Abstract
Background
Despite remarkable activity in epidermal growth factor receptor (EGFR)-mutant lung cancer patients, the clinical efficacy of EGFR tyrosine kinase inhibitors (TKIs) is limited by the emergence of acquired resistance, which is mostly caused by a secondary T790M mutation. Fortunately, newly developed, mutant-selective EGFR-TKIs against T790M have been proven as an effective therapeutic approach although only osimertinib has received the FDA approval until now.
Objective
To determine the in vitro and in vivo efficacy of a new EGFR TKI, OBX1-012 in cells with mutant EGFR.
Methods
Effects of OBX1-012 on cellular viability and EGFR-related signaling were determined in EGFR-mutant non-small cell lung cancer (NSCLC) cells, including cells harboring T790M mutations. In addition, in vivo efficacy of OBX1-012 was evaluated in xenograft models.
Results
We report the discovery and preclinical assessment of another novel, mutant-selective EGFR-TKI, OBX1-012. Compared with other mutant-selective EGFR-TKIs such as olumitinib and osimertinib, it showed similar potency and selectivity for mutant EGFR. OBX1-012 treatment was highly effective against human EGFR-mutant lung cancer models with or without EGFR T790M, not only in vitro but also in vivo. However, OBX1-012 like other EGFR-TKIs failed to exhibit efficacy for the exon 20 insertion mutation or C797S mutation, which was generated by site-directed mutagenesis and stable transfection of Ba/F3 cells.
Conclusions
These results identify OBX1-012 as a highly effective, mutant-selective EGFR-TKI for the treatment of T790M-mediated resistance in NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

1 Introduction
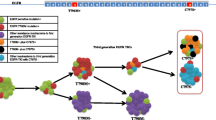
Lung cancer is the leading cause of cancer mortality worldwide and accounts for approximately 20% of all cancer-related deaths [1]. Activating epidermal growth factor receptor (EGFR) mutations are well-known oncogenic drivers for lung cancer [2, 3]. EGFR tyrosine kinase inhibitors (TKIs) show significant benefits in patients with EGFR mutations, and hence, are the standard first-line drug for treatment of EGFR-mutant lung cancer [4,5,6]. However, acquired resistance eventually develops usually within 1 year, with a secondary T790M mutation being the cause of resistance in more than 50% of cases [7,8,9]. This has led to the development of next-generation, mutant-selective EGFR-TKIs, such as AZD9291 (osimertinib), CO-1686 (rociletinib), and HM61713 (olmutinib), which have excellent activity against T790M and minimal effect on wild-type EGFR [10].
Early phase clinical trials showed remarkable response rates of 59% and 61% in patients with T790M-mediated resistance who received rociletinib and osimertinib, respectively [11, 12]. While the development of rociletinib was discontinued because of alleged less impressive efficacy and undesirable side effects, osimertinib succeeded to receive FDA-approval in 2016 for patients with T790M-positive NSCLC in second-line treatment. The randomized, phase III trial using osimertinib in patients with T790M-positive lung cancer (AURA3 study) showed significantly improved progression-free survival (10.1 vs. 4.4 months; hazard ratio 0.30; P < 0.001) and response rate (71% vs. 31%; odds ratio, 5.39; P < 0.001) compared with platinum plus pemetrexed therapy [13]. Moreover, with osimertinib having improved blood-brain barrier penetration, it also showed benefit in the subgroup of patients with CNS metastases [13, 14]. Adverse events leading to discontinuation of medication or life-threatening adverse events were lower indicating acceptable toxicity profiles [11, 13]. In addition, osimertinib has been demonstrated to be highly effective in the first-line setting [15] and has received FDA approval for this indication in April 2018. Thus, this drug is regarded as the standard treatment for T790M-mediated resistance to 1st-line EGFR-TKIs at present.
Although olmutinib, which is another mutant-selective EGFR-TKI, received its first approval in Korea [16], the approval processing in other countries has been delayed, partly because of difficulty in determining the optimal dose. Therefore, osimertinib is the only currently available, FDA-approved EGFR-TKI against T790M, while other potential candidate drugs, including EGF816 and ASP8273, are still under clinical investigation. Although it is true that a number of patients can benefit from osimertinib, its high price poses an economic burden and some intolerable adverse events leading to discontinuation of the drug require the development of additional mutant-selective EGFR-TKIs.
Here we investigated the efficacy of another novel compound capable of selective inhibition of mutant EGFR, including T790M, and compared this drug with osimertinib and olmutinib.
2 Materials and Methods
2.1 Cells and Cultures
Human non-small-cell lung cancer (NSCLC) cell lines (A549 and H1975) and the murine pro-B-cell line Ba/F3 were purchased from the ATCC (Manassas, VA, USA). The PC-9 cell line was a kind gift from Dr. Kazuto Nishio (National Cancer Center Hospital, Tokyo, Japan). All resistant cell lines have been established in previous studies [17,18,19]. Cells were cultured in RPMI1640 medium containing 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) at 37 °C in an atmosphere of 5% CO2. Tests for mycoplasma contamination were negative.
2.2 Cellular Viability Assays
Cells (5 × 103) were seeded in 96-well sterile plastic plates, incubated overnight, and then treated with the drugs. After 72 h, 15 μL 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) solution (5 mg/mL) was added to each well and the plates were incubated for four hours. Crystalline formazan was solubilized by adding 100 μL of 10% (w/v) sodium dodecyl sulfate and incubating for 24 h, after which absorbance at 595 nm was spectrophotometrically recorded using a microplate reader. The results were representative of at least three independent experiments; the error bars signify standard deviation (SD). The IC50 values were calculated using the GraphPad Prism software (La Jolla, CA, USA).
2.3 Cell-Free Kinase Assay
In vitro kinase inhibitory activities were measured using radiometric assays conducted by the Eurofins Pharma Discovery Services UK Limited (Dundee, UK). The IC50 values were calculated based on the dose-response data of 10 points using the GraphPad Prism software.
2.4 Immunoblotting
Cells were lysed in buffer containing 137 mmol/L NaCl, 15 mmol/L egtazic acid (EGTA), 0.1 mmol/L sodium orthovanadate, 15 mmol/L MgCl2, 0.1% Triton X-100, 25 mmol/L MOPS, 100 mmol/L phenylmethylsulfonyl fluoride, and 20 mmol/L leupeptin, adjusted to pH 7.2. Lysis of tumor specimens was performed using the Omni Tissue Homogenizer (TH; Omni International, Kennesaw, GA, USA). Antibodies specific for p-EGFR (Tyr1173), EGFR, protein kinase B (Akt), ERK, and actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and antibodies for p-Akt (Ser473) and p-ERK (Thr202/Tyr204) were purchased from Cell Signaling Technology (Beverly, MA, USA). Proteins were detected using an enhanced chemiluminescence Western blotting kit (Amersham Biosciences, Little Chalfont, UK), according to the manufacturer’s instructions.
2.5 Efficacy of the Drug In Vivo
To establish xenograft models, female severe combined immunodeficiency (SCID) mice (18–20 g, 6 weeks old) were purchased from Charles River Laboratories (Wilmington, MA, USA). All experimental procedures were conducted following a protocol approved by the Institutional Animal Care and Use committee of Asan Institute for Life Sciences (2017-12-200). Tumors were cultured by implanting cells (1–5 × 106 cells/0.1 mL) in 50% Matrigel (BD Biosciences, San Jose, CA, USA) and subcutaneously injected into the right flank of animals. Drug treatment was initiated when the tumors reached a volume of 50–100 mm3. To measure the tumor size, the length (L) and width (W) of each tumor were measured using calipers, and tumor volume (TV) was calculated using the formula TV = (L × W2)/2. All drugs were dissolved in Tween-80 and administered via oral intubation at the indicated times.
2.6 Immunohistochemical Staining (IHC)
Each tumor was harvested from tumor-bearing mice at 3 days following drug treatment. Resected tumors were fixed in 10% formaldehyde and embedded in paraffin. Immunohistochemical staining was performed using a Ki-67-specific primary antibody (DakoCytomation, Los Angeles, CA, USA), EnVision Plus staining kit (DakoCytomation), and an APO-Direct terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay kit (Millipore, Burlington, MA, USA) according to the manufacturer’s instructions. Quantitative analysis of section staining was performed by counting immunopositive cells in five arbitrarily selected fields.
2.7 Site-Directed Mutagenesis and Ba/F3 Cell Stable Transfection
EGFR constructs (EGFR T790M/L858R, EGFR T790M/del746-750, and EGFR D770-N771 insNPG) were purchased from Addgene (Cambridge, MA, USA). EGFR C797S mutation was performed using site-directed mutagenesis conducted by Bioneer Corp. (Daejeon, Korea) and was confirmed by sequencing. The stable Ba/F3 cell lines were established by retroviral infection with each construct followed by interleukin (IL)-3 withdrawal. The stable viral transfectant Ba/F3 cell lines were designated as Ba/F3/insNPG EGFR, Ba/F3/DEL/T790M, Ba/F3/L858R/T790M, Ba/F3/DEL/T790M/C797S, and Ba/F3/L858R/T790M/C797S, respectively.
2.8 Statistics
P values were determined between comparator groups with unpaired t-tests using the GraphPad Prism software.
3 Results
3.1 Development of Novel Mutant-Selective EGFR-TKI
We generated OBX1-012 with a structure similar to that of an osimertinib analog. We synthesized a series of compounds bearing different substituents at the pyrimidine C4 position, keeping the methoxyaniline moiety unchanged, similar to that observed with osimertinib. OBX1-012 was developed as a third-generation inhibitor targeting T790M mutant EGFR; therefore, several other compounds with chemical structures similar to OBX1-012 were also investigated. The initial analysis of the structure−activity relationship (SAR) revealed functional groups important for maintaining activity against the T790M mutant EGFR. Replacement of an N-methyl indole group (osimertinib) to a fused ring moiety containing nitrogen(s) (OBX1-012) barely affected the inhibitory potency against mutant EGFR; however, the introduction of a fused ring moiety containing sulfur at the pyrimidine part in OBX1-012 increased its activity against the T790M mutant EGFR, suggesting that OBX1-012 is more favorable.
OBX1-012 showed substantial selectivity for mutant EGFR compared with wild-type (WT) EGFR in a cell-free kinase assay (Table 1). Particularly, it had high selectivity for T790M mutation (IC50 value of 24 and 2 nM for L858R and L858R/T790M EGFR, respectively).
To further assess the anticancer activity and modulation of EGFR-related signaling by OBX1-012 in various NSCLC cell lines, including cells with acquired resistance to EGFR-TKIs caused by T790M mutation, we performed MTT assays and immunoblotting following treatment with OBX1-012 as well as other current third-generation EGFR-TIKs (olmutinib and osimertinib). H1975 (EGFR; L858R/T790M) cells have shown intrinsic resistance to EGFR-TKIs, such as gefitinib and erlotinib, because of the T790M mutation. PC-9 (EGFR; exon 19 deletion) cells are sensitive to EGFR-TKIs, whereas PC-9/GR (EGFR; exon 19 deletion/T790M) and PC-9/ER (EGFR; exon 19 deletion/T790M) cells were established in our previous studies [18, 19] to have an acquired resistance to gefitinib or erlotinib resulting from the emergence of the T790M mutation (Electronic Supplementary Material Figure 1). As indicated in Fig. 1a and Electronic Supplementary Material Table S1, OBX1-012 treatment exhibited substantial efficacy in EGFR T790M-positive cells in vitro, including PC-9/GR and PC-9/ER sublines with acquired resistance to gefitinib or erlotinib, respectively, and H1975 cells that intrinsically harbored EGFR L858R/T790M. We similarly found that OBX1-012 was effective in these EGFR T790M-positive and EGFR-mutant cells compared with the other third-generation EGFR-TKIs, such as osimertinib and olmutinib. However, all EGFR-TKIs, including OBX1-012, showed no significant impact of treatment on cell viability with either agent in A549 cells, which harbor a WT EGFR.
Preclinical efficacy of OBX1-012 in vitro. a Cells were treated with the indicated doses of mutant-selective EGFR-TKIs for 72 h, and cell viability was determined by the MTT assay. b Cells were treated for 6 h with the indicated doses of mutant-selective EGFR-TKIs. Molecules associated with EGFR signaling activity were detected using immunoblotting
Based on these findings, we next examined the activity of OBX1-012 on EGFR-related signaling. OBX1-012 had minimal impact on downstream signaling in A549 cells, consistent with the minimal activity against WT EGFR observed in the cell-free kinase assay (Fig. 1b and Electronic Supplementary Material Table S2). In contrast, treatment with each agent suppressed the levels of phosphorylated EGFR and the phosphorylation of the downstream signaling proteins Akt and Erk in each EGFR-mutant-expressing cell. These effects of OBX1-012 were comparable with those observed upon treatment with the other third-generation EGFR-TKIs, although subtle differences in activity against individual biomarkers were noted among these inhibitors. Furthermore, treatment with OBX1-012 effectively induced apoptosis signaling, including PARP and caspase-3 activation (Fig. 2). Taken together, these data suggested that OBX1-012 is a novel mutant-selective EGFR-TKI that effectively inhibits mutant EGFR signaling and induces apoptosis in EGFR T790M (−) and (+) EGFR-mutant lung cancer cells.
3.2 Preclinical Efficacy of the Novel Mutant-Selective EGFR-TKI OBX1-012 In Vivo
To further evaluate the efficacy of OBX1-012 in vivo, we used PC-9- and H1975-xenograft models. All drugs (5 mg/kg) were orally administered for 5 days a week. Olmutinib treatment showed only a slight decrease or comparable growth of tumors compared with the control group, whereas osimertinib or OBX1-012 treatment led to substantial growth inhibition (Fig. 3a). The efficacy of OBX1-012 was similar to osimertinib in PC-9 and H1975 tumors, although the tumors showed some regrowth after termination of drug treatment. Moreover, OBX1-012 did not show any overt signs of toxicity in the treated animals as assessed by changes in body weight (Fig. 3b).
Preclinical efficacy of OBX1-012 in vivo. NOD-SCID mice bearing PC-9 or H1975 cells (n = 5 per treatment cohort for each xenograft model) were treated orally with the indicated EGFR-TKIs (5 mg/kg/day) or vehicle control alone over 21 days (five consecutive days/week). Results are indicated as tumor volume (a) or mean body weight (b) over the time course of treatment with vehicle control or each EGFR-TKI and presented ± SD. Each treatment course was initiated at day 0; the red arrow indicates cessation of each therapy with continued measurement of tumor volumes to the endpoint
We next evaluated the changes in EGFR-related signaling molecules and apoptosis in xenografts treated with each drug. As shown in Fig. 4, osimertinib and OBX1-012 treatment completely inhibited EGFR-related signaling, such as EGFR, Akt, and Erk activity, and led to a substantial decrease in tumor cell proliferation (as measured by quantitative analysis of Ki-67 staining in the tumor cells using IHC) and a significant increase in apoptosis (as measured by quantitative analysis of TUNEL staining in the tumor cells using IHC). However, olmutinib showed less activity against EGFR-related signaling molecules and apoptosis, compared with treatment with either osimertinib or OBX1-012. Taken together, our results indicated that OBX1-012 suppresses EGFR T790M (−) and (+) EGFR-mutant lung cancer xenograft growth in mice by inhibiting EGFR signaling, leading to inhibition of cell proliferation and induction of apoptosis of the tumor cells.
The mechanisms of antitumor activity of OBX1-012 in vivo. a and b Each indicated pharmacodynamics biomarker in representative control-treated or EGFR-TKIs-treated tumors was measured using immunoblotting. Results represent at least three independent experiments. c and d Each indicated pharmacodynamics biomarker (c, Ki-67; d, TUNEL staining) was analyzed using immunohistochemistry. Inset on the right indicates quantification of the Ki-67 and TUNEL staining in (c) and (d) under each condition. Tumors from each group were harvested from tumor-bearing mice at 3 days after initiation of therapy. *P < 0.05; **P < 0.005; ***P < 0.0005 for EGFR-TKIs vs control (vehicle-treated) tumors
3.3 Efficacy of the Novel Mutant-Selective EGFR-TKI OBX1-012 on EGFR C797S and Exon 20 Insertion Mutation
Accumulating evidence shows that EGFR exon 20 insertion mutations lead to resistance to first-generation EGFR-TKIs, such as gefitinib and erlotinib, and that the acquired EGFR C797S mutation mediates resistance to mutant-selective EGFR-TKI, such as osimertinib [3, 20,21,22,23]. Thus, we examined whether OBX1-012 could overcome these two EGFR-TKIs resistance-related mutations. To evaluate the efficacy of OBX1-012 on these mutations, we generated stable mutant EGFR-expressing Ba/F3 cell lines under the absence of IL-3. The expression of each mutant EGFR was confirmed using immunoblotting analysis (Fig. 5a, c). OBX1-012 treatment did not inhibit the proliferation of Ba/F3 cells harboring the EGFR exon 20 insertion mutant D770_N771 (insNPG), or the C797S mutation (Fig. 5b, d). However, consistent with previous results, OBX1-012 treatment effectively inhibited the proliferation of Ba/F3 cells harboring EGFR T790M mutations.
The efficacy of OBX1-012 on EGFR C797S and exon 20 insertion mutation. Murine Ba/F3 cells infected with indicated viral particles, including insNPG, DEL/T790M, L858R/T790M, DEL/T790M/C797S, and L858R/T790M/C797S EGFR. a and c The expression of mutant EGFR was confirmed using immunoblotting. b and d Cells were treated with the indicated doses of mutant-selective EGFR-TKIs for 72 h, and cell viability was determined using MTT assay
4 Discussion
Osimertinib was approved by the FDA as a second-line treatment for lung cancer with T790M-mediated resistance based on the AURA3 study comparing it to platinum-based chemotherapy [13]. In addition, results of another large phase III study wherein untreated patients with advanced EGFR-mutant lung cancer were randomized to receive either osimertinib or first-generation EGFR-TKI (erlotinib or gefitinib; FLAURA study) were recently reported [15], showing efficacy superior to that of standard EGFR-TKIs. The median response duration was 17.2 months (95% CI, 13.8–22.0) with osimertinib versus 8.5 months (95% CI, 7.3–9.8) with standard EGFR-TKIs. This has led to the FDA-approval of osimertinib in the first-line setting. The survival benefit in the FLAURA study led to the inclusion of osimertinib as a possible treatment option in first-line therapy for EGFR-mutant lung cancer in the National Comprehensive Cancer Network guidelines (NCCN; NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. V.2.2018). The response duration of first-line osimertinib appears to be similar to that of first−generation EGFR-TKI plus second-line osimertinib in cases of positive T790M. However, when we consider that the failure rate of rebiopsy to confirm the status of T790M is high [24], it is more likely that first-line osimertinib will become the standard treatment for EGFR-mutant lung cancer in the near future. We believe that OBX1-012 with an efficacy similar to that of osimertinib would receive the same indication as that of first-line drugs following confirmatory clinical trials.
Due to the discontinuation of the development of rociletinib and delayed processes of other mutant-selective EGFR-TKIs, osimertinib is currently the only FDA-approved, clinically available drug for T790M-mutated NSCLC. This situation seems to cause some difficulties in real world practice. First, its high price should be mentioned because many patients face financial burden to receive this novel drug. Hence, we are awaiting development of more drugs, which can induce a price cut by proper competition and become affordable to more patients in need. Next, an alternative drug is sometimes required to manage severe side effects. Drugs of the same class may have different toxicities. For example, gefitinib shows more hepatotoxicity compared with erlotinib, whereas erlotinib causes severe skin problems more frequently than gefitinib [25]. If these side effects cannot be managed by the addition of alleviating drugs or adjustment of dose, switching to other drugs of the same class can often solve the problem. In the AURA3 study, adverse events of grade 3 or more occurred in 63 patients (23%) using osimertinib; the permanent discontinuation rate was 7% (19 patients). Similarly, the adverse event rate leading to permanent discontinuation in the FLAURA study was 13% (37 patients). One of the most worrisome side effects are interstitial lung disease (ILD)-like events, because of the high mortality rate and difficulty with rechallenge. Such an event was reported in 10 patients (4%) and one patient died in the AURA3 study. Therefore, we acknowledge that alternative drugs are sometimes necessary to deal with intolerable adverse events leading to the discontinuation of a drug.
As demonstrated in this study, OBX1-012 is another novel, mutant-selective EGFR-TKI that effectively inhibits activating and resistant T790M mutant-EGFRs with nanomolar potency in various cellular assays. It also was effective in PC-9- and H1975-xenograft models. Therefore, we believe that it is a potential candidate to enter clinical trials.
Next, we examined the efficacy of OBX1-012 in C797S mutations after the establishment of a Ba/F3 cell line model using site-directed mutagenesis and stable transfection. C797S mutation is a well-known resistance mechanisms to osimertinib although its reported incidence is somewhat variable because the number of osimertinib-resistant cases is still small [22, 26, 27]. Disappointingly, OBX-012 was also not effective towards the C797S mutation, given that it may share the same drug binding site of C797 with other mutant-selective EGFR-TKIs. All EGFR-TKIs until now cannot control lung cancer with exon 20 insertion mutation of EGFR. Our compound also failed to show efficacy in the established Ba/F3 cell line model with exon 20 insertion mutation of EGFR. Therefore, more efforts to develop new drugs aiming at these resistance mutations are required.
To summarize, as compared with osimertinib and olmutinib, OBX1-012 is a novel, mutant-selective EGFR inhibitor that exhibits comparable efficacy in overcoming T790M-mediated resistance and better selectivity in WT EGFR. It is expected to enter clinical trials soon, which can broaden the choice of mutant-selective EGFR-TKIs and could be an alternative for some patients who cannot afford or are intolerable to the severe side effects of osimertinib and olmutinib.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. https://doi.org/10.3322/caac.20107.
Ji H, Li D, Chen L, Shimamura T, Kobayashi S, McNamara K, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9(6):485–95. https://doi.org/10.1016/j.ccr.2006.04.022.
Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–46. https://doi.org/10.1093/jnci/dji055.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. https://doi.org/10.1056/NEJMoa0810699.
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. https://doi.org/10.1016/S1470-2045(11)70393-X.
Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–34. https://doi.org/10.1200/JCO.2012.44.2806.
Chang YS, Choi CM, Lee JC. Mechanisms of epidermal growth factor receptor tyrosine kinase inhibitor resistance and strategies to overcome resistance in lung adenocarcinoma. Tuberc Respir Dis (Seoul). 2016;79(4):248–56. https://doi.org/10.4046/trd.2016.79.4.248.
Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–92. https://doi.org/10.1056/NEJMoa044238.
Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–7. https://doi.org/10.1158/1078-0432.CCR-12-2246.
Politi K, Ayeni D, Lynch T. The next wave of EGFR tyrosine kinase inhibitors enter the clinic. Cancer Cell. 2015;27(6):751–3. https://doi.org/10.1016/j.ccell.2015.05.012.
Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–99. https://doi.org/10.1056/NEJMoa1411817.
Sequist LV, Rolfe L, Allen AR. Rociletinib in EGFR-mutated non-small-cell lung Cancer. N Engl J Med. 2015;373(6):578–9. https://doi.org/10.1056/NEJMc1506831.
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–40. https://doi.org/10.1056/NEJMoa1612674.
Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, et al. Preclinical comparison of Osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–40. https://doi.org/10.1158/1078-0432.CCR-16-0399.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–25. https://doi.org/10.1056/NEJMoa1713137.
Kim ES. Olmutinib: first global approval. Drugs. 2016;76(11):1153–7. https://doi.org/10.1007/s40265-016-0606-z.
Rho JK, Choi YJ, Jeon BS, Choi SJ, Cheon GJ, Woo SK, et al. Combined treatment with silibinin and epidermal growth factor receptor tyrosine kinase inhibitors overcomes drug resistance caused by T790M mutation. Mol Cancer Ther. 2010;9(12):3233–43. https://doi.org/10.1158/1535-7163.MCT-10-0625.
Rho JK, Choi YJ, Lee JK, Ryoo BY, Na II, Yang SH, et al. The role of MET activation in determining the sensitivity to epidermal growth factor receptor tyrosine kinase inhibitors. Mol Cancer Res. 2009;7(10):1736–43. https://doi.org/10.1158/1541-7786.MCR-08-0504.
Rho JK, Lee IY, Choi YJ, Choi CM, Hur JY, Koh JS, et al. Superior efficacy and selectivity of novel small-molecule kinase inhibitors of T790M-mutant EGFR in preclinical models of lung cancer. Cancer Res. 2017;77(5):1200–11. https://doi.org/10.1158/0008-5472.CAN-16-2432.
Greulich H, Chen TH, Feng W, Janne PA, Alvarez JV, Zappaterra M, et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med. 2005;2(11):e313. https://doi.org/10.1371/journal.pmed.0020313.
Jia Y, Yun CH, Park E, Ercan D, Manuia M, Juarez J, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature. 2016;534(7605):129–32. https://doi.org/10.1038/nature17960.
Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21(6):560–2. https://doi.org/10.1038/nm.3854.
Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13(1):e23–31. https://doi.org/10.1016/S1470-2045(11)70129-2.
Nosaki K, Satouchi M, Kurata T, Yoshida T, Okamoto I, Katakami N, et al. Re-biopsy status among non-small cell lung cancer patients in Japan: a retrospective study. Lung Cancer. 2016;101:1–8. https://doi.org/10.1016/j.lungcan.2016.07.007.
Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88(1):74–9. https://doi.org/10.1016/j.lungcan.2015.01.026.
Arulananda S, Do H, Musafer A, Mitchell P, Dobrovic A, John T. Combination osimertinib and gefitinib in C797S and T790M EGFR-mutated non-small cell lung Cancer. J Thorac Oncol. 2017;12(11):1728–32. https://doi.org/10.1016/j.jtho.2017.08.006.
Ramalingam SS, Yang JC, Lee CK, Kurata T, Kim DW, John T, et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol. 2017; https://doi.org/10.1200/JCO.2017.74.7576.
Acknowledgments
We thank the core facilities of Laboratory of Animal Research at the ConveRgence mEDIcine research center (CREDIT), Asan Medical Center for the use of their shared equipment, services and expertise. The authors would like to thank Enago (http://www.enago.co.kr) for the English language review.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant HI15C0516 and HI15C0972) and a grant of Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1A6A3A01002444).
Conflict of Interest
Dong Ha Kim, Yun Jung Choi, Seon Ye Kim, Jung-Eun Lee, Ki Jung Sung, Young Hoon Sung, Woo Sung Kim, Sung-Eun Kim, Hyung Chul Ryu, Jae Sun Kim, Lu Guangying, Chang-Min Choi, Jin Kyung Rho, and Jae Cheol Lee declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Electronic supplementary material
ESM 1
(PDF 222 kb)
Rights and permissions
About this article
Cite this article
Kim, D.H., Choi, Y.J., Kim, S.Y. et al. Discovery of a Potent and Mutant-Selective EGFR Inhibitor that Overcomes T790M-Mediated Resistance in Lung Cancer. Targ Oncol 13, 389–398 (2018). https://doi.org/10.1007/s11523-018-0568-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-018-0568-z