival (PFS) and worse overall survival after recurrence [32, 33]. Such short treatment-free intervals after recent exposure to oxaliplatin-based adjuvant therapy may further impact the efficacy of first-line metastatic treatment. Additionally, historical data has revealed that the median PFS is approximately 2.5 months among patients receiving second- and third-line therapy [6]. Therefore, initial treatment decision-making remains critical for patients with metastatic disease. Considering the limitations of traditional doublet chemotherapy, it is rational to explore an intensified approach utilizing up-front triplet chemotherapy with fluorouracil, oxaliplatin, and irinotecan (FOLFOXIRI) in combination with bevacizumab for BRAFV600E mCRC. The TRIBE study evaluated FOLFOXIRI + bevacizumab versus FOLFIRI + bevacizumab for treatment-naïve mCRC [34]. The study met its primary endpoint of PFS with a reported 2.4-month benefit for all patients who were exposed to the triplet regimen compared to the doublet. In a subgroup analysis of the 28 enrolled BRAFV600E patients, there was a trend towards survival benefit for the 16 patients randomized to the FOLFOXIRI + bevacizumab (19 months vs. 10.7 months, hazard ratio [HR], 0.55) arm [34]. Although the data did not reach statistical significance due to the small sample size, it is supportive of the use of FOLFOXIRI plus bevacizumab as first-line therapy for BRAFV600E mCRC. As such, for patients with adequate performance status, triplet chemotherapy in combination with bevacizumab is a viable up-front choice of treatment.
However, there are obvious clinical limitations to the widespread application of FOLFOXIRI plus bevacizumab for all patients with BRAFV600E mCRC. Most notably, intensified chemotherapy would be difficult for elderly patients, those with residual peripheral neuropathy, those with poor performance status, those with recent progression on adjuvant chemotherapy, and patients with inherent intolerability to traditional chemotherapy. A previously published meta-analysis of BRAFV600E mCRC patients enrolled in three randomized clinical trials (STEAM, TRIBE, CHARTA) revealed no significant difference in terms of PFS between triplet versus doublet chemotherapy in combination with bevacizumab [35]. Furthermore, recently presented meta-analysis data that included the previously mentioned studies as well as the OLIVIA and TRIBE2 studies evaluated the benefit on OS depending on which first-line option was utilized—triplet chemotherapy plus bevacizumab versus doublet chemotherapy plus bevacizumab [36]. While survival benefit with intensified chemotherapy was noted for all patients with unresectable mCRC, subgroup analysis of the 115 BRAFV600E mCRC patients revealed no additional benefit was achieved from intensification of the chemotherapy backbone, thereby not confirming the previously reported signal noted in the TRIBE study [36]. A population-based study revealed that only 60% of patients with BRAFV600E mCRC receive systemic therapy, with only 26% moving on to second-line treatment [7]. Considering the current treatment sequence in the management of BRAFV600E mCRC, this highlights the need for exposure to more effective regimens early on and the unmet need for a personalized approach to be deployed in the first- line setting. To that effect, ANCHOR-CRC, is an ongoing phase II study investigating the efficacy of a triplet-targeted therapeutic approach utilizing encorafenib (BRAF inhibitor), binimetinib (MEK inhibitor), and cetuximab (EGFR inhibitor) for treatment-naïve BRAFV600E mCRC patients (NCT03693170). This study has a two-stage design with a plan to enroll 95 total patients. Initial data from 40 patients in stage 1 have been presented in abstract form [37]. The primary endpoint of this study is overall response rate (ORR) with secondary endpoints of PFS, OS, safety, and quality of life. Response rates (RRs) were reported at 50% with a median PFS (mPFS) of 4.9 months [37]. These data are encouraging considering use of targeted therapy alone; however, in light of historical outcomes with doublet and triplet chemotherapy reported from BRAFV600E-subgroup analysis in the TRIBE first-line mCRC study (mPFS of 5.5 and 7.5 months, RR 42 and 56%, respectively), a subsequent head-to-head trial is necessary to establish a new standard of care [34]. BREAKWATER is a planned phase III frontline BRAFV600E MSS/pMMR mCRC study (n = 870 patients) that will compare two arms of encorafenib in combination with cetuximab with or without chemotherapy (FOLFOX, FOLFIRI) to a physician’s choice control arm of FOLFOX, FOLFIRI, FOLFOXIRI, CAPOX, ± anti-VEGF antibody with primary endpoints of PFS and secondary endpoints of OS.
Along with intrinsic resistance to standard 5-FU based chemotherapy, the use of anti-EGFR therapy for BRAFV600E mCRC also has significant limitations. Previous data have revealed that both OS and PFS are poor with second-line and greater anti-EGFR-based therapy for BRAFV600E mCRC when compared to BRAF wild-type mCRC patients [26]. While it is established that BRAFV600E mutation status has key prognostic implications, whether or not it could be considered predictive for treatment outcomes proved to be more difficult to determine. Although multiple single-arm analyses had previously suggested diminished efficacy with the use of EGFR monoclonal antibodies, due to their retrospective nature and small sample sizes, firm conclusions could not be made [38, 39]. However, even subgroup analysis of randomized clinical trials revealed conflicting results. The PRIME study reported that BRAFV600E mutations were not reflective of being a predictive biomarker of response for EGFR inhibition when added to chemotherapy [40]. In contrast, both the COIN and PICCOLO studies revealed no benefit with EGFR inhibition in combination with chemotherapy, with suggestion of harm when utilized in later lines of therapy [41, 42]. Additionally, one study revealed patients with BRAFV600E had decreased response rates compared to BRAF wild-type patients when treated with cetuximab and chemotherapy (8.3% vs. 38%, respectively; P = 0.0012) [43]. It is important to highlight a large meta-analysis that included ten clinical trials (nine phase III and one phase II) has been conducted to investigate the clinical impact of EGFR inhibition with a total of 463 BRAFV600E mCRC patients evaluated. These data revealed that the addition of anti-EGFR did not result in improvement in PFS, OS, or response rate when compared to the respective chemotherapy-alone control regimens [44].
Taken together, this conglomerate of data supports the proposal that BRAFV600E mutation is in fact a negative predictive biomarker for anti-EGFR therapy. This finding is in line with signaling biology of the BRAFV600E mutation and its ability to activate the MAPK pathway downstream of EGFR blockade, analogous to RAS mutations [10]. Considering this, NCCN, ESMO, and ESMO Asia all recommend the use of anti-EGFR therapy exclusively for patients who are RAS/BRAF wild-type [45, 46].
3 One Size Does Not Fit All: Limited Efficacy with BRAF Inhibition in BRAFV600E mCRC
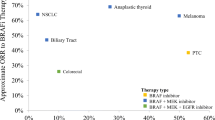
Considering the previously summarized limitations of both traditional chemotherapy and EGFR monoclonal antibodies for patients with BRAFV600E mCRC, there was a pressing need to identify effective targeted therapeutic options. In the era of precision medicine, capitalizing on the potential therapeutic vulnerability of a specific mutation was a favorable approach. Prior success achieved for BRAFV600E-mutant melanoma, non-small-cell lung cancer, and thyroid cancer has resulted in US Food and Drug Administration (FDA) approval with multiple oral BRAF inhibitors including vemurafenib, encorafenib, and dabrafenib [47,48,49,50,51,52]. Unfortunately, similar success was not realized for BRAFV600E mCRC with single-agent BRAF inhibition [53,54,55]. In a phase II study of 21 patients with BRAFV600E mCRC, vemurafenib monotherapy resulted in zero complete responses, one patient with a partial response, and seven patients with stable disease for an overall reported response rate of 5% [53]. In this study, median PFS and OS were noted to be 2.1 and 7.7 months, respectively. A phase 1 trial investigating the use of dabrafenib for patients with advanced solid tumors reported only one partial response out of 11 patients treated with BRAFV600E mCRC [54]. Additionally, a separate phase 1 study evaluated the efficacy of encorafenib monotherapy, and no partial responses were reported in 18 treated BRAFV600E mCRC patients with a 4-month median PFS [55].
Taken together, these studies highlight the stark contrast in efficacy with BRAF monotherapy between BRAFV600E-mutant melanoma and BRAFV600E mCRC, mainly due to the distinct biologic context of these two tumors. Unlike BRAFV600E-mutant melanoma, single-agent BRAF inhibition in BRAFV600E mCRC results in prompt feedback re-activation of EGFR signaling, producing persistent MAPK activation and ongoing tumor cell proliferation [56, 57]. Furthermore, incomplete inhibition of MAPK signaling has been noted in CRC cell lines, with translational studies revealing resistance post-single-agent RAF inhibition is due to acquired mutations in KRAS, NRAS, MAPK1, and copy number amplifications in BRAF [53, 56]. Therefore, considering the limitations of monotherapy with both EGFR and BRAF blockade, a combinatorial approach that simultaneously inhibits multiple effector nodes of the MAPK pathway emerged as a viable next step in the treatment evolution of BRAFV600E mCRC.
4 Overcoming Inherent Resistance to Monotherapy with Novel Combination Approaches in BRAFV600E mCRC
Experience with single-agent EGFR or BRAF biologic therapy has proven to be ineffective for BRAFV600E mCRC. However, a combinatorial approach warranted further investigation, as dual inhibition of EGFR and BRAF may overcome the MAPK feedback loop that results in activation of the EGFR receptor in response to BRAF blockade alone [56, 57]. Considering known resistance mechanisms that develop in response to BRAF-inhibitor monotherapy, pursuing additional novel targets against effectors of the MAPK pathway in combination to facilitate a deeper and more durable response is rational [56]. Several clinical trials exploring the concept of a biologic doublet revealed response rates in the range of 10–22% [58,59,60,61]. Of these, one phase 1 study evaluated the use of dabrafenib in combination with panitumumab with a reported response rate of 10%, median PFS of 3.5 months, and OS of 13.2 months [59]. Nearly half of the patients enrolled experienced a grade 3 or 4 adverse event (AE), with dermatitis acneiform (60%) being the most common AE of all grades. Another study investigated vemurafenib and panitumumab in 15 patients and revealed a response rate of 13% with median PFS of 3.2 months and median OS of 7.6 months [58]. There was a similar AE profile with dermatitis acneiform (53%) being the most common AE of all grades. A phase 1 dose-escalation study utilizing the combination of encorafenib and cetuximab revealed an ORR of 19.2% and disease control rate 76.9% with median PFS of 3.7 months [60]. The subsequent phase II study enrolled 50 patients with this biologic doublet and achieved a median PFS of 4.2 months with an ORR of 22% [61]. Nearly 60% of patients had a grade 3 or 4 AE, with the most common AEs of any grade being nausea (56%), diarrhea (54%), and vomiting (52%). Of note, rash was not one of the more common AEs of any grade with this doublet.
Biologic doublet chemotherapy has been shown to be safe and effective when combined with traditional chemotherapy. An early-phase trial investigating the regimen of vemurafenib in combination with irinotecan and cetuximab (VIC) revealed 6 out of 17 patients with BRAFV600E mCRC achieved a radiographic response with a median PFS of 7.7 months [62]. These encouraging data led to a follow-up randomized phase II trial, the Southwest Oncology Group 1406 (SWOG) study, which compared irinotecan plus cetuximab with or without vemurafenib in patients with BRAFV600E mCRC62. This trial revealed an improved median PFS (4.4 months vs. 2.0 months, [HR, 0.42; 95% confidence interval (CI), 0.26–0.66; P < 0.001]) and ORR (16% vs. 4%) among patients treated with the novel three-drug combination arm compared to those who received irinotecan and cetuximab alone [63]. As expected, grade 3 and 4 AEs of neutropenia, diarrhea, anemia and nausea occurred at a higher rate in the 3-drug combination arm. Based on these results, the VIC regimen was initially inserted into the NCCN guidelines as a treatment option for patients with refractory BRAFV600E mCRC.
Previous translational work has also identified the PI3K/AKT signaling pathway as a potential resistance mechanism to BRAF inhibition [64]. To that effect, a biologic triplet against BRAF, EGFR, and PI3K (encorafenib, cetuximab, and alpelisib) did reveal improved PFS in previously treated patients with BRAFV600E mCRC when compared to encorafenib and cetuximab alone based on interim analysis of a randomized phase II study [61]. However, the difference did not reach statistical significance and the biologic triplet regimen was noted to increase AEs with 79% of patients experiencing grade 3 and 4 AEs compared to 58% with the doublet.
MEK is a downstream effector of BRAF and the addition of a MEK inhibitor to a BRAF inhibitor in BRAFV600E-mutated melanoma revealed significant improvement in response rates when compared to monotherapy [65]. Initial efforts exploring dual inhibition with BRAF and MEK in BRAFV600E-mutated mCRC revealed only limited efficacy, with a 12% response rate and only one patient developing a complete response out of 43 treated patients [66]. Although not mirroring the level of improvement appreciated in corresponding melanoma trials [65], an open-label phase 1 trial investigating the biologic triplet of dabrafenib, panitumumab, and trametinib (a MEK inhibitor) for first-line BRAFV600E mCRC revealed response rates of 21% compared to 10% in those treated with a biologic doublet of dabrafenib plus panitumumab with median PFS of 4.2 and 3.5 months, respectively [59]. Of note, it is important to highlight the combination arm of panitumumab and trametinib in this study revealed a response rate of 0% with the highest rate of (18%) grade 3/4 acneiform dermatitis.
Recently, the BEACON CRC study, the first randomized phase III study and largest clinical trial for previously treated (one or two prior therapies) BRAFV600E mCRC, has been completed [67]. This landmark trial was a global, multicenter, open-label study evaluating the activity of a BRAF inhibitor encorafenib plus cetuximab, with or without the MEK inhibitor binimetinib, in comparison to investigator’s choice control arm of irinotecan combined with cetuximab or FOLFIRI and cetuximab. The primary endpoints for BEACON CRC were OS and independently reviewed confirmed ORRs for the biologic triplet compared to control. Secondary endpoints included OS for the biologic doublet arm compared to the control, as well as PFS, duration of response, and safety signals. With a median follow-up of 18.2 months, initial safety lead in data were published and revealed an ORR of 48% (three complete responses, 11 partial responses), median PFS of 8 months and median OS of 15.3 months, with manageable tolerability among 29 patients who received the triplet regimen, allowing for initiation of the randomized portion of BEACON [68, 69].
Data from the randomized portion of the BEACON CRC study have been previously published with updated survival data presented at the ASCO 2020 Virtual Meeting [67, 70]. Initially published data revealed that the triplet regimen resulted in an improved OS of 9.0 months (95% CI 8.0–11.4 months) compared to 5.4 months (95% CI 4.8–6.6 months) for the control group (hazard ratio = 0.52, 95% CI 0.39–0.70; P < 0.0001) [67]. The ORR for the biologic triplet was 26% compared to 2% for the control regimen (P < 0.0001) [67]. Of note, the response rate for patients with only one prior line of therapy was 34% for the triplet compared to only 2% for the control. Therefore, improved efficacy may be achieved for patients if exposed in earlier lines of treatment. Median PFS was also markedly improved with the triplet regimen at 4.3 months (95% CI 4.1–5.2) compared to 1.5 months (95% CI 1.5–1.7) for the control arm. Overall, the biologic triplet was well tolerated. The biologic doublet of encorafenib plus cetuximab also resulted in improved efficacy compared to control. The tolerability of the doublet and triplet regimens was consistent with known toxicity profiles of the individual agents. Of note, grade 3 AEs were noted in 58% of patients exposed to the triplet regimen, compared to 50% of those exposed to the doublet versus 61% of patients who received the control regimens.
An updated analysis of the BEACON CRC study was presented at the 2020 ASCO Virtual Meeting [70]. The results confirmed encorafenib plus cetuximab with or without binimetinib improved OS, PFS, and ORR in previously treated BRAFV600E mCRC compared to traditional chemotherapy, with a median OS of 9.3 months for both the triplet and doublet regimens [70]. Updated ORR revealed 27% for the triplet, 20% for the doublet, and 2% for the control regimens [70]. Updated median PFS was 4.5 months for the triplet (95% CI 4.2–5.5) compared to 1.5 months (95% CI 1.5–1.9) for the control with HR (95% CI) of 0.42 (0.33–0.53) [70]. Updated median PFS for the biologic doublet was 4.3 months (95% CI 4.1–5.5) compared to 1.5 months for the control (95% CI 1.5–1.9) with a HR (95% CI) of 0.44 (0.35–0.55) [70]. As mentioned, updated median OS for the biologic doublet was 9.3 months (95% CI 8.0–11.3) compared to 5.9 months (95% CI 5.1–7.1) for the control with a HR (95% CI) of 0.61 (0.48–0.77), with no difference in median OS with the utilization of the biologic triplet (9.3 months, 95% CI: 8.2–10.8) [70]. These data confirm that with further follow-up after the primary analysis, the biologic doublet regimen of encorafenib + cetuximab was well tolerated and had similar overall efficacy to the biologic triplet regimen of encorafenib plus cetuximab with binimetinib.
Based on these landmark practice-changing data, FDA approval was granted for the doublet regimen on 8 April 2020. Furthermore, the NCCN has recommended the BEACON regimen of encorafenib in combination with cetuximab as the primary treatment option for previously treated BRAFV600E mCRC. This biologic doublet represents a clear improvement over current standard-of-care options as well as a notable improvement over previously reported BRAF inhibitor combination studies [58,59,60] (Fig. 2). These data represent the first time a completely targeted therapeutic approach has demonstrated survival benefit over standard-of-care treatment for the management of patients with previously treated BRAFV600E mCRC. In summary, encorafenib in combination with cetuximab is FDA-approved for use in patients with previously treated BRAFV600E mutant mCRC and should be the new standard of care.
5 Utilization of Immunotherapy for BRAFV600E mCRC
The use of immune checkpoint blockade for the 5% of patients who are MSI-H in mCRC has dramatically shifted the treatment paradigm for these patients. Agents targeting programmed death-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) revealed durable activity for patients with MSI-H mCRC with 12-month survival rates noted in more than 70% of patients [71, 72]. As previously summarized, a sporadic MSI-H phenotype is isolated in 20–30% of patients with BRAFV600E mCRC and is associated with a response to immune checkpoint blockade agents [71, 72]. The KEYNOTE-164 trial utilized the anti-PD-1 antibody pembrolizumab as monotherapy, with 14/124 (11%) MSI-H mCRC patients having a concomitant BRAFV600E mutation [71]. In the CHECKMATE-142 trial, MSI-H mCRC patients were treated with anti-PD-1 nivolumab with (29% BRAFV600E) or without the anti-CTLA-4 antibody ipilimumab (16% BRAFV600E) [72]. Sustainable responses were seen among patients with BRAFV600E MSI-H mCRC in all respective cohorts. These data support I/O therapy as an additional option for patients with previously treated sporadic MSI-H BRAFV600E mCRC.
Recently, data from the randomized open-label phase III KEYNOTE-177 trial comparing the anti-PD-1 antibody pembrolizumab with investigator’s choice standard-of-care chemotherapy and biologic therapy (± bevacizumab or cetuximab) for treatment-naïve MSI-H mCRC have been presented [73]. This study met one of its primary endpoints of PFS with improvement to 16.5 months with pembrolizumab versus 8.2 months with chemotherapy (HR = 0.60; P = 0.0002) [73]. ORR were noted to be increased in the immunotherapy arm at 43.8% versus 33.1% for chemotherapy (P = 0.0275) [73]. Final OS analysis is pending. Of note, PFS subgroup analysis revealed there were 77 patients with BRAFV600E mCRC treated on trial favoring the pembrolizumab arm (HR 0.48, 95% CI 0.27–0.86) [73]. These positive data support the proposal that pembrolizumab should be the new standard of care for first-line MSI-H mCRC. Considering the sporadic MSI-H BRAFV600E mCRC patients included in this study sustained durable response and benefit, these data support early exposure to I/O therapy for patients with treatment-naïve BRAFV600E is beneficial. The data are interesting in light of recent population-based assessments reporting MSI-H BRAFV600E mCRC patients have a worse median OS when compared to microsatellite stable (MSS) BRAFV600E mCRC (8.9 vs. 17.2 months, HR 1.46, 95% CI 0.96–2.27, P = 0.043), highlighting that early biomarker-based therapeutic interventions remain critical to improve outcomes [7]. Additional work is needed to elucidate the best therapeutic sequential approach for this unique subset of MSI-H BRAFV600E mCRC.
For the majority of BRAFV600E mCRC patients who are MSS, there are numerous ongoing trials investigating agents targeting the MAPK pathway in combination with immunotherapy. To highlight a few key studies: encorafenib in combination with cetuximab and nivolumab (NCT04017650); encorafenib in combination with binimetinib and nivolumab (NCT04044430); and dabrafenib in combination with trametinib and spartalizumab (NCT03668431). Of note, this novel combination approach of BRAF/MEK inhibition with anti-PD-1 (NCT03668431) proved to be well tolerated with a recently reported impressive median duration of response of 5.6 months [74]. We anxiously await the final read-out on these critical studies as we move forward to expand our treatment armamentarium for BRAFV600E mCRC.
6 Rare, but Distinct: Atypical, Non-V600 BRAF-Mutant mCRC
Due to the increasing use of expanded molecular profiling and circulating tumor DNA (ctDNA) in the management of mCRC, awareness has emerged of atypical, non-V600 BRAF (aBRAF) mutations as a unique molecular subset. Previous experience has reported that these aBRAF mCRC patients reflect approximately 2.2% of patients with mCRC with an improved median OS of 60.7 months compared to 11.7 months for patients with BRAFV600E mutations [11]. However, considering the chronicity of aBRAF mCRC, novel treatments are still needed for personalization and in order to maintain quality of life for these patients. Furthermore, aBRAF mCRC has a distinct antithetical clinical profile from BRAFV600E mCRC, with most patients having MSS disease, left-sided primary tumors, lower grade histology, often co-mutated with RAS, and clinical presentations of non-peritoneal spread [11, 75]. As previously summarized, preclinical data have characterized aBRAF into distinct classes that drive ERK activation via unique signaling biology that may guide future trial development and therapeutic vulnerabilities [12] (Fig. 1). Understanding the nuances of these signaling mechanisms will be critical for the development of novel precision-based therapies and highlight why application of the BEACON regimen may not be efficacious in aBRAF mCRC, although efforts are ongoing to that effect (EPOC1703-UMIN000031857; NCT03843775) [76].
To date there are no specific guidelines for the management of patients with aBRAF mCRC, with early-phase clinical trials recommended after failure of traditional systemic chemotherapy. Additionally, deployment of EGFR receptor antibodies remains inconclusive, with data suggesting response may be driven by underlying aBRAF class. Some reports note class III aBRAF may benefit from EGFR inhibition, while subsequent works highlight a lack of durable response and the potential of class II aBRAF reflecting a negative predictive biomarker [13, 14, 77]. Additional data are needed to define the role EGFR inhibition should play in the management of aBRAF mCRC. Of note, in EPOC1703, aBRAF class III patients who are both EGFR receptor antibody naïve and EGFR receptor antibody refractory will be included and treated with the BEACON regimen. The difference in efficacies between these two patient populations may further elucidate the role of EGFR inhibition.
Future trial design must incorporate rational combinatorial treatments that consider specific class biology in order to move the needle forward for this unique molecular subset of mCRC (Fig. 1). Innovative attempts to target the MAPK pathway with novel inhibitors of MEK or ERK alone or in combination with next-generation RAF inhibitors that target dimerization represents one potential path forward in aBRAF mCRC [78, 79]. The nationwide NCI-MATCH study is utilizing a novel ERK1/2 inhibitor called ulixertinib, which may reflect a viable option for patients with previously treated aBRAF mCRC (NCT02465060). Novel RAF dimer inhibitors such as LXH254 (NCT02607813) and BGB-3245 (NCT04249843) are in early-phase clinical trials, with the latter having dosing expansion cohorts that include non-V600 BRAF mutants. PLX8394 (NCT02428712) is another novel agent considered a BRAF-specific dimer breaker that selectively inhibits ERK signaling in cancers driven by dimeric BRAF mutants, such as aBRAF, including fusions and splice variants [80]. Considering the signaling biology of class II aBRAF, such novel RAF inhibitors would represent ideal candidates for potential combination with MEK inhibition. It is important to note that MEK/ERK inhibition in Class III BRAF mutations is limited due to resultant receptor tyrosine kinase (RTK) reactivation [13]. Of note, early-phase clinical trials are utilizing a novel Src homology phosphatase 2 (SHP2) inhibitor (NCT04045496; NCT03518554). SHP2 functions as a major scaffold protein downstream of numerous RTKs to promote the RAS/MAPK signaling pathway in cancers with class 3 BRAF mutations. Therefore, SHP2 inhibition may represent a promising therapeutic approach for patients with refractory class 3 aBRAF mutations, and combinatorial approaches with other MAPK pathway inhibitors, such as MEK or ERK inhibition, may reflect a rational step forward for these subtypes of aBRAF.
7 Future Directions and Conclusions
The treatment landscape for BRAFV600E mCRC has made significant strides in the last 5 years, culminating in FDA approval of the first biologic doublet of encorafenib and cetuximab for patients with previously treated metastatic disease. However, it is important to note that response rates with BRAF inhibitors remain low in comparison to other cancers such as melanoma and NSCLC, with a shorter duration of response. Considering patients with BRAFV600E mCRC develop acquired resistance and subsequent loss of response to BRAF-directed therapy [52, 58], utilization of ctDNA to assess mechanisms of resistance and incorporating RNA signatures to highlight subsets who may benefit from a specific therapy are key areas of future research. Exploratory analysis of the BEACON study is ongoing to investigate noted trends of subsets of patients who may benefit from the addition of binimetinib to encorafenib and cetuximab, based on markers of inflammation and performance status. Furthermore, the favorable safety profile of encorafenib and cetuximab will allow for this regimen to serve as a suitable backbone to evaluate novel chemotherapy combinations, targeted agents, and immunotherapeutic approaches moving forward (NCT04017650). Finally, considering the poor prognosis of the BRAFV600E mutation and how few patients receive second-line treatment, identifying how best to sequence lines of therapy is relevant [7]. Due to inherent resistance to cytotoxic chemotherapy and the urgency of an initial response, early exposure to targeted biologic therapy may result in enhanced patience outcomes. To that end, we await the activation of the frontline BREAKWATER study investigating the biologic doublet of encorafenib and cetuximab alone (Arm A) or in combination with chemotherapy (Arm B) compared to a control arm of chemotherapy alone for treatment naïve MSS/pMMR BRAFV600E mCRC. This trial has a primary endpoint of PFS with secondary endpoints of OS, and will promote additional refinement of the current treatment paradigm.
In conclusion, the treatment landscape for BRAFV600E mCRC has been revitalized by well-conducted preclinical and translational science resulting in practice-changing targeted therapy, establishing the foundation for continued precision moving forward. A similar bench to bedside approach is needed to enhance the management for aBRAF mCRC, and dedicated efforts are underway.
References
Howlader N, Noone AM, Krapcho M, et al. (eds). SEER cancer statistics review, 1975–2016. Bethesda, MD: National Cancer Institute; 2019. https://seer.cancer.gov/csr/1975_2016/.
Nikolouzakis TK, Vassilopoulou L, Fragkiadaki P, et al. Improving diagnosis, prognosis and prediction by using biomarkers in CRC patients (Review). Oncol Rep. 2018;39(6):2455–72.
Zoratto F, Rossi L, Verrico M, et al. Focus on genetic and epigenetic events of colorectal cancer pathogenesis: implications for molecular diagnosis. Tumor Biol. 2014;35:6195–206.
Samowitz WS, et al. Poor survival associated with the BRAFV600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–9.
Clarke CN, Kopetz ES. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies. J Gastrointest Oncol. 2015;6:660–7.
Morris V, Overman MJ, Jiang Z-Q, et al. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin Colorectal Cancer. 2014;13:164–71.
Chu JE, Johnson B, Kugathasan L, et al. Population-based screening for BRAFV600E in metastatic colorectal cancer reveals increased prevalence and poor prognosis. Clin Cancer Res. 2020. https://doi.org/10.1158/1078-0432.ccr-20-1024.
Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):103–19.
Forbes SA, Beare D, Boutselakis H, Bamford S, Bindal N, Tate J, et al. COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017;45(D1):D777–83.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54.
Jones JC, Renfro LA, Al-Shamsi HO, et al. Non-V600 BRAF mutations define a clinically distinct molecular subtype of metastatic colorectal cancer. J Clin Oncol. 2017;35:2624–30.
Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. Tumors with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature. 2017;548(7666):234–8.
Yaeger R, Kotani D, Mondaca S, et al. Response to anti-EGFR therapy in patients with BRAF non-V600-mutant metastatic colorectal cancer. Clin Cancer Res. 2019;25(23):7089–97.
Johnson B, Loree JM, Jacome AA, et al. Atypical, non-V600 BRAF mutations as a potential mechanism of resistance to EGFR inhibition in metastatic colorectal cancer. JCO Precis Oncol. 2019;3:1–10.
Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50(1):113–30.
Schirripa M, Lenz H-J. Biomarker in colorectal cancer. Cancer J. 2016;22(3):156–64.
Lai E, Pretta A, Impera V, Mariani S, Giampieri R, Casula L, et al. BRAF-mutant colorectal cancer, a different breed evolving. Expert Rev Mol Diagn. 2018;18(6):499–512.
Greene C, Atreya CE, McWhirter R, Ikram N, Van Loon K, Venook AP, et al. Differential radiographic appearance of BRAF V600E mutant metastatic colorectal cancer (mCRC) in patients matched by primary tumor location. J Clin Oncol. 2016;34(4_suppl):554.
Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117(20):4623–32.
Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–93.
Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–6.
Dienstmann R, Vermeulen L, Guinney J, et al. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17:79–92.
Tsai JH, Liah JY, Lin YL, et al. Traditional serrated adenoma has two pathways of neoplastic progression that are distinct from the sessile serrated pathway of colorectal carcinogenesis. Mod Pathol. 2014;27:1375–85.
Bettington M, Walker N, Clouston A, et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–86.
Yokota T, Ura T, Shibata N, et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer. 2011;104(5):856–62.
Saridaki Z, Tzardi M, Sfakianaki M, et al. BRAFV600E mutation analysis in patients with metastatic colorectal cancer (mCRC) in daily clinical practice: correlations with clinical characteristics, and its impact on patients’ outcome. PLoS ONE. 2013;8(12):e84604.
Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–12.
Thomsen M, Guren MG, Skovlund E, et al. Health-related quality of life in patients with metastatic colorectal cancer, association with systemic inflammatory response and RAS and BRAF mutation status. Eur J Cancer. 2017;81:26–35.
Network NCC. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): rectal cancer: version 1.2019. 2019. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed Mar 2019.
Network NCC. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): colon cancer: version 1.2019. 2019. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed Mar 2019.
Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65(14):6063–9.
Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28(3):466–74.
Sinicrope FA, Shi Q, Smyrk TC, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015;148(1):88–99.
Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–15.
Shui L, Wu YS, Lin H, Shui P, Sun Q, Chen X. Triplet chemotherapy (FOLFOXIRI) plus bevacizumab versus doublet chemotherapy (FOLFOX/FOLFIRI) plus bevacizumab in conversion therapy for metastatic colorectal cancer: a meta-analysis. Cell Physiol Biochem. 2018;48(5):1870–81.
Cremolini C, Antoniotti C, Stein A, et al. FOLFOXIRI/bevacizumab (bev) versus doublets/bev as initial therapy of unresectable metastatic colorectal cancer (mCRC): a meta-analysis of individual patient data (IPD) from five randomized trials. J Clin Oncol. 2020;38(15_suppl):4015.
Grothey A, et al. ANCHOR CRC: a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E-mutant metastatic colorectal cancer. Ann Oncol. 2020;31(3_suppl):LBA 5.
Loupakis F, Ruzzo A, Cremolini C, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101(4):715–21.
De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753–62.
Douillard J-Y, Oliner KS, Siena S, et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34.
Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377(9783):2103–14.
Seymour MT, Brown SR, Middleton G, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol. 2013;14:749–59.
Zhao B, Wang L, Qiu H, et al. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget. 2017;8:3980–4000.
Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51(5):587–94.
Kristina Gregory NM, Lisa Gurski O, Benson AB, et al. NCCN guidelines version 1.2019 colon cancer continue NCCN guidelines panel disclosures. 2019. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 11 Apr 2019.
Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16.
McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15(3):323–32.
Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–65.
Delord JP, Robert C, Nyakas M, McArthur GA, Kudchakar R, Mahipal A, et al. Phase I dose-escalation and -expansion study of the BRAF inhibitor encorafenib (LGX818) in metastatic BRAF-mutant melanoma. Clin Cancer Res. 2017;23(18):5339–48.
Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, et al. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(9):1272–82.
Planchard D, Kim TM, Mazieres J, Quoix E, Riely G, Barlesi F, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(5):642–50.
Kopetz S, Desai J, Chan E, Hecht JR, O’Dwyer PJ, Maru D, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015;33(34):4032–8.
Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–901.
Gomez-Roca CA, Delord J, Robert C, Hidalgo M, von Moos R, Arance A, et al. Encorafenib (LGX818), an oral BRAF inhibitor, in patients (pts) with BRAF V600E metastatic colorectal cancer (mCRC): results of dose expansion in an open-label, phase 1 study. Ann Oncol. 2014;25(4_Suppl):IV182.
Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2(3):227–35.
Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483(7387):100–3.
Yaeger R, Cercek A, O’Reilly EM, Reidy DL, Kemeny N, Wolinsky T, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res. 2015;21(6):1313–20.
Corcoran RB, Andre T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAF(V600E)-mutant colorectal cancer. Cancer Discov. 2018;8(4):428–43.
van Geel R, Tabernero J, Elez E, Bendell JC, Spreafico A, Schuler M, et al. A phase Ib dose-escalation study of encorafenib and cetuximab with or without alpelisib in metastatic BRAF-mutant colorectal cancer. Cancer Discov. 2017;7(6):610–9.
Tabernero J, Geel RV, Guren TK, Yaeger RD, Spreafico A, Faris JE, et al. Phase 2 results: encorafenib (ENCO) and cetuximab (CETUX) with or without alpelisib (ALP) in patients with advanced BRAF-mutant colorectal cancer (BRAFm CRC). J Clin Oncol. 2016;34(15_suppl):3544.
Hong DS, Morris VK, El Osta B, et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov. 2016;6:1352–65.
Kopetz S, McDonough SL, Morris VK, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG 1406). J Clin Oncol. 2017;35:520.
Mao M, Tian F, Mariadason JM, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res. 2013;19(3):657–67.
Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703.
Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J Clin Oncol. 2015;33:4023–31.
Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAFV600E-mutated colorectal cancer. N Engl J Med. 2019;381:1632–43.
Cutsem EV, Cuyle P-J, Huijberts S, Yaeger R, Schellens JHM, Elez E, et al. BEACON CRC study safety lead-in (SLI) in patients with BRAFV600E metastatic colorectal cancer (mCRC): efficacy and tumor markers. J Clin Oncol. 2018;36(4_suppl):627.
Kopetz S, Grothey A, Yaeger R, Cuyle P-JA, Huijberts S, Schellens JHM, et al. Updated results of the BEACON CRC safety lead-in: encorafenib (ENCO) + binimetinib (BINI) + cetuximab (CETUX) for BRAFV600E-mutant metastatic colorectal cancer (mCRC). J Clin Oncol. 2019;37(4_suppl):688.
Kopetz S, Grothey A, Yaeger R, et al. Encorafenib plus cetuximab with or without binimetinib for BRAF V600E metastatic colorectal cancer: updated survival results from a randomized, three-arm, phase III study versus choice of either irinotecan or FOLFIRI plus cetuximab (BEACON CRC). J Clin Oncol. 2020;38(15_suppl):4001.
Le DT, Kim TW, Van Cutsem E, Geva R, Jager D, Hara H, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38(1):11–9.
Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–9.
Andre T, Shiu Kai-Keen, Kim TW, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: the phase 3 KEYNOTE-177 study. J Clin Oncol. 2020;38(18_suppl):LBA4.
Corcoran R, Giannakis M, Allen J, Chen J, Pelka K, Chao S, et al. Clinical efficacy of combined BRAF, MEK, and PD-1 inhibition in BRAFV600E colorectal cancer patients. Ann Oncol. 2020;31(3_Suppl):SO-26.
Cremolini C, Di Bartolomeo M, Amatu A, et al. BRAF codons 594 and 596 mutations identify a new molecular subtype of metastatic colorectal cancer at favorable prognosis. Ann Oncol. 2015;26(10):2092–7.
Kotani D, Bando H, Taniguchi H, et al. BIG BANG study (EPOC1703): multicentre, proof-of-concept, phase II study evaluating the efficacy and safety of combination therapy with binimetinib, encorafenib and cetuximab in patients with BRAF non- V600E mutated metastatic colorectal cancer. ESMO Open. 2020;5:e000624.
Wang Y, Jones JC, Kipp BR, Grothey A. Activity of EGFR antibody in non-V600 BRAF mutant metastatic colorectal cancer. Ann Oncol. 2019;30(1):147–9.
Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67.
Karoulia Z, Gavathiotis E, Poulikakos PI. New perspectives for targeting RAF kinase in human cancer. Nat Rev Cancer. 2017;17:676–91.
Yao Z, Gao Y, Su W, et al. RAF inhibitor PLX8394 selectively disrupts BRAF dimers and RAS-independent BRAF-mutant-driven signaling. Nature. 2019;25(2):284–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Cancer Center Support Grant (CCSG) CA016672; National Institutes of Health/National Cancer Institute (NIH/NCI) R01 CA187238, R01 CA184843 and MD Anderson Moon Shots.
Conflict of Interest
Dr. Johnson serves as a consultant/clinical advisory board for Gritstone Oncology. Dr. Kopetz serves as a consultant/clinical advisory board for Merck, Navire Pharma, Holy Stone, Biocartis, Natera, Roche, Redx Pharma, Jacobio, Genentech, Lilly, AstraZeneca/MedImmune, EMD Serono, Daiichi Sankyo, Karyopharm Therapeutics, EMD Serono, Symphogen, Novartis, Boston Biomedical, Ipsen, HalioDx, Amgen, Pierre Fabre, Amal Therapeutics, Boehringer Ingelheim, Bayer Health, Lutris, Pfizer.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author Contributions
Benny Johnson and Scott Kopetz provided equally to the contribution of this article.
Rights and permissions
About this article
Cite this article
Johnson, B., Kopetz, S. Applying Precision to the Management of BRAF-Mutant Metastatic Colorectal Cancer. Targ Oncol 15, 567–577 (2020). https://doi.org/10.1007/s11523-020-00747-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00747-5