Abstract
Follower loads are used to simulate physiological compressive loads on the human spine. These compressive loads represent the load-carrying capacity of the human cervical spine and play an important role in maintaining its stability. However, under different follower loads the biomechanical response of the cervical spine is unknown. Therefore, the aim of this study was to determine the effect of follower load on the biomechanics of the cervical spine. A three-dimensional nonlinear finite element (FE) model of the cervical spine (C3–C7) was developed and validated. Using this FE model, we evaluated the effect of different follower loads (0 N, 50 N, 100 N, and 150 N) on the range of motion (ROM), facet joint forces (FJFs), and intradiscal pressure (IDP) in the cervical spine. In addition, a moment of 1 Nm was applied in three anatomical planes (sagittal, coronal, and transverse planes) to simulate different postures. The results indicate that as follower load was increased, the ROM of the cervical spine in extension decreased (4.06°–0.95°), but increased in other postures (flexion 4.19°–6.04°, lateral bending 1.74–3.03°, axial rotation 2.64°–4.11°). Follower loads increased the FJF in all postures (0 N–52 N). In lateral bending (LB), FJFs were only generated in the ipsilateral facet joints. In axial rotation (AR), there was large asymmetry in the FJF, which increased as follower load increased. The IDP of each segment increased nonlinearly with increasing follower load in all postures (0.01 MPa–1.23 MPa). In summary, follower loads caused changes in motion and loading patterns in the cervical spine (C3–C7). Therefore, in common daily activities, we should pay attention to the muscle strength of the neck through exercise to adapt to the biomechanical changes in the cervical spine following an increase in follower load.

Follower load is defined as the compressive load directed approximately along the axis of the spine. The purpose of this investigation was to determine the effect of the follower compressive load on biomechanics of the cervical spine. To do so, a three-dimensional nonlinear FE model of the cervical spine (C3-C7) was built and validated. Using this FE model of the cervical spine, we evaluated the effect of different follower loads (0 N, 50 N, 100 N, 150 N) on range of motion, facet joint force, and IDP in the cervical spine. In this study, the follower load was applied to the finite element model by connector elements. At the same time, a moment of 1 Nm was applied in the three anatomical planes to simulate different postures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The human cervical spine is capable of supporting substantial compressive loads in vivo arising from muscle forces and the weight of the head. These compressive loads can maintain mechanical stability of the cervical spine and play an important role in improving its load-carrying capacity [1]. However, reports of previous research indicate that cadaveric specimens of the cervical spine collapse when exposed to a critical (buckling) load of 11 N, considerably lower than physiological compressive loads experienced in vivo [2], possibly because traditional in vitro testing methods rarely include compressive loads similar to those experienced in vivo, especially in studies of multi-segment cervical spine constructs [3].
Follower loads have been used to simulate physiological compressive loading of the human spine, the majority of previous studies reporting its value in the cervical spine to be between 50 N and 150 N [4,5,6,7,8]. To quantify the biomechanical behavior of the human spine when exposed to a follower load, researchers have performed various in vitro experiments and finite element (FE) studies while applying compressive loads [3, 6, 9,10,11,12,13,14,15,16]. However, most experimental studies to date have focused on the lumbar spine [9,10,11,12], with only a few experimental studies evaluating the effect of compressive load on the cervical spine [3, 6, 13,14,15,16]. For example, Cripton et al. reported that different methods of application of compressive load may affect postures and movements of the cervical spine [13]. Ng et al. investigated the biomechanical effects of variations in orientation angle and magnitude of preload on the cervical spine at the C56 segment [3]. Kevin et al. used a robot test system to realize the application of follower loads on the cervical spine at the C37 segment and used the system to evaluate the effect of follower load on rotational torque parameters and intradiscal pressure (IDP) of the cervical spine [14]. In addition, a number of researchers have conducted biomechanical tests of cervical spine specimens under a follower load of a single magnitude [6, 15, 16]. Nevertheless, there is a lack of FE studies that predict biomechanical response of the cervical spine (C3–C7) to physiological compressive loads.
Regarding of facet joint forces (FJFs), a number of in vitro experiments have demonstrated that the facet joints transmit such loads through the spinal column and limit movement of the vertebral body, especially during axial rotation (AR) and extension [17,18,19]. In addition, Goel et al. examined the characteristics of distribution of FJF and reported that applying a preload influenced the load in particular spinal components [20]. Kuo et al. analyzed the contact behavior of the cervical facet joints and changes in intradiscal pressure associated with different postures [21]. Ung-Kyu et al. studied changes in adjacent segmental FJFs following cervical facet joint replacement and found that the FJF increased after artificial joint replacement [22]. Barrey et al. found that FJFs increased after applying a follower load to the cervical spine [16]. In addition, a number of studies have reported that increased load transmission from one motion segment to another through the facet joints may lead to facet joint pain [23,24,25]. Therefore, understanding the biomechanical effects of follower load on FJF is important in elucidating the basic principles of cervical pain caused by excessive FJF. It is worth noting that Du et al. have studied the effect of follower load on FJF in the lumbar spine in detail [26]. However, the influence of different compressive preloads on FJF in the cervical spine has not been previously examined.
Many previous studies that have measured changes in IDP have focused on the lumbar spine, demonstrating that IDP increases as compressive load is applied, and that this is related to the degree of degeneration in the intervertebral disc [12, 27, 28]. There has been some progress in the study of the effect of follower load on IDP in the thoracic spine [29, 30]. In addition, a number of researchers have evaluated the effect of compressive load on IDP in the cervical spine using in vitro experiments [14, 16]. Thus far, the team of Hattori et al. has been alone in conducting an in vivo study to investigate the IDP of the cervical spine [31]. Because of ethical conflicts and technical barriers associated with in vivo experimental studies conducted by Hattori et al., subsequent experiments have investigated IDP in vitro. However, to date, few researchers have used FE modeling to study the effect of follower load on the IDP of the cervical spine [16]. Compared with experimental methods, FE modeling is more suited to establishing the effect of material properties and performing parameter analysis.
Accordingly, the purpose of this study was to determine the effect of follower load on the biomechanics of the cervical spine. To do so, a three-dimensional nonlinear FE model of the cervical spine (C3–C7) was developed and validated. Using this FE model of the cervical spine, we evaluated the effect of different follower loads (0 N, 50 N, 100 N, and 150 N) on range of motion (ROM), FJF, and IDP in the cervical spine.
2 Materials and methods
2.1 Development of model
The detailed steps for constructing an FE model were obtained from previous studies [26, 32]. Briefly, detailed geometric information of the cervical spine (C3–C7) was derived from computed tomography (CT) images of a healthy male subject (age 30, weight 68 kg, height 173 cm). The subject was scanned using a CT scanner (Brilliance 64, Philips Electronics, Netherlands, resolution 512 × 512) at 0.625 mm slice intervals. This dataset had a voxel size of 0.625 mm × 0.975 mm × 0.975 mm. The CT information was imported into a medical image control system (Mimics 10.0; Materialise Technologies, Leuven, Belgium) in order to complete image segmentation of the cervical spine (C3–C7). Geometric model reconstruction was then completed using reverse engineering scanning software (Geomagic Studio 10.0; Geomagic Inc., NC, USA). Meshes were generated in CAE pre-processing software (Hypermesh 11.0; Altair Engineering Corp., MI, USA) and the final model created using commercial finite element software (Abaqus 6.11; Dassault Systemes Simulia Corporation, PA, USA).
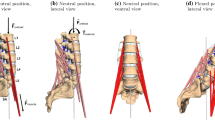
A single vertebra consists of posterior elements, endplates, cortical, and cancellous bone. Intervertebral discs are composed of an annulus (56%) and nucleus pulposus (44%) [33]. The annulus is believed to comprise ground substance reinforced by collagen fibers, with adjacent fiber layers at an angle of approximately ± 25° to the plane of the endplate [34]. The five groups of major vertebral ligaments include the posterior longitudinal ligament (PLL), anterior longitudinal ligament (ALL), ligamentum flavum (LF), capsular ligaments (CL), and interspinous ligaments (ISL). For each spinal segment, the number of elements in the PLL, ALL, LF, CL, and ISL are 5, 5, 6, 16, and 5, respectively. The original gap between facet articular surfaces was approximately 0.5 mm [35]. The thickness of each facet cartilage was estimated from previous observations [36, 37]. A three-dimensional nonlinear FE model of the cervical spine (C3-C7) is shown in Fig. 1.
The material properties of the different cervical spine tissues (Table 1) were those detailed in the literature [23, 32, 38,39,40]. Facet cartilage and each part of the vertebrae were considered isotropic homogeneous elastic materials. The annulus ground material and nucleus pulposus were simulated as incompressible hyper-elastic materials [38]. As specified by Shirazi-Adl et al. [39], fibers were simulated as a tension-only truss. The ligaments were simulated as connector elements with nonlinear properties whose material properties were determined according to previous research [40]. The material properties and element types of each component of the cervical spine model (C3–C7) are shown in Table 1.
2.2 Loading and boundary conditions
A moment of 1 Nm was applied to the node coupled with the superior surface of the C3 superior endplate, and then an additional follower load (0 N, 50 N, 100 N, or 150 N) applied to the FE model. The magnitude of the follower load applied in this study was based on previous research [4,5,6,7,8]. The follower load is a physiological compressive load along the axis of the cervical spine, in which intermediate nodes of each endplate are coupled to the endplate surface and connector units built through these nodes. The follower load was applied to each segment through these connector units [23, 41]. During the loading process, the inferior surface of the C7 endplate was always fully constrained.
2.3 Calibration and validation
The calibration process represented the preliminary stage of the validation process, the accuracy of the final model depending on the validation results. The calibration process was conducted in accordance with a method proposed by Nicole et al. [42]. Correction factors for collagen fibers and ligaments were determined, and the ROM of each segment under the action of moment calculated and compared with experimental results from previous research studies to validate the finite element model [43,44,45,46,47]. Boundary and loading conditions were specified and replicated in vitro. All simulation work was performed using commercial finite element software (Abaqus 6.11; Dassault Systemes Simulia Corporation, PA, USA).
3 Results
3.1 Calibration and validation
The results of the calibration process are shown in Fig. 2a–c and validation results in Figs. 3a–c and 4a–d. The ROM calculated by this FE model was compared with in vitro experimental data [43,44,45,46,47]. As shown in Fig. 4, except for a slightly lower ROM observed in lateral bending (LB) of the C34 segment, the ROM in each posture of the other segments closely matched in vitro experimental data. Therefore, the model was considered calibrated and validated, and used to study the biomechanical response of the cervical spine under different follower loads.
Comparison of FE and experimental studies in six directions under 1 Nm moment (C4–C5) [42]. a Flexion-extension under 1 Nm moment (C4–C5). b Lateral bending under 1 Nm moment (C4–C5). c Axial rotation under 1 Nm moment (C4–C5)
Computational and experimental results in six directions under different moments (C4–C5) [44,45,46,47]. a Computational and experimental flexion–extension results under different moments (C4–C5).
b Computational and experimental lateral bending results under different moments (C4–C5).
c Computational and experimental axial rotation results under different moments (C4–C5)
3.2 Range of motion
The ROM of each segment generated under various follower loads with no postural moment applied is shown in Fig. 5a. No segment rotations exceeded 2.5°, with maximum rotation observed in the C56 segment for all levels of follower load.
The ROM of all segments generated in different postures under various follower loads is shown in Fig. 5b, c. The ROM of each segment decreased with increasing follower load when in extension (4.06°–0.95°), but increased with increasing follower load in all other postures (flexion 4.19°–6.04°, lateral bending 1.74°–3.03°, axial rotation 2.64°–4.11°). As follower loads increased, the difference between ROM in flexion and in extension gradually increased. The largest difference in ROM was at the C56 segment (0.46°–4.34°). The ROM in AR was slightly greater than in LB. The largest difference in ROM was at the C67 segment (0.46°–2.08°).
3.3 Facet joint force
In different postures, the predicted FJFs from different segments when exposed to various follower loads are presented in Fig. 6a–c. In flexion, a number of facet joints experienced no FJF, but the FJF also increased as follower load increased. In extension, the FJF of the C34 segment (15 N–45 N) was higher than observed in other segments (14 N–41 N), while the load transmitted through the facet joint at the C45 segment (14 N–34 N) was least. In addition, FJF increased as follower load increased. For each segment, the FJFs of the facet joints on the left and right sides were not distinctly different.
During LB, FJF was generated only in the ipsilateral facet joint. That is to say, during bending to the right, a FJF was generated only within the right side facet joint, and similarly in the left facet joint during bending to the left. As shown in Fig. 6b, the contact force of the facet joints on left (28 N–52 N) and right (27 N–48 N) during bending did not differ much. During LB, FJF increased as follower load increased. As shown in Fig. 6c, there was large asymmetry in FJF during AR. That is to say, during right axial rotation the FJF on the left side (25 N–49 N) was considerably larger than that on the right side (2 N–20 N). Similarly, during left axial rotation the FJF on the right side (22 N–44 N) was considerably larger than that on the left side (1 N–22 N). This asymmetry was even more apparent as follower load was increased. During AR, the FJF of the facet joints on both sides increased with increasing follower load.
3.4 Intradiscal pressure
Figure 7a–c displays the IDP of all segments in different postures exposed to different follower loads. In different postures, the IDP of all segments increased nonlinearly as follower load increased. The IDP was minimal during extension, while IDP was largest during flexion. The IDP increased substantially as follower load increased during LB and AR. For example, during bending to the right, the IDP of C56 increased from 0.03 MPa to 0.76 MPa, and during left axial rotation, the IDP of C34 increased from 0.04 MPa to 0.77 MPa. The values of the IDP were not much different during LB (0.01 MPa–0.86 MPa) compared with AR (0.01 MPa–0.92 MPa).
4 Discussion
In order to mimic the substantial compressive loads in vivo arising from muscle forces and weight of the head, a follower load technique was employed in this study model. Currently, the majority of literature defines the magnitude of cervical spine follower load at approximately 75 N [23, 32, 35, 48, 49]. Due to differences between different individuals in different occupations, the compressive load on the cervical spine will vary. A value of 75 N is most commonly used, within the range 50 N–150 N tested here (50 N, 100 N, and 150 N), representative of values most commonly presented in the literature as follower load on the cervical spine [4,5,6,7,8, 14]. In this study, a three-dimensional nonlinear FE model of the cervical spine (C3–C7) was developed and validated in order to determine the effect of various follower loads on the biomechanics of the cervical spine.
As shown in Fig. 5a, under pure follower loads, maximum rotation was observed in the C56 segment, followed by that in the C45 segment. This may be due to the C45 and C56 segments being located at the middle of the C37 segment, responsible for the main movements of the cervical spine (C3–C7). Therefore, when exposed to a follower load, a change in motion to the C46 segment is most sensitive.
With increasing follower load, the ROM of all segments increased during flexion, and decreased during extension. Concomitantly, as follower load increased, the difference between the ROM in flexion and extension gradually increased. That is because the follower load restricts motion of the cervical spine when in extension and increases flexibility of the cervical spine during flexion. However, during LB and AR, follower load increased the range of motion of the cervical spine, so the difference in values between them will not be large.
Ng et al. [3] reported that ROM increased from 4.02° to 4.31° and 4.82° respectively during flexion when applying 100 N and 150 N compressive preloads to the C56 segment. Baseline ROM was different, but trend in the change in values was the same as observed in the present study. However, Kevin et al. applied a 100 N follower load to 12 cervical spine specimens (C3–C7) using a robot test system and reported that application of a follower load did not appear to affect the specimen’s magnitude of ROM, but did affect its quality (shape of the curve) [14]. The reason for the difference compared with the present study may be that they calculated the total ROM of the C37 segment and changes in motion of these segments cancelled each other out. Barrey et al. investigated changes to cervical spine intersegmental rotation in flexion when exposed to a 50 N follower load and their findings were also in agreement with our results [16]. Additionally, Puttlitz et al. evaluated the relationship between follower load and cervical spine kinetics, demonstrating that a 44 N follower load decreased ROM of the C45 segment slightly in flexion-extension (14.0 ± 3.4°–13.6 ± 2.5°), consistent with the trend in change observed in the present paper (7.37°–6.61°) [15]. Pelker et al. [50] investigated rotational stability, strength, and failure mechanisms in the cervical spine and found that compressive load appeared related to cervical spine stability. It is worth noting that Moroney et al. [51] measured 35 fresh adult cervical spine cadavers under a 73.6 N follower compressive load and found that stiffness of motion in cervical segments increased in extension. Thus, we hypothesize that the follower load maintains stability in the cervical spine by increasing segmental stiffness.
Current studies of the effect of follower load on segmental flexibility of the cervical spine mostly focus on flexion-extension. That is because in in vitro experiments, absolute compression of a cervical spine segment is probably obtained during both flexion and extension, whereas applying a follower load during LB and AR may lead to a combination of shear and compressive forces, inducing significant changes in the kinematics of the cervical spine [52, 53]. Therefore, the inclusion of LB and AR in the present study makes the results not directly comparable with findings of previously published work.
In the present study, the changes in characteristics of multi-segmental cervical FJF when exposed to a physiological compressive load were studied, and can supplement conventional research of follower loads. Following static analysis, different values of FJF were observed when exposed to follower loads in different postures (Fig. 6a–c). Under a follower load, FJFs were observed only in the ipsilateral facet joints during lateral bending and a large asymmetry was found during axial rotation, possibly related to the physiological structure of the facet joints of the cervical spine. In flexion, the gap between the two articular cartilage faces become larger, so the majority of the facet joints have no contact force. A clear increase in FJF due to follower loading while in an extension posture can be observed in Fig. 6a. This increase may be due to the follower load causing the upper and lower cartilage surfaces of the facet to displace and produce larger deformation. A similar explanation was offered in a study of FJFs in the lumbar spine exposed to follower loading [26]. As shown in Fig. 7a–c, IDP was minimal during extension, while being largest during flexion. IDP in extension is much smaller than IDP in flexion. During extension, with increasing follower load, the posterior facet joints of the cervical spine bear more loads, while the intervertebral discs bear less, leading to the results described above. In all postures, as follower load increased, FJFs also increased. A number of researchers have applied different follower loads to cervical spine specimens exposed to a 2 Nm moment [16, 22], finding that follower loads increased the FJF of the cervical spine. Although they applied a different moment (2 Nm) compared to the present study (1 Nm), the conclusions were the same. However, excessive FJF may lead to pain in the articular facets [23,24,25, 54, 55]. Therefore, it is important that the cervical spine should not bear excessive load for long durations, especially in individuals engaged in physical labor.
Due to the biomechanics of facet joint being sensitive to loading, boundary conditions, and methods of measurement, large differences have been observed in the results between experimental and finite element studies. It is therefore difficult to quantitatively compare the results with other studies. Previous literature has reported that measuring FJF in vivo is difficult or even impractical [56]. That may be because the facet joints of the cervical spine have greater relative slip relative to the lumbar spine, and it is more difficult to obtain stable and reliable experimental measurement data. Facet joints can be monitored in vivo using imaging techniques, but FJF cannot be recorded. In addition, previous studies have measured the spine cadavers, but degeneration of tissue in cadaveric specimens cannot be overlooked [19]. Differences in facet joints between different subjects are also very large. The factors above are the reasons that it is difficult to measure FJFs. It is worth noting that finite element calculations allow data to be collected that is difficult or impossible to obtain experimentally.
Pospiech et al. explored the relationship between muscle strength and pressure in discs and found that muscle strength played a significant role in regulating IDP [57]. Follower load is generated by synergy with muscle tissue. That is to say, an applied follower compressive load results in an increase in cervical spine IDP and the trend in that change is in agreement with the results of the present study. Therefore, for daily activities, individuals should pay attention to the muscle strength of their neck through exercise to adapt to the change in IDP from increasing follower loads. Additionally, Pospiech et al. specifically investigated the IDP of C34 and C56 exposed to muscle load, finding that their values increased 1.2 and 2.7-fold, respectively, in flexion. In addition, Kevin et al. tested twelve human cervical spine cadaveric specimens and found that in the neutral position the IDP of the C45 and C56 segments increased by 4.6-fold (0.017 MPa–0.077 MPa) and 2.6-fold (0.029 MPa–0.076), respectively, after applying a follower load of 100 N [14]. The trends above are consistent with our research, with a significant increase in IDP as follower load is applied. In addition, Barrey et al. evaluated the trend in change of IDP in the cervical spine exposed to a 50 N follower load, finding that IDP increased in all postures under load [16]. It is worth noting that in our study the IDP varied nonlinearly with increasing follower load, possibly because the nucleus pulposus and annulus ground substance were defined as incompressible hyper-elastic materials. In summary, follower loads can cause changes in the motion and loading pattern of the cervical spine (C3–C7).
It is important to emphasize the limitations of the FE model of the cervical spine in this study. Firstly, the current cervical spine FE model was constructed on the basis of anatomical information of the cervical spine from a single individual, and it only reflects trends of change in the mechanical response of the cervical spine under different loads. Moreover, the ligaments of this cervical spine were simulated as unidimensional nonlinear connector elements, the model unable to simulate the true anatomy of the ligaments, which would affect the motion of the cervical spine. Finally, no muscles were simulated in the current model. Although this limitation was mitigated by using the follower load technique, this could not entirely replace the muscles, which might provide a more complex contribution to the spinal response.
5 Conclusions
The results demonstrate that follower loads reduced the ROM of the cervical spine in extension, while causing an increase in other postures. Follower loads increased the ability of the facet joints to transmit loads, and increased FJFs in each segment for all postures. During LB, FJFs were only produced in the ipsilateral facet joints. There was a large asymmetry of FJF during AR, which was amplified with increasing follower load. Application of follower load increased the IDP of each segment in all postures, which varied nonlinearly with increasing follower load. That is to say, follower load restricts motion of the cervical spine in extension and increases its flexibility in other postures. In addition, follower loads increase load on major spinal elements (such as intervertebral discs and posterior facet joints). In summary, follower loads cause changes in the motion and loading patterns of the cervical spine (C3–C7).
Abbreviations
- FE:
-
Finite element
- ROM:
-
Range of motion
- FJFs:
-
Facet joint forces
- IDP:
-
Intradiscal pressure
- CT:
-
Computed tomography
- ALL:
-
Anterior longitudinal ligament
- PLL:
-
Posterior longitudinal ligament
- LF:
-
Ligamentum flavum
- CL:
-
Capsular ligament
- ISL:
-
Interspinous ligament
- LB:
-
Lateral bending
- AR:
-
Axial rotation
References
Patwardhan AG, Havey RM, Ghanayem AJ, Diener H, Meade KP, Dunlap B, Hodges SD (2000) Load-carrying capacity of the human cervical spine in compression is increased under a follower load. Spine 25(12):1548–1554
Panjabi MM, Cholewicki J, Nibu K, Grauer J, Babat LB, Dvorak J (1998) Critical load of the human cervical spine: an in vitro experimental study. Clin Biomech 13(1):11–17
Ng H-W, Teo E-C (2005) Influence of preload magnitudes and orientation angles on the cervical biomechanics: a finite element study. Clin Spine Surg 18(1):72–79
Finn MA, Brodke DS, Daubs M, Patel A, Bachus KN (2009) Local and global subaxial cervical spine biomechanics after single-level fusion or cervical arthroplasty. Eur Spine J 18(10):1520–1527
Finn MA, Samuelson MM, Bishop F, Bachus KN, Brodke DS (2011) Two-level noncontiguous versus three-level anterior cervical discectomy and fusion: a biomechanical comparison. Spine 36(6):448–453
Paxinos O, Ghanayem AJ, Zindrick MR, Voronov LI, Havey RM, Carandang G, Hadjipavlou A, Patwardhan AG (2009) Anterior cervical discectomy and fusion with a locked plate and wedged graft effectively stabilizes flexion-distraction stage-3 injury in the lower cervical spine: a biomechanical study. Spine 34(1):E9–E15
Snyder JT, Tzermiadianos MN, Ghanayem AJ, Voronov LI, Rinella A, Dooris A, Carandang G, Renner SM, Havey RM, Patwardhan AG (2007) Effect of uncovertebral joint excision on the motion response of the cervical spine after total disc replacement. Spine 32(26):2965–2969
Lee S-H, Im Y-J, Kim K-T, Kim Y-H, Park W-M, Kim K (2011) Comparison of cervical spine biomechanics after fixed-and mobile-core artificial disc replacement: a finite element analysis. Spine 36(9):700–708
Patwardhan AG, Havey RM, Meade KP, Lee B, Dunlap B (1999) A follower load increases the load-carrying capacity of the lumbar spine in compression. Spine 24(10):1003–1009
Goel VK, Panjabi MM, Patwardhan AG, Dooris AP, Serhan H (2006) Test protocols for evaluation of spinal implants. JBJS 88(suppl_2):103–109
Shirazi-Adl A (2006) Analysis of large compression loads on lumbar spine in flexion and in torsion using a novel wrapping element. J Biomech 39(2):267–275
Rohlmann A, Neller S, Claes L, Bergmann G, Wilke H-J (2001) Influence of a follower load on intradiscal pressure and intersegmental rotation of the lumbar spine. Spine 26(24):E557–E561
Cripton P (2000) The effects of physiologic compressive preload on the in vitro flexibility of the lower cervical spine. Proceedings of the Cervical Spine Research Society Charleston, SC
Bell KM, Yan Y, Hartman RA, Lee JY (2018) Influence of follower load application on moment-rotation parameters and intradiscal pressure in the cervical spine. J Biomech 76:167–172
Puttlitz CM, Rousseau MA, Xu Z, Hu S, Tay BK, Lotz JC (2004) Intervertebral disc replacement maintains cervical spine kinetics. Spine 29(24):2809–2814
Barrey C (2015) Rousseau M-a, Persohn S, Campana S, Perrin G, Skalli W: Relevance of using a compressive preload in the cervical spine: an experimental and numerical simulating investigation. Eur J Orthop Surg Traumatol 25(1):155–165
Adams MA, Hutton W (1980) The effect of posture on the role of the apophysial joints in resisting intervertebral compressive forces. J Bone Joint Surg Br Vol 62(3):358–362
Shirazi-Adl A, Drouin G (1987) Load-bearing role of facets in a lumbar segment under sagittal plane loadings. J Biomech 20(6):601–613
Schendel MJ, Wood KB, Buttermann GR, Lewis JJ, Ogilvie JW (1993) Experimental measurement of ligament force, facet force, and segment motion in the human lumbar spine. J Biomech 26(4-5):427–438
Goel V, Winterbottom J, Weinstein JN, Kim Y (1987) Load sharing among spinal elements of a motion segment in extension and lateral bending. J Biomech Eng 109(4):291–297
Kuo C-S, Hu H-T, Lin R-M, Huang K-Y, Lin P-C, Zhong Z-C, Hseih M-L (2010) Biomechanical analysis of the lumbar spine on facet joint force and intradiscal pressure-a finite element study. BMC Musculoskelet Disord 11(1):151
Chang U-K, Kim DH, Lee MC, Willenberg R, Kim S-H, Lim J (2007) Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J Neurosurg Spine 7(1):33–39
Cai XY, Sang D, Yuchi CX et al (2019) Using finite element analysis to determine effects of the motion loading method on facet joint forces after cervical disc degeneration. Comput Biol Med 103519
Hussain M, Natarajan RN, An HS, Andersson GBJ (2010) Reduction in segmental flexibility because of disc degeneration is accompanied by higher changes in facet loads than changes in disc pressure: a poroelastic C5–C6 finite element investigation. Spine J 10(12):1069–1077
Hussain M, Natarajan RN, Chaudhary G et al (2012) Posterior facet load changes in adjacent segments due to moderate and severe degeneration in C5-C6 disc: a poroelastic C3-T1 finite element model study. Clin Spine Surg 25(4):218–225
Du C-F, Yang N, Guo J-C, Huang Y-P, Zhang C (2016) Biomechanical response of lumbar facet joints under follower preload: a finite element study. BMC Musculoskelet Disord 17(1):126
Mo Z, Li Q, Jia Z, Yang J, Wong DW-C, Fan Y (2017) Biomechanical consideration of prosthesis selection in hybrid surgery for bi-level cervical disc degenerative diseases. Eur Spine J 26(4):1181–1190
Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE (1999) New in vivo measurements of pressures in the intervertebral disc in daily life. Spine 24(8):755–762
Sis HL, Mannen EM, Wong BM, Cadel ES, Bouxsein ML, Anderson DE, Friis EA (2016) Effect of follower load on motion and stiffness of the human thoracic spine with intact rib cage. J Biomech 49(14):3252–3259
Anderson DE, Mannen EM, Sis HL, Wong BM, Cadel ES, Friis EA, Bouxsein ML (2016) Effects of follower load and rib cage on intervertebral disc pressure and sagittal plane curvature in static tests of cadaveric thoracic spines. J Biomech 49(7):1078–1084
Hattori S (1981) Cervical intradiscal pressure in movements and traction of the cervical spine. Z Orthop 119:568–569
Yuchi C-X, Sun G, Chen C, Liu G, Zhao D, Yang H, Xu B, Deng S, Ma X, Du C-F (2019) Comparison of the Biomechanical Changes After Percutaneous Full-Endoscopic Anterior Cervical Discectomy versus Posterior Cervical Foraminotomy at C5-C6: A Finite Element-Based Study. World Neurosurg 128:e905–e911
Markolf KL, Morris JM (1974) The structural components of the intervertebral disc: a study of their contributions to the ability of the disc to withstand compressive forces. JBJS 56(4):675–687
Goel VK, Clausen JD (1998) Prediction of load sharing among spinal components of a C5-C6 motion segment using the finite element approach. Spine 23(6):684–691
Yu C-C, Liu P, Huang D-G, Jiang Y-H, Feng H, Hao D-J (2016) A new cervical artificial disc prosthesis based on physiological curvature of end plate: a finite element analysis. Spine J 16(11):1384–1391
Womack W, Woldtvedt D, Puttlitz C (2008) Lower cervical spine facet cartilage thickness mapping. Osteoarthr Cartil 16(9):1018–1023
Schwab F, Lafage V, Boyce R, Skalli W, Farcy J-P (2006) Gravity line analysis in adult volunteers: age-related correlation with spinal parameters, pelvic parameters, and foot position. Spine 31(25):E959–E967
Wang Z, Zhao H, Liu J-m, Tan L-w, Liu P, Zhao J-h (2016) Resection or degeneration of uncovertebral joints altered the segmental kinematics and load-sharing pattern of subaxial cervical spine: A biomechanical investigation using a C2–T1 finite element model. J Biomech 49(13):2854–2862
Shirazi-Adl A, Ahmed AM, Shrivastava SC (1986) Mechanical response of a lumbar motion segment in axial torque alone and combined with compression. Spine 11(9):914–927
Yoganandan N, Kumaresan S, Pintar FA (2000) Geometric and mechanical properties of human cervical spine ligaments. J Biomech Eng 122(6):623–629
Du C-F, Guo J-C, Huang Y-P, Fan Y-B (2015) A new method for determining the effect of follower load on the range of motions in the lumbar spine. In: World Congress on Medical Physics and Biomedical Engineering, June 7-12, 2015, Toronto, Canada. Springer, pp 326-329
Kallemeyn N, Gandhi A, Kode S, Shivanna K, Smucker J, Grosland N (2010) Validation of a C2–C7 cervical spine finite element model using specimen-specific flexibility data. Med Eng Phys 32(5):482–489
Wheeldon JA, Pintar FA, Knowles S, Yoganandan N (2006) Experimental flexion/extension data corridors for validation of finite element models of the young, normal cervical spine. J Biomech 39(2):375–380
Panjabi MM, Crisco JJ, Vasavada A, Oda T, Cholewicki J, Nibu K, Shin E (2001) Mechanical properties of the human cervical spine as shown by three-dimensional load–displacement curves. Spine 26(24):2692–2700
Traynelis VC, Donaher PA, Roach RM, Kojimoto H, Goel VK (1993) Biomechanical comparison of anterior Caspar plate and three-level posterior fixation techniques in a human cadaveric model. J Neurosurg 79(1):96–103
Yoganandan N, Pintar FA, Stemper BD, Wolfla CE, Shender BS, Paskoff G (2007) Level-dependent coronal and axial moment-rotation corridors of degeneration-free cervical spines in lateral flexion. JBJS 89(5):1066–1074
Yoganandan N, Stemper BD, Pintar FA, Baisden JL, Shender BS, Paskoff G (2008) Normative segment-specific axial and coronal angulation corridors of subaxial cervical column in axial rotation. Spine 33(5):490–496
Wu T, Meng Y, Wang B, Rong X, Hong Y, Ding C, Chen H, Liu H (2019) Biomechanics following skip-level cervical disc arthroplasty versus skip-level cervical discectomy and fusion: a finite element-based study. BMC Musculoskelet Disord 20(1):49
Rong X, Wang B, Ding C, Deng Y, Chen H, Meng Y, Yan W, Liu H (2017) The biomechanical impact of facet tropism on the intervertebral disc and facet joints in the cervical spine. Spine J 17(12):1926–1931
Pelker RR, Duranceau JS, Panjabi MM (1991) Cervical spine stabilization. A three-dimensional, biomechanical evaluation of rotational stability, strength, and failure mechanisms. Spine 16(2):117–122
Moroney SP, Schultz AB, Miller JA, Andersson GB (1988) Load-displacement properties of lower cervical spine motion segments. J Biomech 21(9):769–779
Cripton PA, Bruehlmann SB, Orr TE, Oxland TR, Nolte L-P (2000) In vitro axial preload application during spine flexibility testing: towards reduced apparatus-related artefacts. J Biomech 33(12):1559–1568
Kim K, Kim YH, Lee S (2011) Investigation of optimal follower load path generated by trunk muscle coordination. J Biomech 44(8):1614–1617
Butler D, Trafimow JH, Andersson GB et al (1990) Discs degenerate before facets. Spine 15(2):111–113
Zhang JG, Wang F, Zhou R, Xue Q (2011) A three-dimensional finite element model of the cervical spine: an investigation of whiplash injury. Med Biol Eng Comput 49(2):193–201
Hussain M, Natarajan RN, An HS, Andersson GBJ (2012) Progressive disc degeneration at C5–C6 segment affects the mechanics between disc heights and posterior facets above and below the degenerated segment: A flexion–extension investigation using a poroelastic C3–T1 finite element model. Med Eng Phys 34(5):552–558
Pospiech J, Stolke D, Wilke HJ, Claes LE (1999) Intradiscal pressure recordings in the cervical spine. Neurosurgery 44(2):379–384
Acknowledgments
Financial support for this work was provided by the National Natural Science Foundation of China (NSFC Nos. 11432016, 11602172, and 11602063) and Sichuan Science and Technology Program (No. 2018SZ0036).
Author information
Authors and Affiliations
Contributions
CXY and YCCX carried out the model development and simulation, data analysis, and drafted the manuscript. DCF participated in the study design. DCF and MZJ participated in revising the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cai, XY., YuChi, CX., Du, CF. et al. The effect of follower load on the range of motion, facet joint force, and intradiscal pressure of the cervical spine: a finite element study. Med Biol Eng Comput 58, 1695–1705 (2020). https://doi.org/10.1007/s11517-020-02189-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-020-02189-7