Abstract
A new multi-purpose Iranian head and neck (MIHAN) anthropomorphic phantom was designed and manufactured to be used in diagnostic and therapeutic applications. Geometry of MIHAN phantom was determined based on the average dimensions acquired by CT scans of twenty patients without any medical problems in their head and neck site. Because the phantom was expected to be used with different modalities with a wide range of photon energies, attenuation coefficients of some selected materials were determined using Monte Carlo simulation. Based on analytical and simulation results, acrylonitrile butadiene styrene (ABS) and polylactic acid (PLA) were found suitable choices for soft and bony tissues, respectively. They were used in the 3D printer to build the phantom. The suitability of the materials was checked by CT number value comparison between the organs included in the phantom and the corresponding body tissues and also film dosimetry of a typical intensity-modulated radiation therapy (IMRT) plan.. Hounsfield Unit agreement and 95% ± 2% pass rate for the IMRT plan verification proved the suitability of material selection. Also, the film dosimetry showed feasibility of using MIHAN in radiotherapy plan verification workflow. In addition, PLA was introduced as a spongy bone tissue substitute for the first time.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Symbol | Name | Definition | Unit |
|---|---|---|---|
μ | Attenuation coefficient | Total linear attenuation coefficient | cm−1 |
E eff | Effective energy | Effective energy of the irradiated photon | keV |
1 Introduction
Tissue-equivalent anatomical phantoms play an essential role in radiation protection, radiobiology, calibration of radiation devices, dose distribution verification in radiation therapy, and to develop and test new imaging modalities. The composition of a tissue substitute chosen for a phantom is selected based on the composition of the body tissue and characteristics of the radiation field [1]. Dosimetry of the heterogeneous body organs is more difficult than the homogenous ones. Head and neck (H&N) region with the skull, vertebra and face bones, and several air cavities, is one of these challenging body sites. Several commercial and non-commercial H&N phantoms with simple cylindrical to complicated geometries have been introduced and used in the clinical settings [2,3,4,5,6,7,8,9,10,11,12,13,14]. While the phantoms with simple cylindrical geometry permit them to be used for a range of clinical body sites, they are not an ideal analogy for the H&N site. Because water is still a standard substance of choice for radiology and radiotherapy dosimetry, some of the developed phantoms have been designed as a hollow shell that can be filled with water or other materials [3, 7]; or in the form of solid water-equivalent slabs [5, 6, 8]; or hollow cylindrical phantoms [2, 4, 9, 10]. Most of them have been developed for one or a defined number of purposes or they provide no or limited available sites to insert desirable organs or include a few number of heterogeneous organs. These limitations restrict their use in different clinical scenarios and increase the clinical costs. Providing a multi-purpose phantom will speed up the dosimetry procedures and give the flexibility in dosimeter selection based on the desired removable 3D-printed detector holders and required resolution and accuracy. The mass or linear attenuation coefficients (μ) of each organ in the phantom should approximate the real one [1]. There are several methods for the selection of the most suitable materials for manufacturing a tissue-equivalent phantom via μ matching. They include Monte Carlo simulation [15], CT number (Hounsfield unit — HU) matching [16], or dosimetry of a pre-determined treatment plan [7, 8].
Herein, a hollow multi-purpose Iranian head and neck (MIHAN) anthropomorphic phantom that can be filled with water and also have the possibility of inserting different H&N organs is introduced. It can be used for radiotherapy quality assurance (QA) purposes, radioiodine dosimetry [17], nuclear accident assessments, calibration/validation of different molecular imaging modalities, testing reconstruction or artifact reduction algorithms, and image distortion evaluations. The phantom incorporates optional 3D-printed heterogeneities, and it includes holders for the insertion of radiochromic films and thermoluminescent detectors (TLDs) for dosimetry purposes. To the best of author’s knowledge, correspondence between the printer materials and soft and bony tissues is evaluated in this study for the first time. It provides flexibility to insert new heterogeneities and dosimeters in the phantom. Because the organ sizes differ among different nations [18], a national hollow H&N phantom based on healthy Iranian people was developed. Herein, the phantom preparation, design, construction, and evaluation steps were presented, and attenuation characteristics of the MIHAN phantom for a wide range of photon energies were investigated. Then suitability of the constructed phantom for quality assurance purposes in intensity-modulated radiotherapy (IMRT) was evaluated by using radiochromic film.
2 Materials and methods
2.1 Determination of phantom geometry
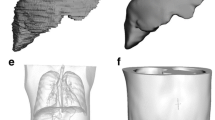
The geometry of MIHAN anthropomorphic phantom was determined based on segmentation of important organs on the CT scan images of 20 patients referred for medical assessment at Shohada Tajrish Hospital (10 males and 10 females with an age range of 48 ± 14 years and head diameter of 167 ± 13 mm) who did not have any problem in their H&N region. Size and position of the internal body structures, including the thyroid gland, spinal canal, cervical vertebra, nasal cavity, mandible, glottic larynx, and trachea, was determined by averaging data obtained from segmenting the desired organs at ISOgray TPS® (DOSIsoft, France). Figure 1 shows a typical three-dimensional (3D) reconstruction of the thyroid gland, mandible, and nasal cavity contours in a patient.
2.2 Selection of phantom material
In order to mimic the radiation attenuation properties of human tissue, materials of the phantom must be chosen in a way that has as close electron density and effective atomic number as possible to body tissues. The materials presented in Table 1 include some of the common materials to substitute soft tissue and bone. The presented values were calculated based on the data provided by Nguyen et al. and Wagner [19, 20] and equations presented in [21].
Polymethyl methacrylate (PMMA) is a common material of choice as equivalent to soft tissue [1, 3,4,5,6] and aluminum as equivalent to bone [1,2,3,4]. There are different methods for phantom manufacturing such as molding [7], use of water equivalent slabs [5, 6, 9], and 3D printing [3, 8, 10]. Due to flexibility provided by 3D printing method, the two common filament materials, namely acrylonitrile butadiene styrene (ABS) and polylactic acid (PLA), are presented in Table 1. SolidWorks (Dassault Systèmes Co.) software was used for the phantom design, and 3D printing was done using a fused deposition modeling (FDM) printer. ABS has been used as a surrogate for the soft tissue [3, 8, 22], whereas PLA has been considered as the brain tissue [23], lung, fat, and muscle by adjusting its infill densities [14] or the thyroid [15]. Based on the fact that MIHAN phantom was intended to be used in radiotherapy and nuclear medicine applications with a predominant photon energy range of more than 100 keV, especially a few MeV, Compton scattering and therefore the electron density were the main factors to be taken into account [1]. Agreement between electron density of ABS to soft tissue (1.2%) and PLA to bone tissue (0.3%) encouraged us to manufacture the phantom by 3D printing method. Afterwards, a thorough investigation of μ matching of the materials mentioned above was done by the Monte Carlo (MC) code MCNPX 2.6.0.
2.3 MC-based confirmation of material selection
MC simulation was used to obtain the linear attenuation coefficient of the materials presented in Table 1 for different photon energy spectra expected to be used in MIHAN phantom manufacturing. The bone and soft tissue compositions and densities were simulated based on the ICRP phantom. Five photon energy spectra were considered including Tc-99 m (140 keV), F-18 (511 keV) radionuclides utilized in nuclear medicine, I-131 spectra [24] for radioiodine therapy, 6 MV X-ray photons to simulate VARIAN radiotherapy linear accelerator (Linac) energy spectrum [25], and 120 kVp X-ray photons used in CT scan [26]. Their effective energy was calculated based on Eq. 1 to find the trend of μ versus effective energy.
where \({\varphi }_{i}\) is the fluence rate in ith energy bin of Ei. Therefore, the obtained effective photon energies were calculated as 66, 140, 373, 511, and 1798 keV for CT, Tc-99 m, I-131, F-18, and Linac, respectively.
Sixty 10 × 10 cm2 slabs with 1-mm thickness from each material was simulated in a good geometry irradiation configuration. The slabs were positioned in vacuum between a point mono-directional photon source and a semi-point (8 × 10−12 mm3) detector with a 20 cm source to detector distance. The slabs were positioned in the radiation field one by one. Therefore, for each material and photon energy spectrum, 60 MC simulations were run, and the photon current on the front surface of the detector was recorded. For each thickness, 200,000 histories were transported to achieve an error of less than 1% within a few minutes. Using the Beer-Lambert law, the μ values were found by fitting the data points for each material and source effective energy. Figure 2 shows the logarithm of primary current to the detected one versus attenuator thickness for 140 keV gamma radiation emitted by Tc-99 m. Fitting a linear equation to the data points resulted in the slope of the lines from which the μ values were deduced. In order to validate the simulation, calculated attenuation coefficients for water and aluminum were compared to their counterparts available at the NIST website [27, 28] for 140 and 511 keV monoenergetic photons.
2.4 Film dosimetry
Gafchromic EBT2 film dosimeter was used to evaluate the applicability and suitability of the manufactured phantom in dosimetry applications. Nineteen 1.5 × 1.5 cm2 pieces of film were exposed using 6 MV X-ray emerged from Unique Varian Linac (Varian medical systems, Palo Alto, CA, USA) for a dose range of 0.5 to 9.5 Gy in 0.5 Gy steps. The dose (D) calibration curve was calculated from the obtained net optical density (NOD) based on the Devic protocol [29] according to the following equation with 1.2% uncertainty (Eq. 2).
Afterwards, CT images were taken by Ingenuity Core CT scanner (Koninklijke Philips co.) from the phantom using the system settings of 120 kVp and 500 mA and slice thickness of 3 mm. Delineation of a hypothetical planning target volume (PTV) was performed as a cylinder with a radius of 2.5 cm and parotid gland as a healthy tissue close to the PTV. An intensity-modulated radiation therapy (IMRT) plan was prepared using five equidistance 6 MV radiation fields with the prescription dose value of 2 Gy per fraction by Eclipse treatment planning system (TPS) (Varian medical systems, Palo Alto, CA, USA) [30]. Configuration of the fields was in such a way that the rays passed through the bone and air inhomogeneities in their paths. The phantom was irradiated while six 5.7 × 5.7 cm2 film pieces were positioned inside the film holder of the phantom. The comparison of the TPS calculated total dose with the measured dose derived from the film pieces was done using the gamma index method [31]. The acceptance criteria were a dose-difference criterion of 3% and distance-to-agreement (DTA) criterion of 3 mm. The ratio of the number of pixels with the gamma index equal to or less than one to the total number of embraced pixels within a 50% isodose curve was determined.
3 Results and discussion
3.1 Phantom material
Fitting linear equations to curves presented in Fig. 2 and finding their slope resulted in the linear attenuation coefficients of different materials for different energy spectra presented in Sect. 2.3. All the obtained fitting R-squared values were more than 0.9980. Table 2 shows the results of validating the obtained values compared to the NIST database for the two monochromatic photon emitters in aluminum and water. The difference between the calculated and the NIST attenuation coefficients was not greater than 2% which confirmed the validity of the simulation.
Illustration of the μ values versus the effective energies implied a power-law equation fitting to the data points as indicated in Eq. (3).
in which μ is the total linear attenuation coefficient in cm−1, Eeff is the effective energy of the irradiated photon in keV and a and b are the fitting parameters. Figure 3 shows the μ values versus effective energy for bone, aluminum, PLA, soft tissue, water, PMMA, and ABS and their fits. It can be seen that PLA can surrogate the bone tissue better than aluminum for the energy range from 66 keV effective energy of CT scanner to about 1.8 MeV effective energy of radiotherapy X-ray photons. The largest difference between bone and aluminum was about 2.6 times the difference between PLA and bone for I-131 energy spectra. It can be seen that the discrepancy is more pronounced for lower energies which limit the use of bone surrogates. It is due to the fact that photoelectric interaction which depends strongly on the atomic number is dominant in lower energies, and based on Table 1, this difference is about 45% between bone and PLA. However, increase of the energy results in Compton scattering interaction dominance which depends on the electron density with a difference of less than 1% for bone and PLA.
On the other hand, while water shows the most similar behavior to the soft tissue, ABS is a better representative of the soft tissue than the ubiquitous PMMA material. Based on Table 1, larger difference is between the effective atomic number of soft tissue and other materials than the electron density. Similar to bone, increase of the effective energy decreases the difference between the soft tissue and other materials. The largest difference between ABS and soft tissue was about 7%, while the smallest difference between PMMA and soft tissue was about 9%. Therefore, manufacturing the phantom using the 3D printer using ABS and PLA filaments in place of soft tissue and bone was confirmed.
Table 3 shows the calculated fitting parameters to Eq. 3 and their 95% confidence interval (CI) as well as their coefficients of determination (R2). Because of the large energy range used in this study (more than two orders of magnitude), it is expected that the fitting parameters can be used for the extrapolated energies, too. Because PLA can act as the spongy bone (discussed in Sect. 3.3), its fitting parameters are not so different from the soft tissue and its substitutes. However, the PLA curve is located above the PMMA.
The MIHAN phantom was constructed to be used in transmission and emission tomography and even magnetic resonance imaging (MRI) image analyses. It is due to the fact that the attenuation coefficients of the phantom materials agree with human tissue for a wide range of energy spectrum and it does not include any ferromagnetic material.
3.2 Phantom geometry
Table 4 presents the mean and standard deviation of the dimensions and volumes obtained from segmentation of organs of interest in 20 healthy individuals.
The phantom was manufactured as a hollow ABS shell with a thickness of 2 mm to fill in with water and desired dosimeters and internal body organs. The internal organs and external shell of the phantom were manufactured based on the mean values presented in Table 4. The internal organs were manufactured from ABS as a substitute for the soft tissue and PLA for the bony structures. The trachea was made of ABS, and it was hollow. Two hollow ABS thyroid lobes and an isthmus in between were manufactured in order to inject radioiodine for dosimetry purposes and monitoring [17]. The nasal cavity and mandible were manufactured from PLA, while the former was hollow and the latter one was filled. All the hollow internal organs have a thickness of 2 mm. The cervical spine and trachea were screwed to the bottom surface of the phantom, and the thyroid was fastened to the trachea. The size of the phantom along coronal, sagittal, and transverse planes were 21 × 33 × 41 cm3 whose mass after filling with water was 6.5 kg. Figure 4 shows a schematic diagram of the internal parts of the phantom and their position in situ.
Four screws in the skull enclosed the reservoir, while a hole on top of the head lets the user to fill it with water. The phantom was designed in such a way to allow the user to measure the dose distribution using radiochromic films and TLDs. The film holder comprised eleven ABS slabs with 3-mm thickness. For the slabs intersecting the mandible, PLA inserts were positioned following the mandible curvature (Fig. 5b). Film holders can rotate in 360 degrees and can be aligned with any Cartesian axes to record the 2D dose distribution in any plane and reconstruct the 3D dose distribution. TLDs can be used for measuring absorbed dose in eye globs, pharynx, clavicle, and cervical spinal cord by two types of TLD (TLD-100 and GR-200). The TLD holder in the spinal canal was manufactured from ABS with TLD position center to center distance of 5 mm. In order to record the absorbed dose in the pharynx, a TLD position was situated on top of the trachea. Herein, the film dosimetry of the phantom has been reported and no TLD measurement was performed. Figure 5 shows three photos of the MIHAN phantom.
a A photo from the MIHAN phantom with a marker on the chin for CT scanning. Internal view of the phantom (b) with and (c) without the film holder. b Trail of the mandible can be seen as a black PLA ellipse and the right curvature is for conforming the ear and recording the parotid dose. c Cervical spine, trachea, thyroid lobes, and mandible can be seen inside the phantom
3.3 Phantom evaluation
In order to double check the validity of material selection after printing, the CT number values of the manufactured phantom were compared to a patient. Figure 6 shows a slice of the phantom as well as the head region of a patient side by side. Three regions of interest (ROI) in the bone and soft tissue parts are shown on the phantom and the human CT images. The corresponding mean and standard deviation of the obtained HUs and corresponding areas are indicated in Table 5. The PLA material which was used for the first time to simulate the human bone showed nearer HU value to real spongy bone compared to a similar study [16].
Applicability of the MIHAN phantom was tested through its application in a radiotherapy verification setting. Implementing a typical H&N IMRT plan and comparing the film measurements to the calculated treatment plans resulted in the gamma pass rates presented in Table 6. The region enclosed by the calculated 50% isodose line was used to report the pass rates.
The mean pass rate for the six film pieces is 95% which is acceptable in IMRT verifications [32]. It is in accordance with the pass rate criteria presented by the developers of Marvin phantom [8]. Figure 7 shows the measured and calculated dose distributions as well as the obtained gamma indices and binary illustration of the failed pixels for film number 4 with the worst performance. Because the significant failed pixels were within the high dose, high dose gradient region, the confidence limit of 10% / 2 mm DTA, in accordance with Palta et al. [33], was tested. Exclusion of 1-mm film border resulted in a 96% pass rate that can be interpreted as another confirmation for suitability of the phantom design and material selection.
Some of the limitations of the study can be summarized as follows. Competency of the phantom in nuclear medicine applications was not investigated. However, trend of the attenuation coefficient curve versus energy for the most prominent diagnostic and therapeutic radiopharmaceuticals was not changed for 99mTc (140 keV) and 131I (373 keV) energies. It is expected that the phantom can be used for nuclear medicine evaluations, as it was done in [17], but further investigation is required. Phantom dimensions were calculated based on the healthy Iranian people, and it may differ from other populations; this may affect phantom usage for others. Unfortunately, presented printed phantom has no insertion for ion chambers, but the flexibility of the phantom geometry can make it possible in the future.
4 Conclusion
An anthropomorphic H&N phantom was designed and manufactured using a 3D printer. The phantom was designed as a hollow shell to be filled with different 3D-printed organs and detectors to make it suitable for radiation dosimetry in radiotherapy and nuclear medicine applications. This design has the required flexibility based on usage, detector variety, and resolution selection, but other phantoms like Alderson Radiation Therapy phantom with solid structure does not provide it. It incorporates TLD and film holders for the dosimetry of several organs of interest. To the best of the author’s knowledge, this was the first study that used PLA as a substitute for the spongy bone tissue and showed its applicability. Attenuation properties of the selected materials were checked through MC calculations. Good agreement between the CT number values of the manufactured phantom and a real patient confirmed the phantom material selection. Gamma analysis of the calculated and measured radiation dose distributions in an IMRT plan using Gafchromic EBT2 films proved the feasibility of using MIHAN in radiotherapy plan verification workflow. Future studies can evaluate the suitability of the MIHAN phantom for stereotactic radiosurgery (SRS) or proton therapy purposes using gel dosimeters. Investigations are necessary in future works to check if this phantom is also suitable for audit purposes in radiotherapy departments or not.
References
White DR, Booz J, Griffith RV, Spokas JJ, Wilson IJ (1989) Tissue substitutes in radiation dosimetry and measurement. J Int Comm Radiat Units Meas (ICRU) Rep 44 os23. https://doi.org/10.1093/jicru/os23.1.Report44
Hermosilla A, Diaz Londono G, García M, Ruíz F, Andrade P, Pérez A (2014) Design and manufacturing of anthropomorphic thyroid-neck phantom for use in nuclear medicine centres in Chile. Radiat Prot Dosim 162:508–514. https://doi.org/10.1093/rpd/ncu022
Alqahtani MS, Lees JE, Bugby SL, Samara-Ratna P, Ng AH, Perkins AC (2017) Design and implementation of a prototype head and neck phantom for the performance evaluation of gamma imaging systems. EJNMMI Phys 4:19. https://doi.org/10.1186/s40658-017-0186-3
Mehdizadeh Naderi S, Sina S, Karimipoorfard M, Lotfalizadeh F, Entezarmahdi M, Moradi H, Faghihi R (2015) Design and fabrication of a multipurpose thyroid phantom for medical dosimetry and calibration. Radiat Prot Dosim 168:503–508. https://doi.org/10.1093/rpd/ncv359
Radaideh KM, Matalqah LM, Tajuddin A, Lee WF, Bauk S, Munem EEA (2013) Development and evaluation of a Perspex anthropomorphic head and neck phantom for three dimensional conformal radiation therapy (3D-CRT). J Radiother Pract 12:272–280. https://doi.org/10.1017/S1460396912000453
Gopishankar N, Vivekanandhan S, Rath G, Laviraj M, Senthilkumaran S, Kale S, Thulkar S, Bisht R, Subramani V (2013) Indigenously developed multipurpose acrylic head phantom for verification of IMRT using film and gel dosimetry. J Appl Clin Med Phys 14:62–76. https://doi.org/10.1120/jacmp.v14i2.4041
Molineu A, Followill DS, Balter PA, Hanson WF, Gillin MT, Huq MS, Eisbruch A, Ibbott GS (2005) Design and implementation of an anthropomorphic quality assurance phantom for intensity-modulated radiation therapy for the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 63:577–583. https://doi.org/10.1016/j.ijrobp.2005.05.021
Aitkenhead AH, Rowbottom CG, Mackay RI (2013) Marvin: an anatomical phantom for dosimetric evaluation of complex radiotherapy of the head and neck. Phys Med Biol 58:6915. https://doi.org/10.1088/0031-9155/58/19/6915
Webster GJ, Hardy MJ, Rowbottom CG, Mackay RI (2008) Design and implementation of a head-and-neck phantom for system audit and verification of intensity-modulated radiation therapy. J Appl Clin Med Phys 9:46–56. https://doi.org/10.1120/jacmp.v9i2.2740
Beaumont T, Ideias PC, Rimlinger M, Broggio D, Franck D (2017) Development and test of sets of 3D printed age-specific thyroid phantoms for 131I measurements. Phys Med Biol 62:4673. https://doi.org/10.1088/1361-6560/aa6514
Han Y, Lee SB, Shin EH, Kim JK, Ju SG, Yoon M, Ahn YC, Lim C, Jeong HK, Oh HJ Development of a head and neck phantom for remote-audit program of IMRT treatment. In: World Congress on Medical Physics and Biomedical Engineering 2006, IFMBE Proceedings, 2007. Springer, Berlin, Heidelberg, pp 2020–2023. https://doi.org/10.1007/978-3-540-36841-0_507
Ibbott G, Beach M, Maryanski M (2002) An anthropomorphic head phantom with a BANG polymer gel insert for dosimetric evaluation of IMRT treatment delivery. International Atomic Energy Agency (IAEA)
Atom Max dental & diagnostic head phantom. http://www.cirsinc.com/products/all/35/atom-max-dental-and-diagnostic-head-phantom/. 2018
IMRT head and neck phantom. http://www.cirsinc.com/products/all/11/imrt-head-and-neck-phantom/. 2018
Alssabbagh M, Tajuddin AA, Abdulmanap M, Zainon R (2017) Evaluation of 3D printing materials for fabrication of a novel multi-functional 3D thyroid phantom for medical dosimetry and image quality. Rad Phys Chem 135:106–112. https://doi.org/10.1016/j.radphyschem.2017.02.009
Mayer R, Liacouras P, Thomas A, Kang M, Lin L, Simone CB (2015) 3D printer generated thorax phantom with mobile tumor for radiation dosimetry. Rev Sci Instrum 86:074301. https://doi.org/10.1063/1.4923294
Hariri Tabrizi S, Ramezani M, Feghhi SAH, Geramifar P (2020) In vitro evaluation of an iodine radionuclide dosimeter (IRD) for continuous patient monitoring. Med Biol Eng Compu 58:763–769. https://doi.org/10.1007/s11517-020-02129-5
Lee C, Lee C, Park SH, Lee JK (2006) Development of the two Korean adult tomographic computational phantoms for organ dosimetry. Med phys 33:380–390. https://doi.org/10.1118/1.2161405
Nguyen TT, Yeom YS, Kim HS, Wang ZJ, Han MC, Kim CH, Lee JK, Zankl M, Petoussi-Henss N, Bolch WE (2015) Incorporation of detailed eye model into polygon-mesh versions of ICRP-110 reference phantoms. Phys Med Biol 60:8695
Wagner H (2002) Reinforcement. Encyclopedia of Polymer Science and Technology
Khan FM, Gibbons JP (2014) Khan’s the physics of radiation therapy. Lippincott Williams & Wilkins
Kumar R, Sharma S, Despande S, Ghadi Y, Shaiju V, Amols H, Mayya Y (2010) Acrylonitrile butadiene styrene (ABS) plastic-based low cost tissue equivalent phantom for verification dosimetry in IMRT. J Appl Clin Med Phys 11:24–32. https://doi.org/10.1120/jacmp.v11i1.3030
Negus IS, Holmes RB, Jordan KC, Nash DA, Thorne GC, Saunders M (2016) Development of a 3D printed subresolution sandwich phantom for validation of brain SPECT analysis. Med phys 43:5020–5027. https://doi.org/10.1118/1.4960003
Cember H (1969) Introduction to health physics. Introduction to health physics
Sheikh-Bagheri D, Rogers D (2002) Monte Carlo calculation of nine megavoltage photon beam spectra using the BEAM code. Med phys 29:391–402. https://doi.org/10.1118/1.1445413
Hsieh J Computed tomography: principles, design, artifacts, and recent advances. In, 2009. SPIE Bellingham, WA,
Aluminum attenuation coefficients. https://physics.nist.gov/PhysRefData/XrayMassCoef/ElemTab/z13.html. 2018
Water Attenuation coefficients. https://physics.nist.gov/PhysRefData/XrayMassCoef/ComTab/water.html. 2018
Devic S, Seuntjens J, Sham E, Podgorsak EB, Schmidtlein CR, Kirov AS, Soares CG (2005) Precise radiochromic film dosimetry using a flat-bed document scanner. Med phys 32:2245–2253. https://doi.org/10.1118/1.1929253
Hong TS, Tomé WA, Harari PM (2012) Heterogeneity in head and neck IMRT target design and clinical practice. Radiother Oncol 103:92–98
Low DA, Dempsey JF (2003) Evaluation of the gamma dose distribution comparison method. Med phys 30:2455–2464. https://doi.org/10.1118/1.1598711
Alber M, Broggi S, De Wagter C, Eichwurzel I, Engström P, Fiorino C, Georg D, Hartmann G, Knöös T, Leal A (2008) Guidelines for the verification of IMRT. ESTRO booklet 7
Palta J, Kim S, Li JG, Liu C (2003) Tolerance limits and action levels for planning and delivery of IMRT. In: Intensity-modulated radiation therapy: the state of the art. Medical Physics, Madison, Wisconsin,
Acknowledgements
We would like to thank Ms. Farahnaz Rahimi for providing suitable CT images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmadi, M., Ramezani Anarestani, M., Hariri Tabrizi, S. et al. Manufacturing and evaluation of a multi-purpose Iranian head and neck anthropomorphic phantom called MIHAN. Med Biol Eng Comput 59, 1611–1620 (2021). https://doi.org/10.1007/s11517-021-02394-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-021-02394-y