Abstract
Organs-on-chips have been tissues or three-dimensional (3D) mini-organs that comprise numerous cell types and have been produced on microfluidic chips to imitate the complicated structures and interactions of diverse cell types and organs under controlled circumstances. Several morphological and physiological distinctions exist between traditional 2D cultures, animal models, and the growing popular 3D cultures. On the other hand, animal models might not accurately simulate human toxicity because of physiological variations and interspecies metabolic capability. The on-chip technique allows for observing and understanding the process and alterations occurring in metastases. The present study aimed to briefly overview single and multi-organ-on-chip techniques. The current study addresses each platform’s essential benefits and characteristics and highlights recent developments in developing and utilizing technologies for single and multi-organs-on-chips. The study also discusses the drawbacks and constraints associated with these models, which include the requirement for standardized procedures and the difficulties of adding immune cells and other intricate biological elements. Finally, a comprehensive review demonstrated that the organs-on-chips approach has a potential way of investigating organ function and disease. The advancements in single and multi-organ-on-chip structures can potentially increase drug discovery and minimize dependency on animal models, resulting in improved therapies for human diseases.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Researchers frequently use primary cell cultures cultivated in standard Petri dishes to evaluate biological ideas. However, the primary concern of these studies has been mostly on the biological reaction of the cells themselves, with no rigorous influence on the surrounding biological environment within which the cells exist. Pharmaceutical firms, on the other hand, frequently test medications on animals, yet a substantial proportion of compounds containing lead do not succeed at the clinical research stage [1]. The human organ-on-chip connects these two methodologies, which will soon revolutionize how academics conduct their study and pharmaceutical firms evaluate their products [2]. Organ-on-chip is a cell culture-based approach for creating miniature tissues or 3D organs that closely mimic their in vivo counterparts, whether they have been larger or smaller in size. The botanical name “chip” describes the principles of design and microfabrication methods used. At the same time, the term “organ” is derived from the adaption of a microenvironment structure that mimics the workings of organs in vivo [3]. Organs-on-chip technology has recently given rise to an innovative new interdisciplinary scientific discipline that has made it possible to create unique in vitro organ and disease models [4,5,6]. The artificial tissues were developed in a microfluidic device, which comprised numerous distinct cell types next to and in contact with one another under precisely monitored conditions. These controlled factors allow for mimicking complicated structures and cellular interactions inside and across diverse in vivo organs and cell types while keeping the culture alive longer. Organ-on-chip technology for human functioning offers extremely beneficial fundamental research and medication advancements in cost-effectiveness and the accuracy of outcomes. The organ-on-chip concept and complete microscope integration enable real-time observation of biological activity. These devices were used to research numerous pathophysiological and physiological issues, find prospective pharmacological specifications, create novel medicines, and evaluate cutting-edge drug delivery strategies [7,8,9,10]. In addition to being a possible alternative to testing on animals, human in vitro models may also provide insight into the variability of human beings due to their small scale and ability to conduct high-throughput tests that are not macroscopically feasible from an economic standpoint. Microfluidics technology has evolved to such an extent that it enables unprecedented manipulation of culture conditions. This method enables precise control over a wide range of stimuli, including spatial homogeneity [11], chemical gradients [12], substrate mechanical characteristics, and time-dependent biochemical stimulations [13]. Over the past decade, several extremely intriguing and diverse scientific advancements have matured, making it feasible to transform the organ-on-chip sector into a cutting-edge technological platform to generate in vitro simulations of human illnesses and organs [14]. The first organ-on-chip devices were created almost concurrently with the advancement of stem cell research. The utilization of induced pluripotent stem cells as system models has experienced significant growth due to these advancements [15]. Artificial pluripotent stem cells enabled the non-invasive and easy production of stem cell lines from healthy people or patients, allowing researchers to study the genetics underlying unique human features and disorders in a stem cell line. While the ethical concerns of embryonic stem cells have been addressed, certain difficulties, such as privacy concerns and the exploitation of artificial pluripotent stem cell lines for commercial purposes, must be addressed. The commencement of human genome sequencing, which holds relevance to the field of organ-on-chip, took place in approximately 2000. This has been another innovative achievement of roughly 10 years [16]. Strong techniques have been created to analyze biochemical reactions in living cells, such as identifying a single cell progeny in a living organism [17, 18]. The pioneering developments in cell culture into microfluidic channels and the investigation of innovative biocompatible and sensitive substrate and scaffold materials drove the fast improvements in microfluidics technology during the previous 10 years [19]. The microfluidic-generated miniaturized tissues may be based on human genetics by employing induced pluripotent stem cell lines acquired from sick or healthy persons. For drug toxicity examinations and other purposes related to the development of drugs, the models have been anticipated to be both repeatable and scalable for higher throughputs.
The rapid development and significance of microfluidic and drug delivery research in the study of human-organ-on-chip analysis are evident from the abundance of reviews discussing various techniques for organ-on-chip manipulation and biochemical analysis. These reviews highlight the importance of organ-on-chip technology in advancing our understanding of single and multi-cell analysis. Each year, numerous reviews and studies on human-on-chip manipulation, microfluidic technique advancements, and diverse analytical methods in the field of organ-on-chip applications have been published (refer to Fig. 1). These reviews offer distinct perspectives, enriching the understanding and knowledge base surrounding this dynamic and evolving area of research. The review will highlight the most recent developments in cancer metastases medication delivery, precision/personalized medicine, and feto-maternal research using single and multi-organ-on-chip techniques. The present research has also reviewed several organ-on-chip methods created for several organs. The advances in organ-on-chip devices and their possible positive and negative aspects will be highlighted. The advancements in microfluidic devices for creating organ-on-chip methods have been finally concluded, while future challenges have been identified and emphasized.
The authors strategically hone in on the integration of multiple organs within organ-on-chip platforms, recognizing that physiological responses often stem from the intricate interplay between various organs. By focusing on multi-organ integration, the manuscript aims to overcome the limitations of studying individual organs in isolation, seeking a more holistic representation of human physiology. Additionally, the authors emphasize the synergy between machine learning and microfluidics, driven by the realization that organ-on-chip generated data can be complex and extensive. This integration aligns with the broader goal of enhancing experimental efficiency and paving the way for personalized medicine by leveraging machine learning algorithms to decipher intricate patterns within the high-dimensional datasets produced by microfluidic devices. Together, these focus areas aim to propel organ-on-chip technology toward more accurate, comprehensive, and personalized in vitro models, fostering advancements in drug development and tailored healthcare solutions.
2 Organ-on-chip method
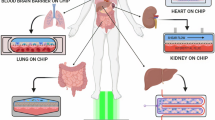
Organ-on-chip refers to a technology that employs microfabrication methods to construct microscale devices, replicating the structure and functionality of human organs. This innovative domain encompasses two basic types of platforms, each adhering to unique but complementary objectives. The first distinct kind of architecture is a single-organ structure, as shown in Fig. 2a, that is meant to replicate the core functioning of an individual organ effectively. These single-organ platforms allow for an emphasized and comprehensive analysis of each organ’s behavior, yielding important insights into organ-specific responses and functionalities. The second category comprises multi-organ tissue platforms, as shown in Fig. 2b, which combine several organs onto a single chip. This complex method mimics interactions and reactions between multiple organ modeling inside a single system. Multi-organ platforms encompass the dynamic interactions of organs, providing a more comprehensive knowledge of physiological processes and systemic reactions. The combination of many organs on a single chip produces a dynamic environment that closely resembles the linked architecture of the human body, giving researchers an excellent device for studying organ interactions, medication reactions, and disease development [22].
These two types of organ-on-chip systems accomplish diverse research goals because of their varying levels of advanced technology. Single-organ structures probe deeply into unique organ characteristics, providing precise insights into their functioning and actions. Multi-organ tissue platforms, on the other hand, provide for an in-depth investigation of systematic relationships, allowing researchers to gain insight into how various organs impact each other in a more realistic and integrated manner. These advances in organ-on-chip technology work together to improve the accuracy and predictability of drug testing, disease research, and personalized medication.
3 Single organ-on-chip
Single organ-on-chip platforms were designed to mimic the shape and working of a single organ, viz., lung [23], liver [24], kidney [25], heart [26], skin [27], or brain [28]. These chips are generally made up of a small space filled with live cells created to duplicate the tissue structure and function of the modelled organ (see Fig. 2a). Investigators may use these devices to analyze whether medications and other therapies interact with the specific organ and how the organ reacts to various physiological situations. The numerous types of cells found in organs have been categorized depending on their function within the human body. Since distinct organs have a variety of functions, the cell structures inside them vary according to cell type, some of which are addressed below.
3.1 Liver-on-chip
The liver is a crucial organ in the human system that performs several vital tasks to keep normal physiological processes running smoothly. In rare circumstances, the injuries are excessively severe, usually due to unfavorable drugs or disease responses. When viable in vitro models, such as those based on organ-on-chip technology, were unavailable, animal testing became the standard drug evaluation method. The liver-on-chip concept is depicted schematically in Fig. 3 [29]. The liver cells have been seeded on a chip by utilizing the organ-on-chip method, and they have then been exposed to chemicals through multiple channels to determine whether the cells will be harmed.
Diagrammatic representation of liver-on-chip method [29]
Furthermore, the liver-on-chip technique stabilizes initial hepatocyte activities by properly interacting with cell-cell, cell-soluble, and cell-ECM molecules [30, 31]. The hepatic system serves as the primary location for medication and toxin metabolism. The liver comprises a number of complex hepatic lesions which provide multicellular physiological linkages [32]. Kane et al. [33] created an initial liver-based device comprising microfluidic channels with a combination of rat liver cells and 3T3-J2 fibroblasts co-cultured to simulate an airway interface. On a chip platform, rat hepatocytes can synthesize albumin and sustain a stable metabolic rate. A chip was created by Lee et al. [24] with an interstitial cell structure made of grown primary liver cells and endothelial cells, with a growth medium infused through the external gap. In the chip, the endothelial space within the hepatocyte cords allowed for isolation from the exterior sinusoidal area and efficient substance interaction. Ho et al. [34] have patterned cells onto spherical polydimethylsiloxane chips using electrophoresis-created radial electric field gradients. These cutting-edge methods mimicked the anatomy of the hepatic lobules. Yum et al. [35] created techniques to investigate hepatocyte’s impact on other cell types. High-throughput tests have been developed to assess the toxicity of medicines on liver cells.
In a similar vein, Kang et al. [36] established a microfluidic device that investigated hepatotropic hepatitis B virus replication using a combination of hepatocytes and endothelial cells in single and double microchannel topologies, with as well as without continuous infusion. The study revealed that for a minimum of 30 days, hepatocytes kept their usual shape and kept on producing urea. Microfluidic chips have been used to build bio-inspired structures for 3D hepatocyte culture procedures that allow in situ infusion of hepatic spheroids to improve physiological models [37]. Lu et al. [38] combined decellularized liver matrixes with gelatin methacryloyl to construct biomimetic liver tumors that mimicked the 3D tumor microenvironment, which accurately mimicked the disease model. Chong et al. [39] devised methods for evaluating drug skin sensitivity by assessing antigen-presenting cell and metabolite activity generation. This technique is significant for detecting substances that cause complex skin reactions in drug testing.
Further, a variety of injuries and medical conditions have been studied. Zhou et al. [40] created a method for simulating alcohol damage. According to the investigation, liver cell damage, apoptosis, and inflammation may be detected using microfluidic co-cultures with embedded biosensors, offering a platform for tracking the liver’s signaling pathway in response to injury. Various liver-on-chip architectures were created to promote the long-term growth of liver cells and make it easier to examine the multiple activities of the liver, such as metabolism, detoxification, and medicine response [41,42,43,44,45,46]. Prodanov et al. [47] designed a microfluidic structure with a porous membrane which separated two compartments and allowed hepatocyte culture to be maintained for 28 days. The studies demonstrated that up to 9 days after treatment, troglitazone significantly altered the morphology of hepatocytes and resulted in substantial cell death. Essaouira et al. [48] proposed a pancreas-liver-on-chip culture design to investigate inter-organ interactions, which allows for physiological simulation and monitoring of insulin production and glucose metabolism. The model can potentially be utilized for studying metabolic disorders and medication toxicity. Polidoro et al. [49] overviewed experimental liver models for studying liver function, disease, and medication toxicity, including classic cell culture techniques and microfluidic organs-on-chip.
The authors examined the benefits and drawbacks of each model, as well as future ideas for enhancing liver models. The study discusses using microfluidic organ-on-chip devices for in vitro simulation of liver disorders. Kanabekova et al. [50] described the benefits of these devices over standard cell culture procedures as well as their potential uses in personalized medicine and drug screening. Moradi et al. [51] examined current achievements in microfluidic organ-on-chip human liver tissue design as well as their potential uses in disease modeling, toxicology, and drug discovery. The authors explore the obstacles and prospects for the future creation of models. The immunological abilities of the liver, including its influence on medicine rejections, have not been explored in existing liver-on-chip designs, making it an important element to address in drug development. In reality, liver damage caused by drugs is the main factor that ends clinical trials and frequently justifies the removal of a drug from the marketplace for distribution [52]. Establishing liver simulators incorporating critical immune system components like Kupffer cells that would vastly improve our knowledge of the primary causes of liver illness and the immune response to medications.
3.2 Heart-on-chip
The most common reason for mortality worldwide is cardiovascular disease. According to predictions, 17.5 million deaths from cardiovascular illnesses occurred globally in 2012, accounting for 31% of the total fatalities [53]. Organs-on-chip technology has demonstrated its significance as a bridge among in vitro models and clinical trials with human participants in cardiovascular disease research [54] (refer to Fig. 4).
Diagram illustrating the construction and implementation of heart-on-chip and vessel-on-chip structures [54]
The human heart circulates blood throughout the body and has a natural characteristic known as auto rhythmicity, controlled by both the neurological and hormonal systems [55]. Challenges in developing cardiovascular drugs include poor predictability of animal models, organism-dependent adverse effects, and lengthy/costly development processes. According to an analysis of AstraZeneca’s drug development pipeline between 2005 and 2010, 82% of concepts were terminated during the preclinical stage due to safety concerns. Cardiovascular failure accounted for approximately 17% of the total organ failures studied [56]. The usage of microfluidic devices allows for working in vitro simulations of cardiovascular organs.
Heart-on-chip studies various heart activities, including cardiac electrophysiology, heart disorders, and electrical stimulation. It has applications in mechanical stimulation, arrhythmogenesis modeling, and hypoxia simulation of cardiac cells. Grosberg et al. [57] developed a surface-textured, elastic polydimethylsiloxane film containing implanted newborn rat cardiomyocytes, which curled when the cells collapsed, allowing them to examine changes in contractile capability depending on the curling degree. Kim et al. [58] established a microfluidic apparatus using a fibrin matrix for modeling vascular networks and discovered that a dynamic environment resulted in more nitric oxide production than static conditions. Zhang et al. [59] developed a micro-organ tissue chip by employing 3D printing methods, combining cardiomyocytes with vascular networks made by vascular endothelial cells. An organ-on-chip has been employed to build a screening platform for cardiovascular medications, endothelial cells in the vascular system to generate vascular structures, and cardiomyocytes to fill the gaps. Xiao et al. [60] developed a microfabricated pharmacological testing bioreactor that utilizes stem cells from human embryos and neonate rat heart cells to construct cardiac biowires. Nitric oxide supplied through the medium into the chamber may cause the cardiac biowires to beat more slowly. Marsano et al. [61] created a cardiac organ system which replicated the mechanical and physiological environment experienced by cardiomyocytes. The development of such a structure is a watershed moment in the field, allowing for standardized operational three-dimensional cardiac models as well as an affordable, distinctive screening tool to enhance the predictive powers of in vitro studies. Zhang et al. [62] demonstrated a heart-on-chip structure which utilized high-speed resistance monitoring to evaluate the efficiency of cardiovascular medications by recording cardiomyocyte contraction, giving a preclinical technique for testing cardiac pharmacological efficacy. Nguyen et al. [63] established a heart-on-chip approach which enables precise and controllable mechanical devices to recreate a simulated physiological environment. This method potentially allows for generating functional cardiac patches in vitro using immature cardiomyocytes to replace damaged cardiac tissues. Schneider [64] designed chips for developing heart tissue in an incubator using human-generated pluripotent stem cells that have proven efficient as well as convenient. Abulaiti et al. [65] described the development of a heart-on-chip microdevice which employs human pluripotent stem cells that have been generated to test the performance of human cardiac tissue in vitro.
A critical aspect of advances in medication development for cardiovascular tissue is the dropping of cardiotoxicity, which commonly occurs in drug trials and has a significant reason for clinical trial termination or drug recall from the market. The authors have created a beating human heart-on-chip by employing micro-engineered cardiac tissue, which may be utilized for estimating hypertrophic alterations to heart cells. The device can create cardiac micro-tissues with improved mechanical and electrical communication between adjacent cells.
3.3 Brain-on-chip
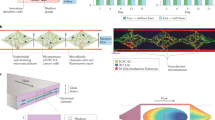
Developing an organ-on-chip design for the brain is recognized as one of the most difficult tasks in this scientific research. Due to brain functions’ very specific and intricate nature, most studies analyze the neurovascular unit, cell ratios, and transport features instead of attempting to predict such complexity. Brain-on-chip designs can be categorized into four groups: compartmentalized models, spheroid models, microfluidic models, and hydrogel-based models. The most prevalent production technologies for the brain on chips are contact printing, 3D printing, mold lithography, and hydrogel casting [66]. Amirifar et al. [67] provided a detailed analysis of the cutting-edge developments in brain-on-chip devices (refer to Fig. 5), focusing on the design and techniques utilized to produce microfluidic models of the brain in both healthy and pathological states. The study additionally examines how these models may be used in drug development and personalized treatment.
Schematic representation of brain-on-chip models [67]
Designs of the blood-brain barrier-on-chip were built and used for investigating the function of neuro-inflammation within degenerative diseases, including Alzheimer’s disorder and antibody trafficking to the brain [68]. Multistep lithography is the technology employed in brain-on-chip cell culture, which has proven successful in growing cells in exposed conditions, like substrates made of glass or transparent Petri dishes. These models have recently been utilized to examine neurodegenerative diseases and their implications on brain cell activity and function [69]. Park et al. [70] studied amyloid toxicity on neural network disintegration. They observed that the amount of destruction was significantly larger when tested under dynamic settings rather than static settings. Kilic et al. [71] successfully created a brain-on-chip prototype to examine the behavior of complex cells and tissues, specifically emphasizing the movement that occurs in human neural progenitor cells in reaction to the brain chemokine CXCL12. An interconnected brain-on-chip system has been designed to study interactions among various neurons in the neurovascular region as well as connections among the brain and other organs [72, 73]. The blood-brain barrier regulates the flow of molecules among the brain and bloodstream, which helps to keep the central nervous system stable. Any disruption within this barrier causes several kinds of neurological diseases. A microfluidic technology, including patient-specific stem cells, has been created to construct a customized human circulatory system that replicates the blood-brain barrier and can predict inter-individual variability.
3.4 Lung-on-chip
Various novel interventions are being explored to improve their treatment to address pulmonary diseases, considering they are a leading cause of death worldwide. Studying cellular interactions, blood flow, and gas circulation inside the pulmonary system is critical for biological studies and medication trials because it includes interchanging external gases, such as carbon dioxide and oxygen, within the blood inside the lungs, an essential organ. It can be challenging to replicate the in vitro alveoli, which govern gas exchange in the lungs. Microfluidics can provide accurate fluid flow and constant gas exchange, allowing extracorporeal lung models to be created and lung disorders to be simulated. Mechanical pulmonary pressure regulation, as well as the influence of shear forces on pathophysiological mechanisms and the blood-brain barrier, are all being studied [74]. Li et al. [75] described the creation of a biomimetic human lung-on-chip to simulate lung diseases (see Fig. 6). Researchers can examine the causes of diseases with the device as it mimics the mechanical and microstructural characteristics of the pulmonary system of humans.
The schematic representation of lung-on-chip model [75]
Huh et al. [8] have developed a lung-on-chip model that precisely reproduces the mechanical and biochemical features found in the human lung. The apparatus allows for the reconstruction of organ-level lung functioning, medication screening, and disease prediction. In comparison to traditional platforms, the lung-on-chip offers a tangible way of examining detailed chemical, mechanical, and biological consequences, allowing for the characterization of dynamic cellular behaviors in vitro and complete drug testing. Stucki et al. [76] established a novel elastic membrane expansion prototype capable of mimicking breathing using a lung-on-chip model. This model emulated 3D recurrent strain to resemble breathing and precisely replicated the lung parenchyma, incorporating an alveolar barrier. Humayun et al. [77] investigated the prospective benefits of using a hydrogel membrane as a physiological model that includes propagated airway epithelium and cells of smooth muscle, coupled with microenvironmental signals and exposure to toxins to indicate long-term lung illness. Blume et al. [78] developed three-dimensional airway culture prototypes utilizing medium and fluid engagement to replicate lung intercellular flow.
The lung-on-chip technology uses microfluidics to deliver pressure to alveoli and capillaries while mimicking pulmonary expansion and lung gas-liquid interactions, resulting in a shear flow profile resembling the actual lung environment. Yang et al. [79] constructed a cell scaffold chip matrix using poly lactic-coglycolic acid electrically spinning nanofiber membranes, emphasizing its potential value in lung tumor high-accuracy treatment and tissue formation. Peng et al. [80] developed a lung assistance apparatus that improves the interchange of gases in the placenta for premature newborns suffering from respiratory insufficiency. This has been the first study that systematically measured umbilical vascular damage caused by catheter expansion. Dabaghi et al. [81] used the microfabrication method to develop microfluidic blood oxygenators with a doul-faced gas supply utilization, resulting in a 343% increase in oxygen absorption compared to single-faced systems. Xu et al. [82] utilized microfluidic devices to culture actual carcinoma cells and cancer cell lines to reproduce the lung cancer microenvironment and assess the efficiency of various chemotherapy treatments. Researchers have utilized microfluidic devices to replicate the lungs-on-chip technique by cultivating lung cells on these platforms. The devices mimic lung functionality by changing pressure, while the cells mimic lung activities, resulting in lung-on-chip simulators [83]. Researchers found that cells on microfluidic devices mimicked the behavior of the lungs in response to pressure changes, including alveolar contraction and expansion and porous membrane expansion and contraction. Various medications may be tested on these models to determine their effectiveness and potential side effects [84]. These organ-on-chip could be utilized to examine the influence of external variables that affect organs and a human airway on a chip to reprocess medications for COVID-19.
Further, the study used human bronchial and pulmonary endothelium to develop a model for circulating immune system cells, viral infection, and cytokine production [85]. Khalid et al. [86] proposed a lung cancer-on-chip device that includes biological sensors to monitor biological processes and assess toxicity. The lung cancer-on-chip technology enables the evaluation of anti-cancer treatment efficacy and physiological toxicity monitoring. At the same time, the nanoparticles are made from fossil fuels, non-biodegradable materials, and nitric oxide. Experiments have demonstrated that prolonged exposure to these non-biodegradable nanoparticle poisons increases the likelihood of developing respiratory problems such as pulmonary fibrosis, asthma, and pulmonary edema. While most organ-on-chip studies concentrate on toxicity and diseases related to the alveoli, there are alternative organ-on-chip models, including those that incorporate 3D printing and construction techniques, which address other regions of the respiratory system [87].
3.5 Kidney-on-chip
The kidney plays an essential role in the metabolism and disposal of drugs; hence drug-induced nephrotoxicity constitutes a few issues that have been addressed the most in drug safety evaluations. The initial kidney-on-chip iteration employed rat tubular cells. It included two compartments, one that simulated the urinary lumen with fluid flow and another that mimicked the interstitial space [88]. The permanent loss of renal filtration caused by kidney toxicity emphasizes the significance of drug screening systems. Microfluidics technology can recreate the fluidic environment required for tubular cell growth. It also has a permeable membrane that retains cell polarity [89]. Like the membrane used in the lung-on-chip device, kidney-on-chip devices use a membrane with holes that include a main flow channel and two smaller sub-channels where kidney and epithelial cells have been cultured on each side. Devices with kidneys-on-chips are schematically depicted in Fig. 7 [90].
Schematic representation of the human kidney-on-chip [90]
Applying inflow-generated shear stress to the epithelial cell layer in kidney-on-chip devices replicates in vivo kidney tubules or promotes primary cilia development and epithelial cell polarization, distinguishing it from conventional microfluidic systems. Jang et al. [91] utilized rat kidney medullary accumulating cells from ducts to create a multi-layered microfluidic system which mimicked the filtering of the kidneys. The microfluidic apparatus created a biomimetic environment that promoted cytoskeletal reorganization and molecular transport. A similar microfluidic system was utilized in 2013 to cultivate initial human epithelial cells from the kidney [92]. These have been the initial kidney cell damage investigations. The device provides novel perspectives not achievable with animals and conventional cultivating cell models. Wilmer et al. [93] successfully utilized microchips for drug-induced nephrotoxicity experiments and built a chip using opossum kidney epithelial cells to examine the albumin transportation process. Musah et al. [94] employed adult human cell podocytes produced by pluripotent-associated stem cells to replicate the albuminuria brought on by adriamycin and evaluate glomerular function. Numerous multi-organ-on-chip designs have featured the kidney-on-chip, which operates as a barrier selective to protein from the plasma and may induce proteinuria. Researchers have attempted to produce podocytes on a chip; however, the technology is difficult due to the need for complex culture processes [95]. Conventional cell culture techniques have a drawback as differentiating cells into functional ones necessitates extended culture times and an external signal detecting device. Schutgens et al. [96] produced robust tubule culture techniques which allowed for sustained growth and examination of human kidney tissue in their studies. This technique has created a flexible initial kidney epithelial cell cultivation method, enabling the rapid and personalized study of cell components, disease simulations, and drug evaluation. Sakolish et al. [97] developed a sustainable microfluidic device for human glomeruli and proximal tubes. This technique enabled cells from the kidney epithelium to flourish in various settings and showed that shear stress might cause nephrotoxicity. Tao et al. [98] developed a robust approach to generate human islet organs from pluripotent stem cells derived from humans. This approach might be applied to regenerative medicine, stem cell-based organ engineering, and other areas. Musah et al. [99] created human being’s glomerular devices by generating pod cells from pluripotent stem cells and cultivating them in organ culture devices. The techniques employed made it possible to mimic the structure and operation of the renal capillary wall, which had never been possible with earlier approaches. The glomerular chip produced by [99] had possible applications in regenerative medicine, disease research, nephrotoxicity assessments, kidney development, and pharmaceutical discovery. These researchers developed a partly physiological environment using a single cell type and numerous biomarkers to characterize their systems.
3.6 Skin-on-chip
Skin is the most significant tissue in a person's body, which acts as the principal barrier against invading viruses and germs. Despite its importance as a barrier, the skin is a successful immune organ that harbors components associated with the adaptive as well as the innate immune systems [100]. A skin-on-chip model has been utilized for dermal diffusion testing, effectiveness testing, inflammation, toxicological investigations, wound healing, shear stress, and repair studies [101]. Kim et al. [102] used a skin-on-chip device to examine coenzyme Q10 and curcumin’s anti-aging abilities without a pump. The two basic layers within the skin that provide immunity are the dermis and epidermis. The epidermis contains essential immune cells such as keratinocytes, which express Toll-like receptors for recognizing pathogenic chemicals, and Langerhans, antigen-presenting dendritic cells [103, 104]. As shown in Fig. 8, platforms have been developed with varying levels of complexity, vascular elements, and cell types incorporated to assist in drug development and testing [105].
Schematics of different skin-on-chip platforms [105]
O'Neill et al. [106] created a skin-on-chip design especially focused on mimicking the epidermis, the skin’s outer layer. Neonatal epidermal keratinocytes were cultivated on collagen-patched microfluidic tubes to replicate a keratinocyte cultivation technique. Sriram et al. [107] created a device prototype by combining epidermal and dermal tissue layers to generate full-thickness skin. The dermal counterpart is created via a fibrin-based material containing human fibroblasts from the dermis, and the epidermal counterpart is created by cultivating keratinocytes on the outermost layer of the dermis. These models have been vascularized with endothelial cells to improve the physiological significance for further research [108, 109]. Mori et al. [109] produced a perfused full-thickness skin model with epidermal and dermal equivalents utilizing nylon wires that penetrate the dermal equivalent. The authors demonstrated the model’s drug development potential by measuring and illustrating the varied penetration of caffeine and isosorbide dinitrate as concept medications. Wufuer et al. [110] created a skin-on-chip device supplied with blood to replicate edema, medication therapy, and inflammation. The replica comprises three polydimethylsiloxane regions seeded with HUVECs, HS27 fibroblasts, and human keratinocytes representing dermal, vascular, and epidermal layers. Abaci et al. [108] utilized endothelial tissue cells created by pluripotent stem cells that had been stimulated to develop a human skin that resembled vascularization. The study found that induced endothelial cells produced by pluripotent stem cells promote new blood vessel formation and encourage vascular invasion from the wound region to the skin surface. Earlier vascularized skin-on-chip models were incomplete as they lacked essential immune cells like dendritic cells and T lymphocytes, leading to inaccurate representation of the skin’s immune response. Ramadan and Ting [111] created the solely available model with immunological components. A porous membrane divides the model’s monocyte and keratinocyte layers between the model, allowing them to communicate in a microfluidic bi-channel arrangement. The researchers illustrated the capability of the model to react to external chemical and physical inputs by subjecting it to Lipopolysaccharide and UV stimulation.
3.7 Gut-on-chip
The chip-based simulated gut with a regulated ecosystem with several human cell types, including immunological, intestinal epithelium, and endothelial cells, has been constructed. The microscopic characteristics of intestine villi were precisely replicated in order to mimic the intestine's in-vivo milieu [112]. Various cell types, including Caco-2, intestinal organoids, immune cells, and several kinds of human endothelial cells, like PBMCs, have been employed for this model. Villi projections and Lieberkühn crypts invaginations characterize the surface of the human gut. A diagrammatic illustration of the human gut-on-chip technology is depicted in Fig. 9 [113].
The diagrammatic illustration of the human gut-on-chip model [113]
One application for gut-on-chip technology is to examine medication host-gut, pharmaceutical kinetics, microbial interactions, and nutritional metabolism in a simulated gut environment [114]. This study used a microfluidic micro hole retaining device to assess the cell permeability of various medications, including naproxen, propranolol, verapamil, furosemide, atenolol, carbamazepine, and antipyrine [115]. A microfluidic model-based device has been created to study the interaction and infection of the host and the Coxsackie B1 virus [116]. Like the skin, the gastrointestinal system frequently exposes to external infections, necessitating efficient defense mechanisms to prevent their entrance into the body’s internal organs [117]. Immunological cells play an important function in the gut in maintaining immunological homeostasis and defending against infections [118]. Important gastrointestinal tract features such as immunological components, an epithelium barrier, a mucus layer, and human-microbial interaction must be included to build a functional gut-on-chip. Kimura et al. [119] developed a fundamental paradigm to facilitate the prolonged cultivation and tracking of epithelium cancer of the colorectal cells that have been utilized in studies of the intestinal processes. The model comprises two distinct pathways divided by a semipermeable, spongy polyester membrane in which cells from Caco-2 have been cultivated. Kim et al. [120] used an identical framework and the same cell line to generate a more realistic in vivo gut simulation, integrating biophysical signals like cyclic strain and shear stress.
The differentiated epithelium improves the function of the gastrointestinal wall and promotes the development of lactic acid bacteria GG, bacteria often present in the human gut. Kim and Ingber [121] used the peristalsis functionality within the gut-on-chip prototype to investigate the proliferation of bipolar epithelium and the formation of villus-like features. The author also revealed that the cells which create villus-like systems in their model are firmly linked with specified secure connections across their interstitial barriers and are covered with brush borders and mucus. During the initial stages of cultivation, cells produced in the gut-on-chip this prototype exhibited a slight rise in the production of a drug-metabolizing mitochondrial enzyme. Still, cells grown on a trans well insert multilayer showed no significant cytochrome activity [121]. Shim and colleagues [122] produced the gut-on-chip model in which villi-like features have been embedded into the microfluidic device rather than emerging in response to physiological stimuli. These models are important platforms for studying human physiology and change due to external stimuli such as viruses or pharmaceutical medications. These devices may test the interactions between immune components and medicines.
3.8 Some other organs-on-chip
Breast-and-spleen-on-chip structures have utilized microfluidic technology; however, their abilities have been restricted. As shown in Fig. 10, breast-on-chip technologies only imitate the placement of physical veins in the breast, not the complete microenvironment comprising vascular, fatty, and glandular tissues [4]. To improve simulations of tumor growth in the breast, the prototype may be coupled to existing tumor-on-chip technologies. Figure 10 depicts a schematic representation of different organ-on-chip [123].
The spleen-on-chip technology replicates the blood-filtering operation of the spleen in vivo using a microfluidic dual-phase flow design. It is challenging to replicate the intricate milieu of bone marrow cells in vitro due to distinct niches with varying components and functions in hematopoiesis [124]. Torisawa et al. [125] created a bone marrow-on-chip system that uses both in vivo tissue engineering and in vitro microfluidic methods to mimic the physiology of the hematological zone in vitro. In the presence of bone marrow infections or disorders, the immune system’s capacity to defend against pathogens is weakened, which can result in serious illnesses or death. In recent years, studies have established the viability of employing organs-on-chip to represent refractory reproductive disorders such as cancer and endometriosis. This section also contains micro-engineered in vitro systems for studying three primary topics: reproductive disorders, assisted reproductive technology, and pregnancy. According to numerous organizations, recent organ-on-chip research and microfluidics breakthroughs have developed 3D micro-engineered devices that reproduce various female reproductive system components [126]. These include human uterine [127] and placental [128, 129] modeling. Recent studies have shown that it has been possible to mimic the 28-day menstrual cycle by integrating various tissues in a complex microfluidic structure [130]. The researchers demonstrate organ-on-chip technology applications to replicate and investigate the relationship of several fetal-maternal interaction elements, including the fetal membranes, maternal tissues, and placenta. Throughout pregnancy, the placenta regulates the flow of both nutrients and oxygen from the mother to the fetus and eliminates waste products to promote fetal growth. It also acts as a protective barrier, shielding the fetus from potentially hazardous diseases and toxins found in the mother’s blood [131, 132]. The latest studies have concentrated on creating placenta-on-chip models to generate multi-layered tissue structures that mimic the normal placental barrier [133]. These models have been used to evaluate the movement of nutrients and materials through the maternal-fetal interface as well as the influence of nanoparticle treatment on placental wall function [134, 135].
Further, these models have been used to study critical regulatory mechanisms that affect placental function and shape. These devices use microfluidic methods to emulate anatomical geometries or biological processes, although they may not completely reproduce the related tissues at the microenvironmental level. The high level of similarity among organ-on-chip technologies and real organs is useful for investigators interested in developing a person-on-chip device, an all-inclusive in vitro platform for investigating the effects of medications on the complete human body. Any disease or infection that impacts the bone marrow hinders the immune system’s ability to eliminate pathogens, which can result in severe illnesses or fatalities.
Table 1 presents a comprehensive summary and illustration of the diverse approaches researchers employ to harness the complete capabilities of technology in their investigations and systematic exploration of single organ-on-a-chip methodologies. Table 1 encompasses a range of organ-on-a-chip methods proposed by various investigators, highlighting the distinctive techniques utilized to unlock the full potential of this technology. To fully understand the effects of medications and drug delivery on the human body, it is crucial to have continuous communication between cells. In order to comprehensively assess the overall effects and conduct a more in-depth examination, it is necessary to integrate several organs into a cohesive, multi-system framework, given their complex interactions. This innovative integration is commonly referred to as a “multi-organ-on-chip,” a technology we delve into more extensively in the following section.
4 Multi organ-on-chip
Organ-on-chip devices have been designed to replicate the interactions of numerous organs in the body of a person to create a more realistic and complete model of human physiology. Multi-organ chips often comprise numerous compartments containing live cells that imitate a single organ’s tissue structure and function, as shown in Fig. 11. Channels or structures connect the chambers, allowing fluid movement and physiological activities between the organs. Multi-organ chips can investigate complicated physiological relationships and the effects of medications and treatments on many organs.
A conceptual representation of interconnected multiorgan chips resembling the Vitruvian Man [4]
4.1 Multiorgan/human-on-chip
Single-organ models have restrictions in mimicking complex interactions between multiple tissues and organs, highlighting the need for multi-organ systems to replicate biological reactions, including organ-organ relationships. Researchers and companies consider the implementation of a sophisticated multiorgan-on-chip or full body-on-chip system for drug discovery and individualized healthcare as a significant achievement. Although single-organ models are useful for studying physiological responses, sophisticated models which integrate multiple organ and tissue types are better for imitating the physiological function of the actual body. The interconnectivity and interaction among various tissues and organs in microfluidic systems distinguishes them and allows for more accurate reproduction of human body function. The multidimensional architecture of the human body makes body scaling and functional activity difficult on these platforms. Human-on-chip techniques can potentially eliminate experiments involving animals in medical research and speed up the therapeutic use of nanomaterials [136]. Multiorgan platforms can enable long-term studies of healthy homeostasis and functioning, and provide insights into the processes of inflammatory diseases affecting many organs. Multi-organs-on-chip allows the concurrent cultivation of many organs linked via channels to explore their interactions and construct a functional system [137, 138]. Though the concept of multi-organs-on-chip is relatively nascent, significant advances have already been achieved, consisting the constructed systems comprising two organs [139, 140], three organs [141, 142], four organs [143, 144], and ten organs on a chip [145]. Multiple organ chips can be combined to construct a body-on-chip platform using various technologies, such as channel and medium-circulating systems. The human body-on-chip technology may improve drug development by estimating drug metabolism, excretion, absorption, and toxicity utilizing in vitro to in vivo translation abilities, as shown in Fig. 12 [146].
Multiorgan-chip devices facilitate disease simulation, drug pharmacokinetics/pharmacodynamics investigation among cells and the development of body-on-chip systems [146]
Microfluidic systems that combine various organs and tissues to mimic the human body can increase drug screening efficacy and precision while eliminating the requirement for testing on animals. Van et al. [139] successfully integrated the liver and the gut tissues into a microfluidic system, demonstrating its potential for studying organ interactions and bile acid synthesis. This approach enabled in vitro experiments and provided data on organ-organ relationships. Modeling multiorgan disorders has been difficult due to the significance of several cell types in metabolic regulation and limited organ accessibility. Recent studies using multiorgan-on-chip systems have revealed metabolic coupling between neurons and microvascular cells, providing insights into the functioning of organs like the blood-brain barrier and the brain. The importance of metabolites such as short-chain fatty acids in liver function, inflammatory gastrointestinal illnesses, and the immunological response has been highlighted by multiorgan-on-chip devices which connect organs such as the liver, and gut cells [147]. A different multiorgan chip simulation represented the lung, liver, and small intestine. The study employing this model demonstrated that it could imitate the effects of three cancer medicines on target cells and reproduce their pharmacokinetics. Multiorgan devices can be an investigative tool for researching complicated illnesses and creating novel treatments [148]. Advancements in chip technology allow for the concentration of multiple organs, enabling in vitro studies and insights into their interactions.
To cultivate cells properly, organ chips need to preserve a steady fluid interaction, prevent contamination by bacteria, and continually evaluate the viability of the cells. Increasing the number of organs on a chip increases system complexity, leading to unexpected results. Lee et al. [149] established a pump-free multiorgan-on-chip that has been simple to assemble and operate. Satoh et al. [150] developed a multiorgan-on-chip technology using a pneumatic pressure-driven media flow mechanism. This method has benefits for drug development, such as the ability to run several organ culture devices simultaneously, compatibility with pipette-based fluid management, versatility in the design of the microfluidic system, analytical methods in microplates, and adaptability to widely used experimental protocols. Lantada et al. [151] designed a transparent chip utilizing an innovative combination of laser technologies which showed to be successful in analyzing human mesenchymal stem cells and facilitating imaging processes. These methods are appropriate for chip mass manufacturing and have practical aerospace, transportation, and energy applications. Table 2 provides an overview and discussion on the relevance of available multiorgan-on-chip platforms in the context of drug development. Specifically, it explores the contributions of these immune organs to developing tolerance toward newly developed drugs. As proof-of-concept systems advance, the emphasis grows on designing devices that are not just theoretically robust but also simple to manufacture and scalable, ensuring their practicality and widespread adoption. In order to create systems that precisely mimic the complexities of human organ systems, it is necessary to use a variety of micromanufacturing methods together with meticulous material selection. The following sections provide a comprehensive analysis of several micromanufacturing processes that are leading the way in creating organ-on-a-chip systems.
5 Manufacturing technology
Organ-on-chip technology represents a rapidly evolving connection with the aim involving the fabrication of miniature, functional models of human organs using microfabrication techniques [152]. These models, also known as organoids or tissue chips, aim to replicate the composition and purpose of real human organs in an organized and customizable approach. The 3D bioprinting for organ-on-chip fabrication (see Fig. 13) includes the capacity to carefully regulate tissue models’ cellular structure, architecture, and mechanical behavior; 3D bio-printed organ-on-chips in drug detection; toxicology testing; disease molding; and personalized medicine [153].
Schematic representation of a advancements in bioprinting techniques for organ-on-chip fabrication and b nozzle-based bio-printed models [153]
3D bio-printing systems constitute extrusion-based, inkjet-based, and laser-based approaches and their applications in creating organ-on-chips. It addresses the many bio-ink materials that may be utilized for 3D bioprinting organ-on-chip models, including hydrogels, cell-laden bio-inks, and composite materials [154, 155]. 3D bio-printed organ-on-chips has the potential to advance personalized medicine by enabling patient-specific disease modeling, medication screening, and regenerative medicine. It also highlights the limitations and challenges of this technology, such as the need for standardized protocols, reproducibility, and regulatory considerations. 3D cell printing technology can generate viable biological tissues and artificial organs [156]. The method has shown promise in drug testing and screening by simulating tissues and disorders such as skin, liver, and cancer [157]. Printing can produce multilayered architectures with required patterns and thicknesses, mimicking the diverse functions of skin that contain various elements in multiple layers [158]. Investigating the liver using 3D-printed chips comprising numerous structures of mini-human liver tissue is possible, as the liver contains parenchymal hepatocytes as well as non-parenchymal auxiliary cells [159]. The article also discusses the use of 3D printing in generating simulated human breast cancer to mimic cancer growth inside the breast stromal tissue [156].
Integrating 3D cell-printed tissue with microfluidic devices has brought the possibility of printing organs-on-chips one step closer [160]. Two main approaches to achieve this integration are a combination of two-step and single-stage manufacturing. In the two-step manufacturing approach, micro-organs are printed separately and assembled onto pre-fabricated microfluidic platforms [161, 162]. This approach allows for traditionally manufactured high-resolution microfluidic tubes and chambers. For example, perfusable liver-on-chip along with multiple micro-liver tissues-on-chip have been successfully printed using extrusion printing and assembled onto pre-fabricated microfluidic platforms [163]. However, this approach still requires manual intervention, which may create mechanization challenges, inaccurate reproduction, and contaminated cultivation, posing challenges for commercialization.
On the other hand, the single-stage manufacturing approach involves the complete chip device, containing the cells and mechanical components, being printed, such as gaskets and microfluidic passage, in a single procedure [162]. This approach has been shown to be an efficient, productive manufacturing procedure. For instance, a spatially heterogeneous liver-on-chip and a nervous structure model with compartmentalized chambers have been successfully printed by employing extrusion-based printing using several materials [163, 164]. Additionally, perfusable and permeable channels made up of live cells, such as a proximal kidney tubule-on-chip, have been printed using fugitive ink in a single-step manufacturing method [165]. Overall, the two-step, as well as single-step manufacturing approaches, have shown promising results in developing organs-on-chips using 3D printing technology. Each approach has its advantages and challenges, and further examination is needed to enhance the printing process, improve automation, and ensure reproducibility and scalability for commercialization.
Integrating machine learning techniques with microfluidic technology in materials science and biomedicine can enable the development of intelligent microfluidic devices to enhance performance along with the efficiency of microfluidic devices in various applications. In microfluidic studies, researchers have commonly set initial conditions and adjusted parameters manually. However, machine learning integration, as demonstrated by autonomous encoders, makes it possible to transform numerous inputs into low-dimensional representations for input reconstruction. Using concentrated emulsions, an unsupervised method has demonstrated efficacy in forecasting emulsion stability based on droplet morphology. Furthermore, machine learning using model-free episodic control and Deep Q-Networks have been applied to automatically optimize volumetric flow rates in microfluidic channels. While observing through an optical microscope and modifying parameters to attain rewards depending on specified variables, these models acquire the appropriate flow rates [166]. The combination of machine learning and microfluidics has resulted in novel applications with significant effects. In drug development, machine learning algorithms automate and improve microfluidic experiments, speeding up the process and improving repeatability. Predictive modeling for fluid dynamics inside microchannels helps the design of microfluidic devices while reducing experiment time. Closed-loop feedback systems use machine learning to make real-time corrections, improving experimental control and flexibility. In diagnostics, microfluidic devices use machine learning to evaluate data, allowing for more accurate illness identification. Overall, this integration accelerates progress in healthcare, biotechnology, materials science, and manufacturing by offering more efficient, automated, and adaptable processes. Arianna et al. [167] discussed the growing significance of machine learning and image analysis in organ-on-chip platforms, which are novel in vitro ideas that simulate human organs’ physiological as well as mechanical properties. They describe how machine learning algorithms are used to analyze the large amounts of data generated by organ-on-chip schemes, including images of cells along with tissues. This allows researchers to identify patterns and relationships that may not be visible to the human eye, make more accurate predictions about how drugs and diseases will interact with the human body, and describe how traditional approaches to analyzing organ-on-chip platforms involved manual observations and measurements, which were time-consuming, labor-intensive, and prone to human bias. In contrast, machine learning algorithms and advanced image analysis techniques can potentially accelerate and enhance organ-on-chip platform analysis. Various machine learning algorithms, such as supervised, unsupervised, and deep learning, are commonly used in organ-on-chip research. It also highlights the importance of data preprocessing, feature extraction, and model validation in ensuring the accuracy and reliability of machine learning-based analyses. It also discusses the challenges and limitations of using machine learning and image analysis in organ-on-chip research, including the need for large annotated datasets, potential biases in training data, interpretability of machine learning models, and standardization of image analysis protocols [167].
Artificial neural networks are used in deep learning, a type of machine learning, to automatically learn representations of data from large datasets, enabling the prediction and analysis of complex patterns [168]. Recurrent neural networks (RNNs) in time-series computation convolutional neural networks (CNNs) in image processing data, and variational autoencoders (VAEs) for generative modeling are among the deep learning approaches often utilized in organ-on-chip research [169, 170]. It incorporates multi-omics data integration for comprehensive analysis, the application of reinforcement learning for automated control of organ-on-chip devices, for the development of personalized medical techniques employing patient-derived organ chips paired with deep learning algorithms [171]. Santbergen et al. [172] emphasized the importance of online and in situ anatomizing techniques in organ-on-chip studies, which enable continuous surveillance and characterization in relation to cellular and tissue responses. This online analysis method includes optical and electrical sensors, microfluidic techniques, and imaging modalities compatible with organs-on-chip technology to continuously monitor physiological parameters, drug metabolism, and cellular behaviors.
Furthermore, the research emphasizes using in situ analytical methods such as immunostaining, gene expression profiling, and proteomics, which enable the assessment of cellular and molecular changes within organs-on-chip devices without altering their milieu. The report also examines the benefits and drawbacks of remote and in situ investigation in organ-on-chip research, such as the capacity to gather real-time data, increase experimental repeatability, and minimize the need for animal models. However, device complexity, compatibility with long-term culture, and data analysis and interpretation are also addressed.
Cho et al. [173] developed a smartphone-based fluorescence microscope in real-time analysis of organ-on-chip devices (refer to Fig. 14a), enabling visualization of cellular structures and monitoring of biomolecules and cells. They propose a dual-mode monitoring system combining time-lapse imagery with fluorescence intensity measurement. Using the time-lapse imaging mode, a portable and low-cost imaging instrument, a smartphone-based microscope, can capture high-quality fluorescence pictures and long-term visualization of biological dynamics, such as cell movement, fertilization, and death. The fluorescence intensity measuring mode measures fluorescence intensity variations, which may be used to track biological responses to medication treatments or other interventions. Combining these two modalities allows an in-depth look at the cellular behavior in organ-on-chip technology. The smartphone-based microscope with mobility and cost makes it accessible to researchers in resource-limited situations, and the real-time monitoring capabilities enable immediate feedback and iterative optimization of organ-on-chip systems [173]. Sun et al. [147] suggested merging additive manufacturing using microfluidics to get around these limitations and enable the creation of another complicated and repeatable organ-on-chips (refer to Fig. 14b). Additive manufacturing methods, like 3D printing, allow for the creation of structures with great complexity and accuracy, while microfluidics allow for controlling fluids and cells within the device.
Combining these two approaches enables the creation of organs-on-chips with complicated geometries and microenvironments that closely resemble in vivo settings. The study shows how additive manufacturing techniques, like stereolithography and fused deposition modeling, have produced microfluidic devices for organ-on-chip applications. The authors also explore the advantages of integrating 3D printing and microfluidics, such as the capacity to generate customized and complicated microenvironments that can simulate the physiological circumstances of certain organs [174]. Table 3 presents various microfabrication techniques commonly utilized to fabricate organ-on-chip microfluidic devices, including microcontact printing, bioprinting technology, replica molding, and soft lithography. Broadening the horizon of technological progress, a crucial need becomes evident—an interdisciplinary intersection of diverse technical approaches and collaborative efforts with the medical field. This integration is crucial to ensure that innovations may be effectively and uniformly adopted and expanded. However, the process of bridging the gap between technological advancements and the stringent realm of medical approvals results in a delay, leading to a challenging and restrictive environment. Moreover, careful material selection becomes critical to chip manufacture, requiring the emulation of human organ-like features for thorough study and practicality. The next section will explore these concerns in more depth, emphasizing the need to use interdisciplinary approaches to effectively navigate this changing environment.
6 Discussion
The present study discusses the challenges, manufacturing technology, and numerous organ-on-chip models in detail. Researchers have created organs-on-chips for a variety of organs during the last decade, including the kidney [25], blood-brain barrier [28], liver [29], smooth and striated muscle [57], heart [61], neuron [71], lung [76], gut [116], skin [101], and bone marrow [125]. The impact of shear stress and fluid movement on cell configuration and activity, along with the resulting gradients of dissolved oxygen together with soluble inflation elements attributable to the depletion of soluble species, have been studied using these devices. The privilege of organ-on-chip engineering science, such as the potential to accurately replicate disease states, screen drugs with greater efficiency and accuracy, and reduce the need for animal testing, has also been discussed in the study. Organ-on-chip specimens, which represent the architecture and functions of real organs, have the potential to advance biomedical research by providing a more precise and reliable method for examining the impacts of medications and illnesses on human tissues. The organ-on-chip field demands the harmony of multiple techniques, such as molecular biology combined with cell biology, organ anatomy, microfluidics, microfabrication, materials science, and clinical disease knowledge. Access to laboratory facilities for cell culture, biochemistry, and microfabrication, along with collaborative efforts, is imperative. The 3D architecture and microenvironment of cells are important factors that need to be accurately replicated to develop models for organ tissues and diseases on a chip. Incorporating softer identity features and a micro incubator with organized fluid perfusion can support a better “in vivo-like” culture environment, as the physical microenvironment of cells in culture significantly impacts cell function. The availability of affordable disposable chip variations for usage in broad culture may result in a rapid adoption proportion among biology labs and potentially revolutionize cell culture. For example, under dynamic flow, in a microfluidic cave, an amalgamated population of primary hepatocytes developed periportal- and perivenous-like structures and cell compartments within 1 day. The “zonation” response in the microfluidic chamber with mixed primary hepatocytes was primarily determined by oxygen tension, as the flow produced dissolved oxygen gradients with accountable growth factors [175].
Significant progress has been made in the microfluidic design, which serves as a foundational element in organ-on-chip platforms. Studies have demonstrated the development of intricate architectures that closely replicate the intricate microenvironments found in human organs. These technological developments have resulted in improved interactions between cells in the microfluidic environment, affecting cell behavior, survival, and specialization. Advanced biomaterials and cutting-edge methods, like as 3D printing, have improved cell culture in organ-on-chip systems. This allows for the replication of tissue-specific functions and architecture. Notably, studies have shown the efficient differentiation of stem cells into particular cell types, attaining a degree of biological relevance critical for meaningful in vitro modeling. The primary objective of organ-on-chip systems is to accurately replicate disease circumstances, and recent research has achieved significant advancements in this area. From replicating the microenvironment of cancers to simulating cardiovascular disorders and neurodegenerative conditions, these models offer unprecedented insights into disease progression and responses to therapeutic interventions. Organ-on-chip technology represents a major advancement in personalized medicine, allowing for customized treatment strategies that take into account individual disease characteristics. Furthermore, by the integration of several chips specialized to various organs, scientists have developed more extensive models that replicate systemic interactions and reactions, thus advancing our progress toward a full depiction of human physiology. These integrated platforms provide opportunities to investigate the connections between many organs, drug metabolism, and toxicity in a way that accurately represents the intricate nature of the human body.
Also, organ-on-chip technology nourishes a unique stage toward disease research, advancing diagnosis and treatment techniques to improve global health. This method reduces the requirement for in vivo animal testing and saves time and money in medication development. Organ-on-chip enables 3D culture and cell-cell communications, as well as the application of mechanobiological stimuli, networked testing intensive care, and reduced experimentation costs. However, critics have worried about organ-on-chip accuracy, particularly in reproducing micro-physiological settings. Bioengineered liver, cardiac, and lung tissues have been integrated into a multi-tissue organ-on-chip platform in a different recirculating perfusion system. The platform enables biosensing capabilities and allows for studying intricate connections between various bodily tissues. The technology is demonstrated for a wide range of applications, including the characterization of individual organoid/tissue models, liver insult, and preventative measures research, a two-organoid interdependent dual drug response, a three-organoid detection of an unexpected drug undesirable effect, and a liver insult and countermeasure study. The article emphasizes the importance of bioengineered tissues and their supportive microenvironment in achieving high tissue functionality toward the future of the multi-tissue organ-on-chip platform in drug development and toxicity testing. Microchannels are scaffold-like structures that, when combined with suitable cell culture techniques, are ideal for examining vessels. Flexible materials are utilized in organ-on-chip to resemble the mechanical motions peculiar to organs, as flow is a crucial aspect of the technology. Material compatibility alongside the cultural environment and test configurations is essential in organ-on-chip technology, and surface modification procedures can mitigate the impact of the material on the test mixtures. The accomplishment of organ-on-chip technology depends on continued multidisciplinary research to develop new chips, identify suitable materials and microfabrication techniques, and make cost-effective designs available in the market.
However, the success of effective in vitro models such as organ-on-chip technology remains limited, leading to the prevalent utilization of animals for testing drugs for various illnesses and injuries. These models stabilize early hepatocyte activity, allowing researchers to determine if exposing cells to toxins would result in their destruction via various channels [24, 34,35,36]. Such models have proved beneficial in developing drug testing models, researching viral replication of the hepatitis B virus, assessing medicine skin sensitivity, and imitating alcohol harm [39]. However, the current liver-on-chip models do not assess the liver and immunological competence and its influence on medicine rejection, which is crucial in drug development. Notably, drug-induced liver toxicity is the leading cause of the end of medical therapeutic studies and is the primary justification for removing drugs from the market [52]. Incorporating essential immune system characteristics like Kupffer cells into liver simulators would enhance our acquaintance with the root causes of liver disease. Microfluidic organ-on-chip models, accompanied by personalized medicine, are promising for medication screening, toxicity, and disease modeling. Heart-on-chip technology has been utilized within cardiovascular research on the road to developing innovative research methodologies. Researchers have developed surface-textured, elastic polydimethylsiloxane films containing implanted newborn rat cardiomyocytes to examine changes in contractile capability [57]. A microfluidic device with a fibrin matrix has been created to simulate vascular networks, and a micro-organ tissue chip has been developed by merging cardiomyocytes with vascular endothelial cell networks [58, 59]. A cardiac organ platform that matches cardiomyocytes' mechanical and physiological environment has been established, allowing for direct viewing and quantitative evaluation previously unattainable in cell cultures or animal models [61]. One critical component of advancements in cardiovascular medicine research is the reduction of cardiotoxicity, which regularly happens in drug trials and has been a primary reason for clinical trial discontinuation or drug recall from the market. To address this, a functioning micro-engineered cardiac tissue-based pulsating heart on a chip was constructed to forecast hypertrophic alterations in cardiac cells. The technology can produce cardiac microtissues accompanied by better mechanical and electrical connectivity between nearby cells. Brain-on-chip technology is often created using contact printing, 3D printing, mold lithography, and hydrogel casting [66]. Recent advances have focused on developing brain microfluidic simulations in both healthy as well as diseased states, yielding promising results in drug discovery and tailored therapy for neurological illnesses. Models of the blood-brain barrier-on-chip have been built to investigate the function of neuro-inflammation in degenerative diseases such as Alzheimer’s syndromes with antibody trafficking into the central nervous system [68]. Furthermore, multistep lithography has shown beneficial in growing cells in open environments, allowing for experiments on axon regeneration and axon-specific pharmacological therapies. For example, a brain-on-chip idea was developed to investigate the harmful effect of amyloid on neuronal network breakdown. An integrated brain-on-chip system has also been built to research interactions between neurovascular cells and linkages between the brain and organs [72, 73].
A microfluidic technique that includes patient-specific stem cells has been developed to build a personalized brain-blood barrier chip in order to duplicate the blood-brain barrier and to anticipate inter-individual variability. Microfluidics and organ-on-chip technology can enhance our comprehension of pulmonary, renal, skin, as well as gastrointestinal illnesses and treatments. In the case of lung disorders, replicating the intricate alveolar system in a laboratory setting has been challenging, but by using microfluidics, the success of extracorporeal lung models has shown promise. Kidney-on-chip devices have enabled researchers to analyze drug-induced nephrotoxicity, albumin transport, glomerular function, and kidney development. Skin-on-chip technologies are useful for considering numerous aspects of skin biology along with pathology, including inflammation, toxicological research, and wound healing. Gut-on-chip models have permitted the creation of a regulated microenvironment that imitates the human intestine and in-vivo microenvironment. Although breast-on-chip and spleen-on-chip technologies have been created, their capabilities are still limited. By combining organ-on-chip approaches with other technologies, like tumor-on-chip technology, we may enhance the simulation of tumor development in the breast and the microenvironment of bone marrow in vitro. These advancements in microfluidics technology provide a potential method for tailoring drug screening systems and enhancing greater comprehension of the underlying molecular mechanisms of organ function and illness [74, 75, 93,94,95,96,97,98, 106, 107, 114,115,116]. The application of 3D-printed organ-on-chips is growing in popularity, and present research has stated new features and trends in their fabrication. The progress of organ-on-chip models leans on developing efficient and automated initial production processes, modular evolution for creating complex organ-on-chips, and improved personified applications. Developing a multi-organ technique, or body-on-chips, is also a promising application for 3D printing technology, which has shown great potential in amalgamating organs and other operating parts in a modular fashion. Designing standardized interfaces, ensuring reliable chip-to-world connection sealing, and finding suitable sterilization methods are experimental works for 3D-printed organ-on-chips. Numerous potential applications for microfluidic systems have also been shown in assisting 3D bio-printing. Therefore, as a consequence, the combination of microfluidic chips and cell biology has aided in the advancement of multi-organs-on-chip. These systems visualize physiology and disease while reproducing tissue shape and complicated organ function. The developed system’s repeatability, dependability, and sensitivity will be continuously assessed. This method is a proof-of-concept, and further improvements to gene expression and regulation analysis may be necessary.
The study explores the real-world application of the stated technology, going beyond theoretical boundaries. The manuscript extends its exploration beyond theoretical advancements to delve into practical applications where organ-on-a-chip technology has showcased substantial impact. By scrutinizing real-world scenarios, the manuscript provides valuable insights into the transformative potential of organ-on-chip platforms. Examples may include their use in drug development, where precise mimicking of human organ responses aids in the identification and testing of pharmaceutical compounds, ultimately expediting the drug discovery process. Additionally, the application of organ-on-chip technology in disease modeling allows for a nuanced understanding of pathophysiological mechanisms, offering a platform for personalized medicine strategies. Through these practical applications, the manuscript not only elucidates the theoretical strides made in organ-on-chip technology but also underscores its tangible contributions to revolutionizing various facets of biomedical research and healthcare.
7 Challenges
Creating organ-on-chip models presents a hopeful route toward studying brain physiology and disease in a controlled setting and for preclinical drug identification and development. These models can be employed alone or as part of a larger system to examine pathological states in the brain, such as inflammation, infection, traumatic brain injury, degenerative illnesses, brain tumors, and others. However, numerous biological and technical difficulties need to be addressed and solved to make organ-on-chip feasible as preclinical platforms. Some biological issues involve the creation of global media, tissue vascularization, co-culture on various cell types, cell density control, and immune response recapitulation. The technological challenges include drug and biomarker adsorption, sensor module integration, proper oxygenation and nutrition delivery, cell-cell interaction, and viability monitoring. Several fundamental challenges must be overcome before body-on-chip-based technologies be used to their full potential. These include combining many cell types growing for integration inside a single device to mimic organ acts in an imperceptible device, issues caused by PDMS dependence, methods for effective, repeatable microfluidic chip manufacture, etc., and incorporating on-chip sensors.
Despite recent advancements, it has proven difficult to maintain sustainable in vitro blended cultures of more than two or three distinct kinds of cells; hence, finding a universal medium capable of maintaining multicellular networks is essential. While numerous materials containing PMMA, COC, and PEGDA, have been investigated as substitutes for PDMS, every single one has its drawbacks, and new materials need to be studied; scaling organ-on-chip models offers another significant difficulty. While a basic engineering strategy can suffice to mimic the role of some organs, such as merging a peristaltic micropump to resemble pulsatile blood flow, studying drug potential toxicity in the body or acquaintance with syndrome and etiology necessitates vital response between multiple organs and specialized microenvironments. Physiological barriers such as the BBB, skin, GI tract, along lung require different engineering together with perfusion procedures than parenchymal tissues such as fat, kidney, heart, adrenal glands, liver, spleen, and pancreas, which are best modeled utilizing 3D culture approaches. The material used to build multi-organ-on-chip platforms is critical, with PDMS being the most popular in the academic community. However, questions have been expressed concerning its absorbency and compatibility with large-scale fabrication and device commercialization. By simulating in vivo-like tumor microenvironments, the microfluidic organ-on-chip prospect of technology development is promising for screening anticancer drugs, cellular and nanotechnology-based therapies, and optimizing therapeutic conditions. However, optimizing the functional connection of numerous organ systems to precisely simulate drug adsorption, distribution, metabolism, and excretion remains a substantial issue. Organ-on-chip outperforms animal and earlier static models, like spheroids and organoids, by allowing for clinical pharmacokinetics, precise control of cell-culture parameters, and toxicities of drugs. The capacity to modify crucial factors such as concentration gradients and tissue-organ interplays placed organ-on-chip among the World Economic Forum and top ten developing technologies. Technical robustness, on the other hand, remains an issue, and clinical studies take time. Recent breakthroughs in organ-on-chip technology include creating biopolymer-based materials for scalable systems and an effective, integrated microfluidic grade generator for mechanical stimulus and medication administration.
Organ-on-chip can potentially speed up drug development, notably in targeting cancer stem cells, and provide significant insights into multicellular interactions and drug resistance mechanisms. Commercializing prefabricated tumor chips will most likely simplify medical research for cancer therapies. The creation of intricate 3D organ-imitating cell constructions on chips is made possible by 3D bioprinting. However, there are drawbacks and difficulties with current 3D bioprinting technologies, including high costs and low cell survival. Future research may focus on creating fresher 3D bioprinting techniques or merging existing ones to solve these problems. Further developing iPSC-derived cells can also raise the population variety necessary for effective drug screening. Incorporating enhanced molecular reporters, nanoscale biosensors, and superior imaging technologies into microfluidic chips can overcome difficulties in detecting, sampling, and monitoring results. The performance of the chips can be impacted by the biomaterials employed to make them, and the high protein adsorption on the surface of popular materials like PDMS can limit interactions using the cells inside the chip. The collaboration among cells and natural ECM may be impacted by the mechanical qualities that synthetic biomaterials have over native ECM. Furthermore, the lack of human tissue cells, which may necessitate intrusive collecting methods, limits the patient specificity of organ chips. Organ chips can only be cultured for a few weeks at a time, making it impossible to examine long-term consequences. Device design calls for technical expertise, and there is no integration with high-throughput screening methods. Additionally, there are issues with organ chip validation and quality control, which need thorough testing for robustness and reproducibility before moving on to the translational stages. Finally, organ mimetic devices are challenging to adapt and set up due to the expensive and specialized equipment needed to apply mechanical forces and circulate fluid through microfluidic channels.
However, substantial constraints restrict their accuracies, such as removing crucial characteristics that contribute to disease models, physiology, and pathology. Gut-on-chip models, for example, lack supporting cells and tissues seen in the human intestine, creating an inaccurate illustration of the human gut’s complexity and activities. Furthermore, gut-on-chip models have various manufacturing limitations, such as cell line longevity, alternate sensor connectivity, and an alternate material than PDMS. In addition, adding iPSC-derived cells and a mucus layer to gut-on-chip models can increase their functioning. Although liver-on-chip systems have been created to help in drug screening and development, many lack in vivo complexity and suitable cell sources. Primary human hepatocytes are viable for modeling complicated liver illnesses, but their scarcity and donor-to-donor variability offer problems. Human-specific hepatocytes produced out of iPSCs have the potential for personalized medical methods, but their extensive effectiveness and immense manufacturing process, accompanied by great consistency, are still unknown. Although microfluidic technologies may replicate liver zonation via biochemical, oxygen, and hormone gradients, other biological features, such as hepatocyte polarity, must be addressed. Although liver chips can aid in creating bile canalicular networks, bile efflux collection channels are required to prevent cell toxicity. Miniaturization and parallelization can aid in developing low-cost liver analogs with good organotypic. To research the host impact on infections in the microenvironment of a liver chip, hepatocytes must merge cultures with alternative cells along with tissue kinds prevalent in the liver, like immune cells and non-parenchymal cells (NPCs). These NPCs are required for medicinal delivery, pharmacokinetic examination, and disease forming. Immune cells are useful for simulating complicated disease pathways such as hepatitis B microbe contagion, immune-mediated drug-induced liver damage (DILI), and liver fibrosis. Indirect medication toxicity, such as that caused by trovafloxacin and methotrexate, necessitates immune cells and their reactions because they produce hepatocyte destruction and prompt immune-inflammatory cell cytokine release, resulting in fibrotic injury responses.
8 Future perspectives
Drug discovery, disease modeling, and personalized medicine all stand to benefit greatly from organ-on-chip and multi-organs-on-chip systems, but these technological advances are still in the proof-of-concept stage, are challenging to use and construct, and have low throughput. Commercially available organ-on-chip systems are simpler than the latest academic devices, which often exhibit greater complexity. There are limitations on what researchers can do when assembling many chips into multi-organ systems using only one supplier because commercial systems are incompatible. The adoption of organ-on-chip by users would be substantially facilitated by implementing standards in their design and operation, and these requirements can be met via a standard user interface and an open platform instrumentation, data standards, compound sets for model validation, technology interfacing, and form factors are all examples of areas where standardization is applicable.
A broadly embraced standardized approach will make transferring new gadgets and features from creators to consumers easier, hasten commercialization, and promote innovation. The technical elements of the systems for organs on chips and microfluidic operation are principally the topic of this viewpoint review. To initiate the process of achieving this equilibrium evolutionarily and cooperatively, a proposal has been put forward to explore an open platform approach that incorporates standardized interface communication. This idea is currently under consideration and being carefully considered. Additionally, it would offer a standardized and automated method of running devices together with a database, making it simpler to test models quickly without needing device-specific control and readout systems. Bubbles are introduced to cellular integrity while attaching organ-on-chips to microfluidic tubing for system perfusion proliferation and jeopardize system sterility. Aside from these technological challenges, the application of organ-on-chip technology in medication research and testing is a challenge that must be solved. The technique must be thoroughly examined before any regulative body can approve it as an effective preclinical strategy for evaluating freshly manufactured medicines. To do this, researchers, regulators, and industry must collaborate closely. Creating a body on a chip necessitates considering inter-organ scaling, shared medium and flow rate, as well as the interdependent operation of several organs. Even though this technology is utilized to depict important organs, relatively little research has been done on other organs, such as adipose tissue, the retina, and the placenta. A recent study has found a link between the microbiome and host metabolism, emphasizing the necessity of integrating organ-on-chip technology and microbiome research. Regulative bodies should establish standards for verifying this technology for various uses, including creating disease models. High throughput organ-on-chip technology must be developed to speed up the screening procedure.
Model parallelization, standardized and adaptable platforms, assurance, automated processes, and online data analysis are all critical issues for designing high-throughput organ-on-chip models. According to recent research, North America is expected to have the largest share (49%) of the organ-on-chip technology market as it transitions from 2D to 3D cell cultures. The key segments that might gain from this technology include pharmaceutical firms, biotechnology corporations, and academic and research institutes. The growing number of cell-based therapy clinical examinations will drive global growth in pharmaceutical/biotechnology research funding and the organ-on-chip sector. Newer systems have improved functionalities, fusion, automation, production, and individualized precision medicine to be compatible with ordinary biological laboratory studies and the pharmaceutical business. Future organ-on-chip systems must enhance and standardize the production process and improve automation, high throughput, and parallel operation. Human-derived materials, such as decellularized ECM and human tissues, will be employed in personalized precision medicine. Utilizing induced patient-specific stem cells with pluripotent properties and drug screening will allow for the production of patient-specific organ chips for individualized disease modeling. Machine learning may also help analyze massive amounts of data obtained from organ-on-chip platforms. Gravity-driven or auto-perfusion systems, paper-based cell culture platforms, and magnetic levitation for cell separation/isolation/characterization are other viable alternatives. The primary goal is to develop a body-on-chip system allowing patient-specific illness inquiry and medication screening across multiple or all organs. It is difficult to find high-quality human cells, but the availability of human-specified driven pluripotent stem cells and organoids has made it easier. The restricted throughput of most organ chips makes statistically significant tests with many copies difficult, while the advent of higher-throughput organ chips or automated devices could overcome this problem. Though using established cell lines is not ideal, it may be advantageous in some circumstances. The idea that the PDMS material utilized to produce several organ chips would be inefficient for drug research has not proven to be as problematic as previously feared. The most challenging issue is conceptual because many researchers hesitate to change their practices. However, with organizations like the IQ Consortium and the FDA, benchmarks are being established to confirm the usage of humankind organ chips as animal alternatives and initial improvements in this field are being seen.
9 Summary
The current paper delves into organ-on-chip technology, covering its problems, manufacturing procedures, and several organ models. Researchers have successfully developed over the past 10 years organ-on-chip models for several different organs, including the kidney, blood-brain barrier, liver, muscle, heart, neuron, lung, gut, skin, and bone marrow. These models examine how fluid flow, shear stress, oxygen, and growth affect each other factor gradients on cell function. The study emphasizes the benefits of organ-on-chip technology, including its ability to effectively reproduce disease states, enhance drug screening efficiency, eliminate animal testing, and give a more reliable technique for examining the impact of drugs and illnesses on human tissues. Interdisciplinary collaboration and competence in biology, physiology, microfluidics, materials science, and clinical knowledge are required to create model organs on chips. Accurately recreating the 3D structure and cellular microenvironment is crucial for developing realistic organ and disease models on a chip. Cost-effective manufacturing technologies are needed to produce versatile microfluidic cell culture chips in bulk, allowing wider access and adoption in biology labs. The development of diagnosis and therapy methods through the use of organs on chips offers promise for illness research and reduces the reliance on animal testing. However, challenges remain in accurately reproducing micro-physiological conditions. Integrating multiple tissues into a multi-organ platform has shown promise in studying complex interactions and developing drug development and toxicity testing applications. Material compatibility, surface modifications, and microfabrication techniques play a significant role in organ-on-chip design. Models with a heart and liver on chips have shown potential in drug testing and studying cardiotoxicity. Lung, kidney, skin, and gut-on-chip technologies have also offered insights into numerous diseases and therapies, while brain-on-chip models have been employed for neurological research and drug discovery. Models of organs on chips are being created using 3D printing, and attempts are still being made to enhance the manufacturing process and develop modular and customized systems. The advancement of organ-on-chip technology offers new possibilities for studying organ function, disease mechanisms, and personalized medicine.
Data availability
No data is available.
References
Engle SJ, Puppala D (2013) Integrating human pluripotent stem cells into drug development. Cell Stem Cell 12(6):669–677. https://doi.org/10.1016/j.stem.2013.05.011
Huh D, Hamilton GA, Ingber DE (2011) From 3D cell culture to organs-on-chips. Trends Cell Biol 21(12):745–754. https://doi.org/10.1016/j.tcb.2011.09.005
van der Meer AD, van den Berg A (2012) Organs-on-chips: breaking the in vitro impasse. Integr Biol 4(5):461–470. https://doi.org/10.1039/c2ib00176d
Brownell L (2018) FDA expands award to Wyss Institute for radiation treatment studies using Organ Chips, Wyss Institute
Goldstein Y, Spitz S, Turjeman K, Selinger F, Barenholz Y, Ertl P, Benny O, Bavli D (2021) Breaking the third wall: implementing 3D-printing techniques to expand the complexity and abilities of multi-organ-on-a-chip devices. Micromachines 2(6):627. https://doi.org/10.3390/mi12060627
Boeri L, Izzo L, Sardelli L, Tunesi M, Albani D, Giordano C (2019) Advanced organ-on-a-chip devices to investigate liver multi-organ communication: focus on gut, microbiota and brain. Bioeng 6(4):91. https://doi.org/10.3390/bioengineering6040091
Giese C, Lubitz A, Demmler CD, Reuschel J, Bergner K, Marx U (2010) Immunological substance testing on human lymphatic micro-organoids in vitro. J Biotechnol 148(1):38–45. https://doi.org/10.1016/j.jbiotec.2010.03.001
Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE (2010) Reconstituting organ-level lung functions on a chip. Science 328(5986):1662–1668. https://doi.org/10.1126/science.1188302
Baker M (2011) A living system on a chip. Nature 471:661–665. https://doi.org/10.1038/471661a
Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatta D, Coskun AU, Feldman CL, Wagner DD, Ingber DE (2012) Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science 337(6095):738–742. https://doi.org/10.1126/science.1217815
Weibel DB, Whitesides GM (2006) Applications of microfluidics in chemical biology. Curr Opin Chem Biol 10(6):584–591. https://doi.org/10.1016/j.cbpa.2006.10.016
Luni C, Feldman HC, Pozzobon M, De Coppi P, Meinhart CD, Elvassore N (2010) Microliter-bioreactor array with buoyancy-driven stirring for human hematopoietic stem cell culture. Biomicrofluidics 4(3):034105. https://doi.org/10.1063/1.3380627
Schwarz US, Bischofs IB (2005) Physical determinants of cell organization in soft media. Med Eng Phys 27(9):763–772. https://doi.org/10.1016/j.medengphy.2005.04.007
Richardson L et al (2020) Fetal Membrane Organ-On-Chip: An Innovative Approach to Study Cellular. Interactions. 27(8):62–69. https://doi.org/10.1007/s43032-020-00184-9
Mummery C, Wilmut I, van de Stolpe A, Roelen BAJ (2011) Stem cells, scientific facts, and fiction. Elsevier Books
van de Stolpe A, den Toonder J (2013) Workshop meeting report Organs-on-chips: human disease models. Lab Chip 13:3449–3470. https://doi.org/10.1039/C3LC50248A
Barker N, Clevers H (2010; Chapter 5: Unit5A) Lineage tracing in the intestinal epithelium. Curr Protoc Stem Cell Biol. https://doi.org/10.1002/9780470151808.sc05a04s13
Snippert HJ, Schepers AG, Delconte G, Siersema PD, Clevers H (2011) Slide preparation for single-cell-resolution imaging of fluorescent proteins in their three-dimensional near-native environmen. Nat Protoc 6(8):1221–1228. https://doi.org/10.1038/nprot.2011.365
Kim J, Hayward RC (2012) Mimicking dynamic in vivo environments with stimuli-responsive materials for cell culture. Trends Biotechnol 30:426–439. https://doi.org/10.1016/j.tibtech.2012.04.003
Singh D, Mathur A, Arora S, Roy S, Mahindroo N (2022) Journey of organ on a chip technology and its role in future healthcare scenario. Appl Surf Sci 9:100246. https://doi.org/10.1016/j.apsadv.2022.100246
Danku AE, Dulf EH, Braicu C, Jurj A, Neagoe LB (2022) Organ-on-a-chip: a survey of technical results and problems. Front Bioeng Biotechnol 10. https://doi.org/10.3389/fbioe.2022.840674
Ronaldson-Bouchard K, Vunjak-Novakovic G (2018) Organs-on-a-chip: a fast track for engineered human tissues in drug development. Cell Stem Cell 22(3):310–324. https://doi.org/10.1016/j.stem.2018.02.011
Zamprogno P, Wüthrich S, Achenbach S (2021) Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun Biol 4:168. https://doi.org/10.1038/s42003-021-01695-0
Lee PJ, Hung PJ, Lee LP (2007) An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol Bioeng 97:1340–1346. https://doi.org/10.1002/bit.21360
Lee J, Kim S (2018) Kidney-on-a-Chip: a new technology for predicting drug efficacy, interactions, and drug-induced nephrotoxicity. Curr Drug Metab 19(7):577–583. https://doi.org/10.2174/1389200219666180309101844
Liu H, Bolonduro OA, Ning Hu JJ, Rao AA, Duffy BM, Huang Z, Black LD, Timko BP (2020) Heart-on-a-Chip model with integrated extra and intracellular bioelectronics for monitoring cardiac electrophysiology under acute hypoxia. Nano Lett 20:2585–2593. https://doi.org/10.1021/acs.nanolett.0c00076
Lukacs B, Bajza A, Kocsis D, Csorba A, Antal I, Ivan K, Laki AJ, Erdo F (2019) Skin-on-a-Chip device for ex vivo monitoring of transdermal delivery of drugs-design, fabrication, and testing. Pharmaceutics 11(9):445. https://doi.org/10.3390/pharmaceutics11090445
Raimondi L, Izzo L, Tunesi M, Comar M, Albani D, Giordano C (2020) Organ-on-a-chip in vitro models of the brain and the blood-brain barrier and their value to study the microbiota-gut-brain axis in neurodegeneration. Front Bioeng Biotechnol 10(7):435. https://doi.org/10.3389/fbioe.2019.00435
Dalsbecker P, Adiels CB, Goksör M (2022) Liver-on-a-chip devices: the pros and cons of complexity. Am J Physiol Gastrointest Liver Physiol 323(3):188–204. https://doi.org/10.1152/ajpgi.00346.2021
Fraczek J, Bolleyn J, Vanhaecke T, Rogiers V, Vinken M (2013) Primary hepatocyte cultures for pharmaco-toxicological studies: at the busy crossroad of various antidedifferentiation strategies. Arch Toxicol 87(4):577–610. https://doi.org/10.1007/s00204-012-0983-3
LeCluyse EL (2001) Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci 13(4):343–368. https://doi.org/10.1016/s0928-0987(01)00135-x
Mccuskey RS (2008) The hepatic microvascular system in health and its response to toxicants. Anat Rec 291(6):661–671. https://doi.org/10.1002/ar.20663
Kane BJ, Zinner MJ, Yarmush ML, Toner M (2006) Liver-specific functional studies in a microfluidic array of primary mammalian hepatocytes. Anal Chem 78:4291. https://doi.org/10.1021/ac051856v
Ho CT, Lin RZ, Chen RJ, Chin CK, Gong SE, Chang HY, Peng HL, Hsu L, Yew TR, Chang SF (2013) Liver-cell patterning lab chip: mimicking the morphology of liver lobule tissue. Lab Chip 13:3578–3587. https://doi.org/10.1039/c3lc50402f
Yum K, Hong SG, Healy KE, Lee LP (2014) Physiologically relevant organs on chips. Biotechnol J 9(1):16–27. https://doi.org/10.1002/biot.201300187
Kang YBA, Sodunke TR, Lamontagne J, Cirillo J, Rajiv C, Bouchard MJ, Noh M (2015) Liver sinusoid on a chip: long term layered coculture of primary rat hepatocytes and endothelial cells in microfluidic platforms. Biotechnol Bioeng 112(12):2571–2582. https://doi.org/10.1002/bit.25659
Ma LD, Wang YT, Wang JR, Wu JL, Meng XS, Hu P, Mu X, Liang QL, Luo GA (2018) Design and fabrication of a liver on- a-chip platform for convenient, highly efficient, and safe in situ perfusion culture of 3D hepatic spheroids. Lab Chip 18:2547–2562. https://doi.org/10.1039/c8lc00333e
Lu S, Cuzzucoli F, Jiang J, Liang LG, Wang Y, Kong M, Zhao X, Cui W, Li J, Wang S (2018) Development of a biomimetic liver tumor-on-a-chip model based on decellularized liver matrix for toxicity testing. Lab Chip 18:3379–3392. https://doi.org/10.1039/c8lc00852c
Chong LH, Li H, Wetzel I, Cho H, Toh YC (2018) A liver-immune coculture array for predicting systemic drug-induced skin sensitization. Lab Chip 18:3239–3250. https://doi.org/10.1039/c8lc00790j
Zhou Q, Patel D, Kwa T, Haque A, Matharu Z, Stybayeva G, Gao Y, Diehl AM, Revzin A (2015) Liver injury-on-a-chip: microfluidic cocultures with integrated biosensors for monitoring liver cell signaling during injury. Lab Chip 15:4467–4478. https://doi.org/10.1039/C5LC00874C
Schütte J, Hagmeyer B, Holzner F, Kubon M, Werner S, Freudigmann C, Benz K, Böttger J, Gebhardt R, Becker H, Stelzle M (2011) Artificial micro-organs—a microfluidic device for dielectrophoretic assembly of liver sinusoids. Biomed Microdevices 13(3):493–501. https://doi.org/10.1007/s10544-011-9517-7
Domansky K, Inman W, Serdy J, Dash A, Lim MHM, Griffith LG (2010) Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 10:51–58. https://doi.org/10.1039/B913221J
van Midwoud PM, Verpoorte E, Groothuis GMM (2011) Microfluidic devices for vitro studies on liver drug metabolism and toxicity. Integr Boil 3(5):509–521. https://doi.org/10.1039/c0ib00119h
Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, Upadhyaya A, Kurzawski P, Wack KE, Stolz DB, Kamm R, Griffith LG (2002) A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng 78(3):257–269. https://doi.org/10.1002/bit.10143
Khetani SR, Bhatia SN (2008) Microscale culture of human liver cells for drug development. Nat Biotechnol 26:120–126. https://doi.org/10.1038/nbt1361
Lee SA, No DY, Kang E, Ju J, Kim DS, Lee SH (2013) Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte-hepatic stellate cell interactions and flow effects. Lab Chip 13(18):3529–3537. https://doi.org/10.1039/C3LC50197C
Prodanov L, Jindal R, Bale SS, Hegde M, McCarty WJ, Golberg I, Bhushan A, Yarmush ML, Usta OB (2016) Long-term maintenance of a microfluidic 3D human liver sinusoid: maintenance of a microfluidic 3D human liver sinusoid. Biotechnol Bioeng 113(1):241–246. https://doi.org/10.1002/bit.25700
Essaouiba A, Okitsu T, Kinoshita R, Jellali R, Shinohara M, Danoy M, Legallais C, Sakai Y, Leclerc E (2020) Development of a pancreas-liver organ-on-chip coculture model for organ-to-organ interaction studies. Biochem Eng J 164:107783. https://doi.org/10.1016/j.bej.2020.107783
Polidoro MA, Ferrari E, Marzorati S, Lleo A, Rasponi M (2021) Experimental liver models: From cell culture techniques to microfluidic organs-on-chip. Liver Int 41(8):1744–1761. https://doi.org/10.1111/liv.14942
Kanabekova P, Kadyrova A, Kulsharova G (2022) Microfluidic organ-on-a-chip devices for liver disease modeling in vitro. Micromachines (Basel) 13(3):428. https://doi.org/10.3390/mi13030428
Moradi E, Jalili-Firoozinezhad S, Solati-Hashjin M (2020) Microfluidic organ-on-a-chip models of human liver tissue. Acta Biomater 116:67–83. https://doi.org/10.1016/j.actbio.2020.08.041
Chen M, Vijay V, Shi Q, Liu Z, Fang H, Tong W (2011) FDA-approved drug labeling for the study of drug-induced liver injury. Drug Discov Today 16(15-16):697–703. https://doi.org/10.1016/j.drudis.2011.05.007
World Health Organization. Cardiovascular diseases (CVDs). Available from: http://www.who.int/mediacentre/factsheets/fs317/en/
Liu Y, Lin L, Qiao L (2023) Recent developments in organ-on-a-chip technology for cardiovascular disease research. Anal Bioanal Chem. https://doi.org/10.1007/s00216-023-04596-9
Reinecke M (1985) Neurotensin. Immunohistochemical localization in central and peripheral nervous system and endocrine cells and its functional role as neurotransmitter and endocrine hormone. Prog Histochem Cytochem 16(1):1–172
Ribas J, Sadeghi H, Manbachi A, Leijten J, Brinegar K, Zhang YS, Ferreira L, Khademhosseini A (2016) Cardiovascular organ-on-a-chip platforms for drug discovery and development. Appl In Vitro Toxicol 2(2):82–96. https://doi.org/10.1089/aivt.2016.0002
Grosberg A, Nesmith AP, Goss JA, Brigham MD, McCain ML, Parker KK (2012) Muscle on a chip: in vitro contractility assays for smooth and striated muscle. J Pharmacol Toxicol Methods 65:126–135. https://doi.org/10.1016/j.vascn.2012.04.001
Kim S, Lee H, Chung M, Jeon NL (2013) Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13(8):1489–1500. https://doi.org/10.1039/C3LC41320A
Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell’Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A, Khademhosseini A (2016) Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 110:45–59. https://doi.org/10.1016/j.biomaterials.2016.09.003s
Xiao Y, Zhang B, Liu H, Miklas JW, Gagliardi M, Pahnke A, Thavandiran N, Sun Y, Simmons C, Keller G, Radisic M (2014) Microfabricated perfusable cardiac biowire: a platform that mimics native cardiac bundle. Lab Chip 14(5):869–882. https://doi.org/10.1039/c3lc51123e
Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, Votta E, Cerino G, Redaelli A, Rasponi M (2016) Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 16:599–610. https://doi.org/10.1039/c5lc01356a
Zhang X, Wang T, Wang P, Hu N (2016) High-throughput assessment of drug cardiac safety using a high-speed impedance detection technology-based heart-on-a-chip. Micromachines (Basel) 7(7):122. https://doi.org/10.3390/mi7070122
Nguyen MD, Tinney JP, Ye F, Elnakib AA, Yuan F, El-Baz A, Sethu P, Keller BB, Giridharan GA (2015) Effects of physiologic mechanical stimulation on embryonic chick cardiomyocytes using a microfluidic cardiac cell culture model. Anal Chem 87(4):2107–2113. https://doi.org/10.1021/ac503716z
Schneider O, Zeifang L, Fuchs S, Sailer C, Loskill P (2019) User-friendly and parallelized generation of human induced pluripotent stem cell-derived microtissues in a centrifugal heart-on-a-chip. Tissue Eng Part A 25:786–798. https://doi.org/10.1089/ten.TEA.2019.0002
Abulaiti M, Yalikun Y, Murata K, Sato A, Sami MM, Sasaki Y, Fujiwara Y, Minatoya K, Shiba Y, Tanaka Y, Masumoto H (2020) Establishment of a heart-on-a-chip microdevice based on human iPS cells for the evaluation of human heart tissue function. Sci Rep 10:19201. https://doi.org/10.1038/s41598-020-76062-w
Haring AP, Johnson BN (2020) Brain-on-a-chip systems for modeling disease pathogenesis. Organ-on-a-Chip:215–232. https://doi.org/10.1016/B978-0-12-817202-5.00006-1
Amirifar L, Shamloo A, Nasiri R, de Barros NR, Wang ZZ, Unluturk BD, Libanori A, Ievglevskyi O, Diltemiz SE, Sances S, Balasingham L, Seidlits SK, Ashammakhi N (2022) Brain-on-a-chip: recent advances in design and techniques for microfluidic models of the brain in health and disease. Biomaterials 285:121531. https://doi.org/10.1016/j.biomaterials.2022.121531
Wevers NR, Kasi DG, Gray T, Wilschut KJ, Smith B, van Vught R, Shimizu F, Sano Y, Kanda T, Marsh G, Trietsch SJ, Vulto P, Lanz HL, Obermeier B (2018) A perfused human blood-brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 15(23). https://doi.org/10.1186/s12987-018-0108-3
Bang S, Jeong S, Choi N, Kim HN (2019) Brain-on-a-chip: a history of development and future perspective. Biomicrofluidics 13:051301. https://doi.org/10.1063/1.5120555
Park J, Lee BK, Jeong GS, Hyun JK, Lee CJ, Lee SH (2015) Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer’s disease. Lab Chip 15:141–150. https://doi.org/10.1039/c4lc00962b
Kilic O, Pamies D, Lavell E, Schiapparelli P, Feng Y, Hartung T, Bal-Price A, Hogberg HT, Quinones-Hinojosa A, Guerrero-Cazares H, Levchenko A (2016) Brain-on-a-chip model enables analysis of human neuronal differentiation and chemotaxis. Lab Chip 16:4152–4162. https://doi.org/10.1039/c6lc00946h
Maoz BM, Herland A, Fitzgerald EA, Grevesse T, Vidoudez C, Pacheco AR, Sheehy SP, Park TE, Dauth S, Mannix R, Budnik N, Shores K, Cho A, Nawroth JC, Segre D, Budnik B, Ingber DE, Parker KK (2018) A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat Biotechnol 36:865–874. https://doi.org/10.1038/nbt.4226
Oleaga C, Bernabini C, Smith AST, Srinivasan B, Jackson M, Mclamb W, Platt V, Bridges R, Cai Y, Santhanam N, Berry B, Najjar S, Akanda N, Guo X, Martin C, Ekman G, Esch MB, Langer J, Ouedraogo G et al (2016) Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep 6:20030. https://doi.org/10.1038/srep20030
Guenat OT, Berthiaume F (2018) Incorporating mechanical strain in organs-on-a-chip: lung and skin. Biomicrofluidics 2:42207. https://doi.org/10.1063/1.5024895
Li K, Yang X, Xue C, Zhao L, Zhang Y, Gao X (2019) Biomimetic human lung-on-a-chip for modeling disease investigation. Biomicrofluidics 13(3):031501. https://doi.org/10.1063/1.5100070
Stucki AO, Stucki JD, Hall SRR, Felder M, Mermoud Y, Schmid RA, Geiser T, Guenat OT (2015) A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip 15:1302–1310. https://doi.org/10.1039/c4lc01252f
Humayun M, Chow CW, Young EWK (2018) Microfluidic lung airway-on-a-chip with array able suspended gels for studying epithelial and smooth muscle cell interactions. Lab Chip 18:1298–1309. https://doi.org/10.1039/c7lc01357d
Blume C, Reale R, Held M, Millar TM, Collins JE, Davies DE, Morgan H, Swindle EJ (2015) Temporal monitoring of differentiated human airway epithelial cells using microfluidics. PLoS One 10:e0139872. https://doi.org/10.1371/journal.pone.0139872
Yang X, Li K, Zhang X, Liu C, Guo B, Wen W, Gao X (2018) Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip 18:486–495. https://doi.org/10.1039/c7lc01224a
Peng J, Rochow N, Dabaghi M, Bozanovic R, Jansen J, Predescu D, DeFrance B, Lee SY, Fusch G, Selvaganapathy PR, Fusch C (2018) Postnatal dilatation of umbilical cord vessels and its impact on wall integrity: prerequisite for the artificial placenta. Int J Artif Organs 41(7):393–399. https://doi.org/10.1177/0391398818763663
Dabaghi M, Fusch G, Saraei N, Rochow N, Brash JL, Fusch C, Selvaganapathy PR (2018) An artificial placenta type microfluidic blood oxygenator with double-sided gas transfer microchannels and its integration as a neonatal lung assist device. Biomicrofluidics 12(4):44101. https://doi.org/10.1063/1.5034791
Xu Z, Gao Y, Hao Y, Li E, Wang Y, Zhang J, Wang W, Gao Z, Wang Q (2013) Application of a microfluidic chip-based 3D coculture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials 34:4109–4117. https://doi.org/10.1016/j.biomaterials.2013.02.045
Wang D, Cong Y, Deng Q, Han X, Zhang S, Zhao L, Luo Y, Zhang X (2021) Physiological and disease models of respiratory system based on organ-on-a-chip technology. Micromachines 12:1106. https://doi.org/10.3390/mi12091106
Kimura H, Sakai Y, Fujii T (2018) Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab Pharmacokinet 33:43–48. https://doi.org/10.1016/j.dmpk.2017.11.003
Si L, Bai H, Rodas M, Cao W, Oh CY, Jiang A, Moller R, Hoagland D, Oishi K, Horiuchi S, Uhl S, Blanco-Melo D, Albrecht RA, Liu W-C, Jordan T, Nilsson-Payant BE, Golynker I, Frere J, Logue J et al (2021) A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nat Biomed Eng 5(8):815–829
Khalida MAU, Kima YS, Alia M, Leeb BG, Choc YJ, Choi KH (2020) A lung cancer-on-chip platform with integrated biosensors for physiological monitoring and toxicity assessment. Biochem Eng J 155:107469. https://doi.org/10.1016/j.bej.2019.107469
Lu RXZ, Radisic M (2021) Organ-on-a-chip platforms for evaluation of environmental nanoparticle toxicity. Bioactive Mater 6:2801–2819. https://doi.org/10.1016/j.bioactmat.2021.01.021
Jang KJ, Suh KY (2010) A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 10:36–42. https://doi.org/10.1039/b907515a
Bhatia SN, Ingber DE (2014) Microfluidic organs-on-chips. Nat Biotechnol 32:760–772. https://doi.org/10.1038/nbt.2989
Paoli R, Samitier J (2016) Mimicking the kidney: a key role in organ-on-chip development. Micromachines (Basel) 7(7):126. https://doi.org/10.3390/mi7070126
Jang KJ, Suh KY (2010) A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 10:36–42. https://doi.org/10.1039/b907515a
Jang KJ, Mehr AP, Hamilton GA, Mcpartlin LA, Chung S, Suh KY, Ingber DE (2013) Human kidney proximal tubule on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol (Camb) 5:1119–1129. https://doi.org/10.1039/c3ib40049b
Wilmer MJ, Ng CP, Lanz HL, Vulto P, Suter-Dick L, Masereeuw R (2016) Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol 34(2):156–170. https://doi.org/10.1016/j.tibtech.2015.11.001
Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, Fatanat-Didar T, Koshy S, Weaver JC, Church GM, Ingber DE (2017) Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng 1. https://doi.org/10.1038/s41551-017-0069
Friedrich C, Endlich N, Kriz W, Endlich K (2006) Podocytes are sensitive to fluid shear stress in vitro. Am J Physiol Renal Physiol 291:856–865. https://doi.org/10.1152/ajprenal.00196.2005
Schutgens F, Rookmaaker MB, Margaritis T, Rios A, Ammerlaan C, Jansen J, Gijzen L, Vormann M, Vonk A, Viveen M et al (2019) Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol 37:303–313. https://doi.org/10.1038/s41587-019-0048-8
Sakolish CM, Philip B, Mahler GJ (2019) A human proximal tubule-on-a-chip to study renal disease and toxicity. Biomicrofluidics 13:14107. https://doi.org/10.1063/1.5083138
Nieskens TTG, Sjögren AK (2019) Emerging in vitro systems to screen and predict drug-induced kidney toxicity. Semin Nephrol 39:215–226. https://doi.org/10.1016/j.semnephrol.2018.12.009
Musah S, Dimitrakakis N, Camacho DM, Church GM, Ingber DE (2018) Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a glomerulus chip. Nat Protoc 13:1662–1685. https://doi.org/10.1038/s41596-018-0007-8
Salmon JK, Armstrong CA, Ansel JC (1994) The skin as an immune organ. West J Med 160:146–152
Ponmozhi J, Dhinakaran S, Varga-Medveczky Z, Fonagy K, Bors LA, Ivan K, Erdo F (2021) Development of Skin-On-A-Chip platforms for different utilizations: factors to be considered. Micromachines (Basel) 12:294. https://doi.org/10.3390/mi12030294
Kim J, Kim K, Sung GY (2020) Coenzyme Q10 efficacy test for human skin equivalents using a pumpless Skin-on-a-Chip system. Int J Mol Sci 21(22). https://doi.org/10.3390/ijms21228475
Matejuk A (2018) Skin Immunity. Arch Immunol Ther Exp 66:45–54. https://doi.org/10.1007/s00005-017-0477-3
B.S. Baker, J.M. Ovigne, A.V. Powles, S. Corcoran, L. Fry, Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis, Br J Dermatol,148(4) (2003) 670–679. https://doi.org/10.1046/j.1365-2133.2003.05287. x.
Sutterby E, Thurgood P, Baratchi S, Khoshmanesh K, Pirogova E (2020) Microfluidic Skin-on-a-chip models: toward biomimetic artificial skin. Small 16(39):2002515. https://doi.org/10.1002/smll.202002515
O’Neill AT, Monteiro-Riviere NA, Walker GM (2008) Characterization of microfluidic human epidermal keratinocyte culture. Cytotechnology 56(3):197–207. https://doi.org/10.1007/s10616-008-9149-9
Sriram G, Alberti M, Dancik Y, Wu B, Wu R, Feng Z, Ramasamy S, Bigliardi PL, Bigliardi QM, Wang Z (2018) Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater Today 21(4):326–340. https://doi.org/10.1016/j.mattod.2017.11.002
Abaci HE, Guo Z, Coffman A, Gillette B, Lee WH, Sia SK, Christiano AM (2016) Human skin constructs with spatially controlled vasculature using primary and iPSC-derived endothelial cells. Adv Healthc Mater 5:1800–1807. https://doi.org/10.1002/adhm.201500936
Mori N, Morimoto Y, Takeuchi S (2017) Skin integrated with perfusable vascular channels on a chip. Biomaterials 116:48–56. https://doi.org/10.1016/j.biomaterials.2016.11.031
Wufuer M, Lee G, Hur W, Jeon B, Kim BJ, Choi TH, Lee S (2016) Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci Rep 6:37471. https://doi.org/10.1038/srep37471
Ramadan Q, Ting FCW (2016) In vitro micro-physiological immune-competent model of the human skin. Lab Chip 16:1899–1908. https://doi.org/10.1039/C6LC00229C
Sung JH, Yu J, Luo D, Shuler ML, March JC (2011) Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 11:389–392. https://doi.org/10.1039/c0lc00273a
Thomas DP, Zhang J, Nguyen NT, Ta HT (2023) Microfluidic gut-on-a-chip: fundamentals and challenges. Biosensors 13:136. https://doi.org/10.3390/bios13010136
Y. Xiang, H. Wen, Y. Yu, M. Li, X. Fu, S. Huang, Gut-on-chip: recreating human intestine in vitro, J. Tissue Eng., 11 (2020), 2041731420965318. https://doi.org/10.1177/2041731420965318.
Yeon JH, Park JK (2009) Drug permeability assay using microhole-trapped cells in a microfluidic device. Anal Chem 81(5):1944–1951. https://doi.org/10.1021/ac802351w
Villenave R, Wales SQ, Hamkins-Indik T, Papafragkou E, Weaver JC, Ferrante TC, Bahinski A, Elkins CA, Kulka M, Ingber DE (2017) Human Gut-on-a-Chip supports polarized infection of Coxsackie B1 virus in vitro. PLoS One 12:e0169412. https://doi.org/10.1371/journal.pone.0169412
Mahler GJ, Esch MB, Glahn RP, Shuler ML (2009) Characterization of a gastrointestinal tract microscale cell culture analog used to predict drug toxicity. Biotechnol Bioeng 104(1):193–205. https://doi.org/10.1002/bit.22366
McDermott AJ, Huffnagle GB (2014) The microbiome and regulation of mucosal immunity. Immunology 142(1):24–31. https://doi.org/10.1111/imm.12231
Kimura H, Yamamoto T, Sakai H, Sakai Y, Fujii T (2008) An integrated microfluidic system for long-term perfusion culture and on-line monitoring of intestinal tissue models. Lab Chip 8:741–746. https://doi.org/10.1039/B717091B
Kim HJ, Huh D, Hamilton G, Ingber DE (2012) Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12:2165–2174. https://doi.org/10.1039/C2LC40074J
Kim HJ, Ingber DE (2013) Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol 5(9):11–30. https://doi.org/10.1039/c3ib40126j
Shim KY, Lee D, Han J, Nguyen NT, Park S, Sung JH (2017) Microfluidic gut-on-a-chip with three-dimensional villi structure. Biomed Microdevices 19(2):37. https://doi.org/10.1007/s10544-017-0179-y
Chou DB, Frismantas V, Milton Y et al (2020) On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat Biomed Eng 4(4):394–406. https://doi.org/10.1038/s41551-019-0495-z
Yu VWC, Scadden DT (2016) Hematopoietic stem cell and its bone marrow niche. Curr Top Dev Biol 118:21–44. https://doi.org/10.1016/bs.ctdb.2016.01.009
Torisawa Y, Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T, Collins JJ, Ingber DE (2014) Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat Methods 11:663–669. https://doi.org/10.1038/nmeth.2938
Mancini V, Pensabene V (2019) Organs-on-chip models of the female reproductive system. Bioengineering 6(4):103. https://doi.org/10.3390/bioengineering6040103
Wei-Xuan LI, Liang GT, Wei YAN, Zhang Q, Wei WANG, Xiao-Mian ZHOU, Da-Yu LIU (2013) Artificial uterus on a microfluidic chip. Chin J Anal Chem 41(4):467–472. https://doi.org/10.1016/S1872-2040(13)60639-8
Blundell C, Tess ER, Schanzer AS, Coutifaris C, Su EJ, Parry S, Huh DA (2016) A microphysiological model of the human placental barrier. Lab Chip 16:3065–3073. https://doi.org/10.1039/C6LC00259E
Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong JS, Huh D (2016) Placenta- on-a-chip: A novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med 29(7):1046–1054. https://doi.org/10.3109/14767058.2015.1038518
Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, Malpani SS, Arnold-Murray CA, Chen K, Jiang M, Bai L, Nguyen CT, Zhang J, Laronda MM, Hope TJ, Maniar KP, Pavone ME, Avram MJ, Sefton EC et al (2017) A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 8:14584. https://doi.org/10.1038/ncomms14584
Norwirz ER, Schust DJ, Fisher SJ (2001) Implantation and the survival of early pregnancy, N. Engl J Med 345(19):1400–1408. https://doi.org/10.1056/NEJMra000763
Whisler JA, Chen MB, Kamm RD (2014) Control of perfusable microvascular network morphology using a multiculture microfluidic system. Tissue Eng C Methods 20:543–552. https://doi.org/10.1089/ten.tec.2013.0370
Gude NM, Roberts CT, Kalionis B, King RG (2004) Growth and function of the normal human placenta. Thromb Res 114:397–407. https://doi.org/10.1016/j.thromres.2004.06.038
Park YK, Tu TY, Lim SH, Clement IJM, Yang SY, Kamm RD (2014) In vitro microvessel growth and remodeling within a three-dimensional microfluidic environment. Cell Mol Bioeng 7:15–25. https://doi.org/10.1007/s12195-013-0315-6
Abbas Y, Oefner CM, Polacheck WJ, Gardner L, Farrell L, Sharkey A, Kamm R, Moffett A, Oyen ML (2017) A microfluidics assay to study invasion of human placental trophoblast cells. J R Soc Interface 14. https://doi.org/10.1098/rsif.2017.0131
Rodrigues RO, Sousa P, Rocha LA, Du J, Lima R, Minas G (2020) Organ-on-a-chip: a preclinical microfluidic platform for the progress of nanomedicine. Small 16(51):2003517. https://doi.org/10.1002/smll.202003517
Palaninathan V, Kumar V, Maekawa T, Liepmann D, Paulmurugan R, Eswara JR, Ajayan PM, Augustine S, Malhotra BD, Viswanathan S, Renugopalakrishnan V, Kumar DS (2018) Multi-organ on a chip for personalized precision medicine. MRC 8(3):652–667. https://doi.org/10.1557/mrc.2018.148
Zhao Y, Kankala RK, Wang SB, Chen AZ (2019) Multi-organs-on-chips: towards long-term biomedical investigations. Molecules 24(4):675. https://doi.org/10.3390/molecules24040675
van Midwoud PM, Merema MT, Verpoorte E, Groothuis GMM (2010) A microfluidic approach for in vitro assessment of interorgan interactions in drug metabolism using intestinal and liver slices. Lab Chip 10:2778–2786. https://doi.org/10.1039/c0lc00043d
Tsamandouras N, Wen LKC, Edington CD, Stokes CL, Griffith LG, Cirit M (2017) Integrated gut and liver microphysiological systems for quantitative in vitro pharmacokinetic studies. AAPS J 19(5):1–14. https://doi.org/10.1208/s12248-017-0122-4
Skardal A, Murphy SV, Devarasetty M, Mead I, Kang HW, Seol YJ, Zhang YS, Shin SR, Zhao L, Aleman J, Hall AR, Shupe TD, Kleensang A, Dokmeci MR, Lee SJ, Jackson JD, Yoo JJ, Hartung T, Khademhosseini A et al (2017) Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep 7:8837. https://doi.org/10.1038/s41598-017-08879-x
Maschmeyer I, Hasenberg T, Jaenicke A, Lindner M, Lorenz AK, Zech J, Garbe LA, Sonntag F, Hayden P, Ayehunie S, Lauster R, Marx U, Materne EM (2015) Chip-based human liver-intestine and liver-skin co-cultures—a first step toward systemic repeated dose substance testing in vitro. Eur J Pharm Biopharm 95:77–87. https://doi.org/10.1016/j.ejpb.2015.03.002
Maschmeyer I, Lorenz AK, Schimek K, Hasenberg T, Ramme AP, Hübner J, Lindner M, Drewell C, Bauer S, Thomas A, Sambo NS, Sonntag F, Lauster R, Marx U (2015) A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 15:2688–2699. https://doi.org/10.1039/c5lc00392j
Oleaga C, Bernabini C, Smith AST, Srinivasan B, Jackson M, McLamb W, Platt V, Bridges R, Cai Y, Santhanam N, Berry B, Najjar S, Akanda N, Guo X, Martin C, Ekman G, Esch MB, Langer J, Ouedraogo G et al (2016) Multi-organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep 6:20030. https://doi.org/10.1038/srep20030
Edington CD, Chen WLK, Geishecker E, Kassis T, Soenksen LR, Bhushan BM, Freake D, Kirschner J, Maass C, Tsamandouras N, Valdez J, Cook CD, Parent T, Snyder S, Yu J, Suter E, Shockley M, Velazquez J, Velazquez JJ et al (2018) Interconnected microphysiological systems for quantitative biology and pharmacology studies. Sci Rep 8:4530. https://doi.org/10.1038/s41598-018-22749-0
Jalili-Firoozinezhad S, Miranda CC, Cabral JMS (2021) Modeling the human body on microfluidic chips. Trends Biotechnol 39(8):838–852. https://doi.org/10.1016/j.tibtech.2021.01.004
An F, Qu Y, Liu X, Zhong R, Luo Y (2015) Organ-on-a-chip: new platform for biological analysis. Anal Chem Insights 10:39–45. https://doi.org/10.4137/aci.s28905
Singh D, Mathur A, Arora S, Roy S, Mahindroo N (2022) Journey of organ on a chip technology and its role in future healthcare scenario. Appl Surf Sci 9:100246. https://doi.org/10.1016/j.apsadv.2022.100246
Lee H, Kim DS, Ha SK, Choi I, Lee JM, Sung JH (2017) A pumpless multi-organ-on-a-chip (MOC) combined with a pharmacokinetic-pharmacodynamic (PK-PD) model. Biotechnol Bioeng 114:432–443. https://doi.org/10.1002/bit.26087
Satoh T, Sugiura S, Shin K, Onuki-Nagasaki R, Ishida S, Kikuchi K, Kakiki M, Kanamori T (2017) A multi-throughput multiorgan-on-a-chip system on a plate formatted pneumatic pressure-driven medium circulation platform. Lab Chip 18:115–125. https://doi.org/10.1039/c7lc00952f
Lantada AD, Pfleging W, Besser H, Guttmann M, Wissmann M, Plewa K, Smyrek P, Piotter V, García-Ruíz JP (2018) Research on the methods for the mass production of multi-scale organs-on-chips. Polymers (Basel) 10(11):1238. https://doi.org/10.3390/polym10111238
Dabbagh SR, Sarabi MR, Birtek MT, Mustafaoglu N, Zhang YS, Tasoglu S (2022) 3D Bioprinted Organ-On-Chips. Aggregate 4(1). https://doi.org/10.1002/agt2.197
Miri AK, Mostafavi E, Khorsandi D, Hu SK, Malpica M, Khademhosseini A (2019) Bioprinters for Organs-On-Chips. Biofabrication 11(4):042002. https://doi.org/10.1088/1758-5090/ab2798
He Y, Wu Y, Fu JZ, Gao Q (2016) Developments of 3D printing microfluidics and applications in chemistry and biology: a review. Electroanalysis 28(8):1658–1678. https://doi.org/10.1002/elan.201600043
Galateanu B, Hudita A, Biru EL, Iovu H, Zaharia C, Simsensohn E, Costache M, Petca RC, Jinga V (2022) Applications of Polymers for Organ-On-Chip Technology in Urology. Polymers 14(9):1668–1668. https://doi.org/10.3390/polym14091668
Yi HG, Lee H, Cho DW (2017) 3D printing of organs-on-chips. Bioengineering 4(4):10. https://doi.org/10.3390/bioengineering4010010
Groll J, Boland T, Blunk T, Burdick JA, Cho DW, Dalton PD, Derby B, Forgacs G, Li Q, Mironov VA, Moroni L, Nakamura M, Shu W, Takeuchi S, Vozzi G, Woodfield TBF, Xu T, Yoo JJ, Malda J (2016) Biofabrication: Reappraising the definition of an evolving field. Biofabrication 8(1):013001. https://doi.org/10.1088/1758-5090/8/1/013001
Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo SS, Dai G, Karande P (2013) Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods 20:473–484. https://doi.org/10.1089/ten.TEC.2013.0335
Nguyen DG, Funk J, Robbins JB, Crogan-Grundy C, Presnell SC, Singer T, Roth AB (2016) Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS One 11:e0158674. https://doi.org/10.1371/journal.pone.0158674
Matsusaki M, Sakaue K, Kadowaki K, Akashi M (2013) Three-dimensional human tissue chips fabricated by rapid and automatic inkjet cell printing. Adv Healthc Mater 2(4):534–539. https://doi.org/10.1002/adhm.201200299
Knowlton S, Yenilmez B, Tasoglu S (2016) Towards single-step biofabrication of organs on a chip via 3D printing. Trends Biotechnol 34(9):685–688. https://doi.org/10.1016/j.tibtech.2016.06.005
Lee H, Cho DW (2016) One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 16:2618–2625. https://doi.org/10.1039/C6LC00450D
Bhise NS, Manoharan V, Massa S, Tamayol A, Ghaderi M, Miscuglio M, Lang Q, Zhang YS, Shin SR, Calzone G, Annabi N, Shupe TD, Bishop CE, Atala A, Dokmeci MR, Khademhosseini A (2016) A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 8(1):014101. https://doi.org/10.1088/1758-5090/8/1/014101
Johnson BN, Lancaster KZ, Hoque IB, Meng F, Kong YL, Enquist LW, Mcalpine MC (2016) 3D printed nervous system on a chip. Lab Chip 16(8):1393–1400. https://doi.org/10.1039/c5lc01270h
Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA (2016) bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 6:34845. https://doi.org/10.1038/srep34845
Galan EA, Zhao H, Wang X, Dai Q, Huck WTS, Ma S (2020) Intelligent microfluidics: the convergence of machine learning and microfluidics in materials science and biomedicine. Matter 3(6):1893–1922. https://doi.org/10.1016/j.matt.2020.08.034
Mencattini A, Mattei F, Schiavoni G, Gerardino A, Businaro L, Natale CD, Martinelli E (2019) from petri dishes to organ on chip platform: the increasing importance of machine learning and image analysis. Front Pharmacol 10. https://doi.org/10.3389/fphar.2019.00100
Li J, Chen J, Bai H, Wang H, Hao S, Ding Y, Peng B, Zhang J, Li L, Huang W (2022) An Overview of organs-on-chips based on deep learning. Research 2022. https://doi.org/10.34133/2022/9869518
Mahdi Y, Daoud K (2017) Microdroplet size prediction in microfluidic systems via artificial neural network modeling for water-in-oil emulsion formulation. J Dispers Sci Technol 38(10):1501–1508
Han S, Kim T, Kim D, Park YL, Jo S (2018) Use of deep learning for characterization of microfluidic soft sensors. IEEE Robot Autom Lett 3(2):873–880. https://doi.org/10.1109/LRA.2018.2792684
Parlato S, Ninno AD, Molfetta R, Toschi E, Salerno D, Mencattini A, Romagnoli G, Fragale A, Roccazzello L, Buoncervello M, Canini I, Bentivegna E, Falchi M, Bertani FR, Gerardino A, Martinelli E, Natale C, Paolini R, Businaro L, Gabriele L (2017) 3D Microfluidic model for evaluating immunotherapy efficacy by tracking dendritic cell behavior toward tumor cells. Sci Rep 7(1):1093. https://doi.org/10.1038/s41598-017-01013-x
Santbergen MJC, van der Zande M, Bouwmeester H, Nielen MWF (2019) Online and in situ analysis of organs-on-a-chip, TrAC, trends anal. Chem 115:138–146. https://doi.org/10.1016/j.trac.2019.04.006
Cho S, Islas-Robles A, Nicolini AM, Monks TJ, Yoon JY (2016) In situ, dual-mode monitoring of organ-on-a-chip with smartphone-based fluorescence microscope. Biosens Bioelectron 86(15):697–705. https://doi.org/10.1016/j.bios.2016.07.015
Sun H, Jia Y, Dong H, Dong D, Zheng J (2020) Combining additive manufacturing with microfluidics: an emerging method for developing novel organs-on-chips. Curr Opin Chem Eng 28:1–9. https://doi.org/10.1016/j.coche.2019.10.006
Allen JW, Bhatia SN (2003) Formation of steady-state oxygen gradients in vitro: application to liver zonation. Biotechnol Bioeng 82(3):253–262. https://doi.org/10.1002/bit.10569
Author information
Authors and Affiliations
Contributions
Dhiraj Kumar: methodology, literature review, investigation, data curation, result analysis, writing-original draft preparation. Rahul Nadda: methodology, investigation, data curation, writing-original draft preparation. Ramjee Repaka: methodology, reviewed, and edited.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, D., Nadda, R. & Repaka, R. Advances and challenges in organ-on-chip technology: toward mimicking human physiology and disease in vitro. Med Biol Eng Comput 62, 1925–1957 (2024). https://doi.org/10.1007/s11517-024-03062-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-024-03062-7