Abstract
Hepatocellular carcinoma (HCC) is one of the most common and deadliest cancers worldwide with a rising incidence in the Western world. HCCs are characterized by high resistance to systemic therapies induced by phenotypic and molecular heterogeneity. For almost 10 years, the tyrosine kinase inhibitor sorafenib was the only approved treatment for advanced HCCs in patients with preserved liver function, and until 2016, no new compounds tested in large phase III studies have led to a survival benefit. The tyrosine kinase inhibitor regorafenib, a fluorinated sorafenib analog, was the first substance that showed a significant improvement in overall survival after failure of sorafenib treatment, which subsequently led to its regulatory approval in a second-line setting in 2017. In addition, the non-inferiority of lenvatinib in comparison with sorafenib opened another therapeutic first-line option in the same year. Furthermore, several other compounds showed promising results in recent phase III studies, including ramucirumab in patients with elevated alpha-fetoprotein (AFP) levels as well as cabozantinib in second- and third-line settings. In addition, promising early reports of the immune checkpoint inhibitors nivolumab and pembrolizumab, with objective response rates of 15–20%, paved the way for immuno-oncological interventions for HCC and these will probably gain increasing attention as mono- and combination therapies. In summary, following the approval of sorafenib in 2007 and almost 10 years of therapeutic stagnation, results from recent clinical trials in first- and further-line settings for the first time demonstrated efficacy of several active compounds in advanced HCCs. Thus, a sequential approach should now be implemented in HCC treatment and will improve the survival of HCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Hepatocellular carcinoma (HCC) ranks among the most common and deadliest cancers worldwide [1]. Incidence rates nearly doubled over the last decades and, to date, HCC is one of the fastest growing causes of cancer-related deaths in the USA. Despite effective surveillance options, < 20% of patients are eligible for curative treatment, such as liver transplantation, resection, or radiofrequency ablation, at the time of diagnosis [2]. These observations clearly indicate that liver cancer is evolving to become a major healthcare problem in the United States and Europe, thus highlighting the critical need for improved understanding of the pathophysiology and development of novel treatment options for this deadly disease [3]. During the past 10 years, drug development in advanced HCC stages has been significantly hampered by a pronounced phenotypic and molecular heterogeneity of the tumors, as well as a high toxicity of active compounds under clinical evaluation [4]. The majority of patients with advanced HCC present with a severely impaired liver function, which further requires a fine balance between anti-tumor activity and drug-induced toxicity [5]. Until recently, the multi-tyrosine kinase inhibitor sorafenib was the therapeutic standard of care in a first-line setting since 2007 [6]. Until 2016 no survival benefit could be demonstrated for any of the compounds evaluated in large phase III studies (Table 1), either in comparison with sorafenib, in combination with sorafenib, or after sorafenib failure [4]. In 2017, results of the RESORCE trial (Study of Regorafenib After Sorafenib in Patients With Hepatocellular Carcinoma) led to approval of regorafenib as a second line therapy [7]. Since then, several other active substances as well as immunotherapeutic checkpoint inhibitors showed promising results in both first- and second-line treatment. Thus, a sequential treatment approach is now feasible and should be implemented and evaluated in advanced HCC. Despite the promising developments in systemic therapies, the overall survival (OS) of HCC patients remains decisively low and further novel therapeutic strategies need to be developed. The current review provides a summary of the most recent results from large phase III clinical trials and will delineate future developments and potential pitfalls for clinical implementation. The work is based on a selective literature review that reflects the current clinical context and promising future developments in the field.
2 First-Line Therapies
2.1 Sorafenib—The Gold Standard in First-Line Therapy for 10 Years
Until 2007, no effective treatment for patients diagnosed with advanced HCC or patients who progressed into this stage after failure of other therapies was available. The positive results of the randomized, controlled phase III SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol) trial evaluating sorafenib, an oral multi-tyrosine kinase inhibitor (TKI) with activity against vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), and RAF kinase for advanced HCC in a mainly Western cohort provided first evidence for the efficacy of anti-angiogenetic strategies in advanced HCC [6]. Median OS in the sorafenib arm was 10.7 months versus 7.9 months in placebo-treated patients (hazard ratio [HR] 0.69; 95% CI 0.55–0.87; p = 0.00058). Similar results were not only demonstrated in a parallel phase III study involving mainly Asian, predominantly hepatitis B-infected patients [8], but also in eight subsequent phase III studies in which sorafenib served as the control treatment. Importantly, none of the trials could demonstrate superiority over sorafenib. On the basis of the positive results from both trials, sorafenib was approved and became the systemic standard of care across different therapeutic lines. Notably, although currently no predictive biomarkers for response exist, several clinical factors including chronic hepatitis C infection or side effects including early dermatological events or hypertension favor a better response to the treatment [9, 10]. Despite approval for all stages of liver disease, large non-interventional observational studies have shown that the survival of patients with CHILD class B cirrhosis is significantly shorter than those of patients with CHILD A cirrhosis. Since these studies did not provide conclusive evidence for a benefit in CHILD B patients, the use of sorafenib should in general be limited to patients with compensated stages of cirrhosis [11]. The spectrum of adverse effects of sorafenib is well described and requires close monitoring of the patients, specifically during the first weeks of treatment. The majority of these adverse effects can be controlled and attenuated by supportive measures and dose reductions, but require close monitoring of the patients, specifically at the beginning of treatment. For instance, diarrhea, hypertension and hand-foot syndrome occur in a number of patients. Interestingly, early skin reactions and diarrhea seem to be predictors of a better response to therapy [9]. Furthermore, required dose reductions (i.e., < 800 mg/day) do not seem to impair overall outcome of patients [12].
2.2 Lenvatinib—REFLECT Trial
Lenvatinib is another oral multi-tyrosine kinase inhibitor with activity against VEGFR1–3, fibroblast growth factor receptor (FGFR) 1–4, PDGF, RET and KIT. A recent open-label phase III study involving mainly Asian patients was conducted to demonstrate non-inferiority of lenvatinib in comparison with sorafenib in a first-line setting [13]. The study achieved its primary endpoint with a median OS of 13.6 months in the experimental lenvatinib arm versus 12.3 months in the sorafenib arm (HR 0.92; 95% CI 0.79–1.06). An interesting observation of this trial was the high objective response rate (ORR) for lenvatinib with 24.1% versus 9.2% for sorafenib despite the similar OS (independent review: ORR modified Response Evaluation Criteria in Solid Tumors [mRECIST] 40.6% vs 12.4%, ORR RECIST: 18.8% vs 6.5%). Further, surrogate characteristics for survival such as progression-free survival (PFS) and time to progression (TTP) were consistently higher in the lenvatinib group than in the sorafenib group (PFS: 7.4 months vs 3.7 months; TTP: 8.9 months vs 3.7 months). Adverse effects were overall slightly more pronounced in lenvatinib-treated patients, particularly hypertension and thrombocytopenia. Notably, time on treatment was also significantly higher in the experimental arm (5.7 months for lenvatinib vs 3.7 months for sorafenib) and time-on-treatment–adjusted adverse event rates were similar in both arms. Importantly, the study excluded patients with adverse prognostic tumor characteristics such as main branch portal vein thrombosis or > 50% tumor occupation of the liver. Nevertheless, results from the trial encouraged the use of lenvatinib as an effective first-line therapy in advanced HCC, leading to its inclusion in recent European Association for the Study of the Liver (EASL) and European Society For Medical Oncology (ESMO) guidelines [3, 14]. Consequently, approval for lenvatinib in first-line treatment was recently granted by the US Food and Drug Association (FDA) and European Medicines Agency (EMA).
2.3 Compounds in First-Line Treatment with No Therapeutic Benefits in Phase III Trials
Following the approval of sorafenib, several other first-line substances have been tested either against sorafenib (brivanib, linifanib, sunitinib) or in combination with sorafenib (sorafenib plus erlotinib, sorafenib plus doxorubicin). Despite positive signals from phase II trials, none of the studies achieved their primary endpoint and demonstrated a meaningful survival benefit over sorafenib alone. Similarly, systemic chemotherapies such as FOLFOX seem to be ineffective in this setting [4]. Based on several reports indicating a survival benefit in advanced disease stages, different systemic therapies (i.e., sorafenib, brivanib, and orantinib) were evaluated in combination with local transarterial chemoembolization (TACE) treatment in four phase III studies [15,16,17,18]. However, none of the studies showed improved patient survival so that the sequential use of TACE followed by systemic therapy remains standard.
3 Second-Line Therapies
3.1 Regorafenib—RESORCE Trial
Regorafenib is an oral fluorinated sorafenib analog with a similar spectrum of molecular targets. Besides a profound anti-proliferative effect on the tumor cells, regorafenib significantly inhibits neo-angiogenesis and, thus, modulates the tumor microenvironment. The randomized controlled RESORCE phase III trial evaluated the role of regorafenib in patients with advanced HCC that progressed under sorafenib therapy [7]. The main inclusion criteria were a preserved liver function (CHILD A), progressive disease under sorafenib as well as tolerability to sorafenib (defined as receiving sorafenib ≥ 400 mg for at least 20 days of the last 28 days of treatment). The study further rigorously stratified for region, portal-vein thrombosis, alpha-fetoprotein (AFP) levels and extrahepatic tumor manifestation. This highly selective strategy was performed to avoided toxicity and unequal distribution of prognostically adverse characteristics. The study reached its primary endpoint and demonstrated a significant improved OS for regorafenib (10.6 months) versus placebo (7.8 months) (HR 0.63; 95% CI 0.50–0.79; p < 0.0001) as well as an increase in the median TTP (3.2 months vs 1.5 months; HR 0.44; 95% CI 0.36–0.55; p < 0.001). In addition, regorafenib significantly extended the tumor control (65.2% vs 36.1%; p < 0.001) as well as ORR (10.6% vs 4.1%; p = 0.005). The spectrum of adverse events was comparable to side effects described for sorafenib, including hypertension, hand-foot syndrome, fatigue, and diarrhea, but was overall manageable. Based on the results of the RESORCE trial, regorafenib was approved by the FDA and the EMA in patients with advanced HCCs previously treated with sorafenib. Notably, a retrospective evaluation of the sequential treatment effect of sorafenib followed by regorafenib revealed a median OS from the beginning of the systemic therapy of 26 months versus 19.6 months for placebo [19]. These data obtained in a well selected patient population provided, for the first time, evidence that sequential application of systemic therapies in Barcelona clinic liver cancer–stage C (BCLC-C) patients can reach comparable survival times observed in phase III trials of TACE in BCLC-B patients. Thus, this strategy should be prospectively implemented and evaluated in suitable patients.
3.2 Cabozantinib—CELESTIAL Trial
Cabozantinib is a multi-tyrosine kinase inhibitor with activity against MET, VEGFR2, and RET. Following its approval for the treatment of thyroid and renal cell carcinomas by both the EMA and the FDA, cabozantinib has most recently been granted approval as a second-line treatment in HCC Child–Pugh A patients by the EMA and the FDA. The phase III CELESTIAL trial compared the benefit of cabozantinib (60 mg daily) with placebo in second- and third-line treatment for advanced HCC with preserved liver function and good performance status (i.e., Child–Pugh A, Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0/1). The study was stopped after the second interim analysis due to proven efficacy [20]. Overall, an improvement in OS from 8.0 months to 10.2 months could be demonstrated for cabozantinib compared with placebo. Mean PFS was 5.4 months versus 1.9 months (HR 0.44; 95% CI 0.36–0.52; p < 0.001). Further, the disease control rate was 64% for cabozantinib versus 33.4% in placebo (p < 0.001) with a low ORR rate of 4% versus 0.4% according to RECIST 1.1 (p = 0.0086). Similar to the other TKIs, grade 3/4 side effects occurred in 68% of patients and predominantly involved hand-foot syndrome (17 vs 0%), hypertension (12 vs 2%), transaminase elevation (12 vs 7%), and fatigue (10 vs 4%). Interestingly, nearly 30% of patients in the study had received more than one pre-treatment, albeit most of these patients had been treated with chemotherapy in addition to sorafenib. Nevertheless, the results of the CELESTIAL study suggest that cabozantinib could also have a place in later therapy lines. Interestingly, a recent analysis confirmed the efficacy of cabozantinib over placebo in patients with different AFP levels, but most prominently in patients with AFP levels ≥ 400 ng/mL, which determines a poor prognosis subgroup of patients. In this cohort, the median OS was 8.5 months compared with 5.2 months with cabozantinib or placebo, respectively (HR 0.71; 95% CI 0.54–0.94) [21].
3.3 Ramucirumab—REACH-2
Ramucirumab is a recombinant monoclonal antibody that specifically binds to the VEGFR2 domain, thereby preventing the binding of VEGF ligands. Similar to other compounds, such as sunitinib and brivanib, ramucirumab initially showed promising results in a small phase II study for advanced HCC. Based on these results, the randomized controlled phase III REACH study was initiated as a second-line therapy after sorafenib failure [22]. However, the REACH study failed to demonstrate a significant improvement in median OS for all patients and did not meet its primary endpoint. Despite these initial discouraging results, a subgroup analysis suggested that ramucirumab improves survival in patients with elevated baseline AFP levels above 400 ng/ml. Subsequently, the REACH II study was initiated in this patient population. In this selected cohort, ramucirumab improved the median OS from 7.3 months to 8.5 months versus placebo (HR 0.710; 95% CI 0.53–0.95; p = 0.019) and PFS from 1.6 months to 2.8 months (HR 0.452; 95% CI 0.40–0.60; p < 0.0001) [23]. A combined analysis of the REACH I and II study confirmed the survival benefit of ramucirumab compared with placebo (Delta: 3.1 months; HR 0.69; 95% CI 0.57–0.84; p = 0.0002). Thus, ramucirumab is an interesting second-line option in patients with high AFP levels and a poor prognosis. Notably, ramucirumab is the first intravenous, non-TKI drug with proven anti-angiogenetic efficacy in advanced HCC. Accordingly, the side-effect spectrum deviates substantially from multi-tyrosine kinase inhibitors. With respect to grade 3/4 side effects, only hypertension (12.7% vs 3.8%) and proteinuria (1.3% vs 0%) occurred more frequently with ramucirumab compared with placebo.
3.4 Compounds in Second-Line with No Therapeutic Benefits in Phase III Trials
Several compounds were evaluated against placebo in second-line settings for advanced HCC [4]. Neither brivanib nor everolimus nor tivantinib showed a significant improvement in OS.
4 Checkpoint Inhibition in HCC
Based on the recent success of checkpoint blockade in a range of different tumor entities, the Nobel Prize in medicine was awarded to two pioneers of immunotherapy, Tasuko Honjo and James Allison, in 2018. In contrast with classical chemotherapy or molecular therapies that targets cancer cells directly, immunotherapies aim to block immune-escape mechanisms of tumors and, consecutively, induce a strong and predominantly T cell mediated immune response against cancer cells [24]. The most successful form of immunotherapy to date has been the blockade of the immune checkpoints CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) and PD-1/PD-L1 receptors (programmed cell death protein 1/programmed death-ligand 1), which were first described in 1992 and 1995 [25, 26]. Only 10 years after their initial description, first-in-class checkpoint inhibitors were approved for the treatment of malignant melanoma, renal cell carcinoma, and non-small-cell lung cancer [27]. Given the inflammatory background of most HCC, immunotherapy in this cancer entity also seems to be a reasonable approach. Initial results from preclinical and early clinical trials in HCC are encouraging and the landscape of clinical trials in HCC has experienced a shift towards this field. To date, several clinical trials evaluate the efficacy of antibodies against PD-1, PD-L1, and CTLA-4, both as monotherapy as well as in combinatorial therapy approaches in patients with advanced HCC [28].
4.1 Anti-CTLA-4 Inhibition
Clinical studies with the anti-CTLA-4 antibody tremelimumab showed first positive results in a pilot trial with 27 patients with advanced HCC and hepatitis C infection [29]. Anti-tumor response was assessable in 17 and toxicity in 20 patients. Disease control rate was 76.4% and three patients had a partial response. Median TTP was 6.48 months (95% CI 3.95–9.14) and median OS was 8.2 months (95% CI 4.64–21.34). Interestingly, in addition to its anti-tumor activity, tremelimumab exerted antiviral effects and led to a significant decline in viral load. The most common adverse events were skin rash and pruritus consistent with immune-related dermatitis (65%) followed by fatigue (55%) and diarrhea (30%). After the first dose of tremelimumab a significant increase of serum transaminases in more than half of all patients was the predominant cause of grade 3/4 treatment-related adverse events. Nevertheless, elevation of liver enzymes was reversible with dose interruption or the use of steroids and did not lead to a sustained impairment of liver function [29].
4.2 Anti-PD1/PD-L1 Inhibition
Nivolumab, pembrolizumab, tislelizumab, and camrelizumab are fully humanized monoclonal antibodies against PD-1, whereas durvalumab and atezolizumab target PD-L1. The efficacy of these antibodies is currently being investigated in various clinical trials as mono- or combination therapy in advanced HCC. Initial clinical trials were performed with nivolumab; a large dose-escalation and expansion study for patients with advanced hepatocellular carcinomas demonstrated both safety and efficacy of the anti-PD-1 antibody [30]. High disease control rates and ORR were observed across all investigated subgroups, including ‘poor prognosis patients’ with impaired liver function (Child–Pugh A), extrahepatic tumor burden, or in patients after treatment with sorafenib. Results from the dose-escalation phase indicated that nivolumab is safe in patients with and without chronic hepatitis C and B infections. Most importantly, severe deterioration of liver function or death were not observed and none of the patients experienced flares of the hepatitis B virus infection. In the dose-expansion cohort, 214 patients were included and treated with a dose of nivolumab 3 mg/kg. Notably, this cohort included 145 patients (68%) that had experienced progression or were intolerant to sorafenib. The disease control rate reached 64% (95% CI 58–71) and ORR was 15–20% (95% CI 15–26), including three complete responses and 39 partial responses. Remarkable was a 9-month OS rate of 74% (95% CI 67–79) and expected median OS of 28.6 months in non-pretreated and 15.6 months in pretreated patients. The most common treatment-related adverse events were fatigue, rash, pruritus, and increase of serum transaminases. Grade 3/4 treatment-related adverse events occurred in 12 of 48 patients, and included adrenal insufficiency, diarrhea, hepatitis, and acute kidney injury. Importantly, the safety profile of nivolumab was consistent among the dose-escalation and expansion cohorts and did not differ from the observed safety profiles reported for other tumor entities [31,32,33]. Based on these findings, nivolumab was approved by the FDA for second-line therapy of advanced HCC. However, a request to the EMA has been withdrawn in Europe until results of the ongoing, controlled phase III study are available, which are expected in 2019. Similar results have also been shown for other checkpoint inhibitors such as pembrolizumab and durvalumab alone or in combination with tremelimumab. In the KEYNOTE-224 study, median OS was similar to nivolumab in the second line at 12.9 months with a disease control rate of 61% and ORR of 18% [34]. Consequently, the FDA granted provisionary approval for pembrolizumab as second-line treatment for advanced HCC, pending results from the randomized phase III trial. Tislelizumab and camrelizumab have thus far been mainly tested in the Asian population. At ESMO 2018, a phase II study evaluating two treatment schedules of camrelizumab in Chinese patients who failed or were intolerant to systemic therapy revealed a promising ORR of 13.8% and a median OS of 14.4 months in line with data presented for pembrolizumab and nivolumab in Caucasian patients [35]. There were no new safety signals and the spectrum of treatment-related adverse events was similar to previous reports. A first-in-human, phase 1A/1B study demonstrated that single-agent tislelizumab was generally well tolerated and showed evidence of antitumor activity in patients with advanced solid tumors, including HCC (NCT02407990). Based on these data, a global, phase III, randomized, multicenter, non-inferiority study currently evaluates the efficacy and safety of tislelizumab compared with sorafenib as a first-line treatment of unresectable HCC (NCT03412773).
In addition to monotherapy, promising combinatorial treatment strategies involving immune checkpoint inhibitors are under investigation both for concurrent and sequential use. These include dual checkpoint inhibition, combination with kinase inhibitors or loco-regional therapies, such as radiofrequency ablation, TACE, and irradiation [36]. Preclinical studies and early clinical studies indicate that the combination results in stronger and more durable anti-tumor effects than single therapies by synergistically modulating the immune-cell composition within the immunosuppressive tumor microenvironment, thereby potentiating the anti-tumor efficacy of checkpoint inhibitors [37, 38]. The effectivity of dual checkpoint blockade using anti-PD-1/PD-L1 and anti-CTLA-4 antibodies has been already demonstrated in malignant melanomas [39]. First results of a phase I/II trial for hepatocellular carcinomas, in which durvalumab (anti-PD-L1) was combined with tremelimumab (anti-CTLA-4), were presented at the annual meeting of the American Society of Clinical Oncology (ASCO) in 2017. ORR of 40 patients in phase I was 15% and disease control rate was 57.5% with a satisfactory safety profile [40]. Based on these results, a large phase III trial has been initiated to evaluate the efficacy and safety of the combination and durvalumab alone in comparison with sorafenib in first-line therapy. Furthermore, preclinical studies indicate that molecular targeted agents inhibit signaling pathways for maintaining an immunosuppressive environment. Combination of checkpoint inhibitors with molecular targeted agents can therefore induce changes in the tumor microenvironment, which facilitates a penetration and infiltration of cytotoxic T-lymphocytes into tumor tissue [41]. Interesting phase I/II data have already been reported for lenvatinib in combination with pembrolizumab, and atezolizumab together with bevacizumab. Overall, these studies suggest synergistic effects of the combinations with a favorable safety profile in HCC patients.
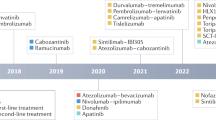
5 Sequential Treatment Strategies for Advanced HCC and the Stage Migration Concept
The approval of regorafenib in 2016 enabled HCC patients to continue on systemic targeted therapy after progression on sorafenib. Within the last 2 years, additional compounds have entered the clinic and are available for first- and second-line therapeutic approaches (Fig. 1). Exploratory analyses of the reported first- and second-line trials indicate that the sequential use of sorafenib and lenvatinib with subsequent second-line therapies (e.g., regorafenib, cabozantinib, and ramucirumab) as well as the early use of immunotherapy can achieve cumulative OS of over 20 months in BCLC-C patients with maintained liver function. HCC is a malignancy that requires interdisciplinary evaluation to develop individualized and tailored treatment concepts. The reasonable combination of appropriate surgical approaches, interventional/loco-regional treatment strategies, and various lines of systemic therapies will consistently improve patient survival. Although they will remain a mainstay of HCC therapy, recent studies advocate for a more cautious use of surgical and locoregional therapies. Indeed, many patients receive repeated surgical and/or loco-regional therapies regardless of tumor stage, which may result in a progressive impairment of liver function, rendering the initiation of effective and long-term systemic treatment impossible [42]. In line with this, it has recently been shown that in clinical routine < 20% of patients receive systemic therapy following repeated local therapies [43]. Thus, potential benefits of a treatment should be critically weighed against a potential adverse effect on liver function, which may seriously affect the prognosis. In this respect, there is increasing evidence that outcome with TACE in unselected patient populations is significantly worse than the results of the pivotal phase III study. A recent meta-analysis of 101 studies with > 10,000 patients revealed a median OS of only 18 months in mainly BCLC-B patients [44]. On the other hand, 5-year survival rate was 25% in these studies, highlighting the anti-tumor efficacy in well selected patients. To identify patients that will likely benefit from TACE, prognostic scores such as the mHAP-III score should be more routinely employed [45].
An interdisciplinary dialogue of all disciplines involved in the treatment of HCC patients is imperative to improve patient outcome. Sequential application of different treatments and an early switch of therapeutic modalities in the absence of deep response (e.g., after TACE) should be considered and may improve the outcome of patients. In this context, it is mandatory that the rapid development in the field of systemic HCC therapy is continuously communicated and the full spectrum of new treatment options is incorporated into routine clinical practice.
6 Conclusions and Outlook
Sorafenib was the standard of care for the systemic treatment of advanced HCC for many years. Following the failure of several large phase III clinical trials, lenvatinib, regorafenib, cabozantinib, and ramucirumab are now available and viable options in first- as well as second-line settings. Overall, the results of these studies confirm safety and efficacy of targeted therapy in HCC. Accumulating evidence from several phase I/II studies also suggest promising efficacy for immunotherapy in HCC, with six already-tested compounds highlighting the rapid evolution of this field. Results from the ongoing phase III trials are urgently awaited. Most noticeably, all the recent studies underlined the critical importance of a well preserved liver function as well as a limited tumor burden for successful administration of systemic therapy. Therefore, new therapeutic compounds require a fine balance between anti-tumor activity and toxicity, similar to requirements for local therapies. Advances and convincing results in the field of immuno-oncology will become increasingly important in this context and point to a combination of systemic and local or ablative therapy in the future. Accordingly, different immuno-oncological combination therapies as well as the combination of local, immunomodulatory approaches with checkpoint inhibition seem particularly attractive [46]. More data supporting this approach are urgently needed. Furthermore, with the exception of high AFP values for ramucirumab and potentially cabozantinib, the field still lacks reliable predictive biomarkers that can be used to guide the proper selection of therapy in routine clinical practice. In addition, several prognostic and clinical factors that challenge a one-size-fits-all approach in HCC need to be considered. Although several treatment modalities and drugs are now available to choose from, it is still entirely unclear which sequence of different therapeutic strategies might be most suitable for individual patients. For future approaches it might, therefore, be beneficial to implement clinical and/or molecular enrichment of patients instead of continuing the un-selected ‘all-comer’ strategies performed in the previous trials [47]. In addition, it seems logical to extend our translational efforts and include information from pre-clinical studies and representative models into future clinical trial design. In summary, the availability of new active compounds extended the ‘continuum-of-care’ in HCC across different stages of disease. A sequential application and critical evaluation of efficacy in the available treatment modalities should be implemented and may lead to a sustained improvement of the patient outcome.
References
Global Burden of Disease Cancer Collaboration. Global, Regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–48.
Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
Worns MA, Galle PR. HCC therapies–lessons learned. Nat Rev Gastroenterol Hepatol. 2014;11(7):447–52.
Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 008;100(10):698–711.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90.
Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34.
Rimola J, Diaz-Gonzalez A, Darnell A, Varela M, Pons F, Hernandez-Guerra M, et al. Complete response under sorafenib in patients with hepatocellular carcinoma: Relationship with dermatologic adverse events. Hepatology. 2017. https://doi.org/10.1002/hep.29515.
Jackson R, Psarelli EE, Berhane S, Khan H, Johnson P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35(6):622–8.
Marrero JA, Kudo M, Venook AP, Ye SL, Bronowicki JP, Chen XP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol. 2016;65(6):1140–7.
Reiss KA, Yu S, Mamtani R, Mehta R, D’Addeo K, Wileyto EP, et al. Starting dose of sorafenib for the treatment of hepatocellular carcinoma: a retrospective. Multi-Institutional Study. J Clin Oncol. 2017;35(31):3575–81.
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73.
Vogel A, Cervantes A, Chau I, Daniele B, Llovet J, Meyer T, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supplement_4):iv238–iv55.
Kudo M, Cheng AL, Park JW, Park JH, Liang PC, Hidaka H, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol. 2018;3(1):37–46.
Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology. 2014;60(5):1697–707.
Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–75.
Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64(5):1090–8.
Finn RS, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69(2):353–8.
Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63.
Kelly RK, El-Khoueiry AB, Meyer T, Rimassa L, Merle P, Chan SL, et al. Outcomes by baseline alpha-fetoprotein (AFP) levels in the phase III CELESTIAL trial of cabozantinib (C) versus placebo (P) in previously treated advanced hepatocellular carcinoma (HCC). Ann Oncol. 2018. https://doi.org/10.1093/annonc/mdy282.085.
Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–70.
Zhu AX, Galle PR, Kudo M, Finn RS, Qin SK, Xu YH, et al. A study of ramucirumab (LY3009806) versus placebo in patients with hepatocellular carcinoma and elevated baseline alpha-fetoprotein (REACH-2). J Clin Oncol. 2018;36(4):TPS538-TPS.
Schmidt N, Thimme R. Role of immunity in pathogenesis and treatment of hepatocellular carcinoma. Dig Dis. 2016;34(4):429–37.
Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–95.
Krummel MF, Allison JP. Pillars article: CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. J Immunol. 2011;187(7):3459–65.
Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–82.
Greten TF, Wang XW, Korangy F. Current concepts of immune based treatments for patients with HCC: from basic science to novel treatment approaches. Gut. 2015;64(5):842–8.
Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81–8.
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627–39.
Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–84.
Brown ZJ, Heinrich B, Steinberg SM, Yu SJ, Greten TF. Safety in treatment of hepatocellular carcinoma with immune checkpoint inhibitors as compared to melanoma and non-small cell lung cancer. J Immunother Cancer. 2017;5(1):93.
Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–52.
Qin SK, Ren ZG, Meng ZQ, Chen ZD, Chai XL, Xiong JP, et al. A randomized multicentered phase 2 study to evaluate SHR-1210 (PD-1 antibody) in subjects with advanced hepatocellular carcinoma (HCC) who failed or intolerable to prior systemic treatment. Ann Oncol. 2018. https://doi.org/10.1093/annonc/mdy424.029.
Heinrich B, Czauderna C, Marquardt JU. Immunotherapy of hepatocellular carcinoma. Oncol Res Treat. 2018;41(5):292–7.
Stein S, Pishvaian MJ, Lee MS, Lee KH, Hernandez S, Kwan A. Safety and clinical activity of 1L atezolizumab plus bevacizumab in a phase Ib study in hepatocellular carcinoma (HCC). J Clin Oncol. 2018;36(15):4074.
Ikeda M, Sung MW, Kudo M, Kobayashi M, Baron AD, Finn RS. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol. 2018;36(15):4076.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
Kelley RK, Abou-Alfa GK, Bendell JC, Kim TY, Borad MJ, Yong WP, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J Clin Oncol. 2017;35(15_suppl):4073
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGF-β signalling attenuates tumour response to PD-L1 checkpoint blockade by contributing to retention of T cells in the peritumoural stroma. Ann Oncol. 2017. https://doi.org/10.1093/annonc/mdx760.001.
Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014;68(5):609–17.
Kirstein MM, Schweitzer N, Winter T, Lappas K, Graen N, Kunstmann I, et al. Patterns and challenges of treatment sequencing in patients with hepatocellular carcinoma: experience from a German referral center. J Gastroenterol Hepatol. 2017;32(10):1730–8.
Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–16.
Cappelli A, Cucchetti A, Cabibbo G, Mosconi C, Maida M, Attardo S, et al. Refining prognosis after trans-arterial chemo-embolization for hepatocellular carcinoma. Liver Int. 2016;36(5):729–36.
Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66(3):545–51.
Nault JC, Galle PR, Marquardt JU. The role of molecular enrichment on future therapies in hepatocellular carcinoma. J Hepatol. 2018;69(1):237–47.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Jens U. Marquardt has received honoraria from Bayer, Roche and Pfizer. Arndt Vogel has received honoraria from Bayer, Roche, Lilly, EISAI, Ipsen, BMS, and MSD. Anna Saborowski and Carolin Czauderna declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Rights and permissions
About this article
Cite this article
Marquardt, J.U., Saborowski, A., Czauderna, C. et al. The Changing Landscape of Systemic Treatment of Advanced Hepatocellular Carcinoma: New Targeted Agents and Immunotherapies. Targ Oncol 14, 115–123 (2019). https://doi.org/10.1007/s11523-019-00624-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-019-00624-w