Abstract
Increased intrathecal IgG and oligoclonal bands (OCB) are seminal features of multiple sclerosis (MS). Although no such differences in MS blood total IgG antibodies have been reported, serum OCB are a common and persistent finding in MS and have a systemic source. Recent studies showed that IgG3+ B cells and higher levels of serum IgG3 are linked to the development of MS. Additionally, intrathecal IgG synthesis in MS is associated with IgG3 heavy chain gene single nucleotide polymorphisms, and there is a strong relationship between susceptibility to MS and an IgG3 restriction fragment length polymorphism. These studies support the role of IgG3 in disease pathogenesis. Using multiple immunoassays, we investigated levels of total IgG, IgG1, and IgG3 in sera and CSF of 102 MS patients (19 paired CSF and sera), 76 patients with other neurological disorders (9 paired CSF and sera), and 13 healthy controls. We show that higher levels of total IgG and IgG3 antibodies were detected in MS serum, but not in CSF, which distinguishes MS from other inflammatory and non-inflammatory neurological disorders, with Receiver Operating Characteristic (ROC) Curves 0.79 for both IgG3 & total IgG. Our data support the notion that IgG3 antibodies may be a potential candidate for MS blood biomarker development.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most consistent and characteristic feature of multiple sclerosis (MS) is the increased synthesis of IgG and the presence of oligoclonal bands (OCBs) in the cerebrospinal fluid (CSF). Over 20 times more IgG can be extracted from MS plaques than from control brain tissue (Glynn et al. 1982). OCBs are associated with increased levels of disease activity/disability, higher levels of cortical lesion load, and greater brain atrophy (Joseph et al. 2009; Ferreira et al. 2014; Farina et al. 2017). OCB in MS have been shown to target ubiquitous intracellular antigens released in cellular debris (Brandle et al. 2016), and antigens to OCBs are known to be patient-specific (Graner et al. 2020).
The glycoprotein IgG is the most abundant isotype in healthy human plasma and can be separated into four subclasses: IgG1 (60–70% in plasma), IgG2 (20–30%), IgG3 (5–8%), and IgG4 (1–3%) (Vidarsson et al. 2014). Using multiple immunoassays, we undertook a detailed analysis of levels of total IgG (H + L), IgG1, and IgG3 subclasses in serum and CSF of MS patients. The rationale for using multiple methods is based on the following evidence:
-
1)
In MS CSF, the predominant subclasses are IgG1 and IgG3 (Keir et al. 1982; Vartdal and Vandvik 1982; Grimaldi et al. 1986; Mehta 1988; Losy et al. 1992).
-
2)
In MS, there is no correlation between the number of oligoclonal bands and CSF IgG concentration, but there is a strong correlation between serum and CSF IgG (Beseler et al. 2017).
-
3)
There are contradictory findings regarding MS serum total IgG antibodies. A study of 334 patients with MS and other neurological disorders found no difference in serum IgG between MS and controls (Fischer-Williams and Roberts 1971). Another study of 772 MS patients and 226 neurological controls again showed that there was no difference in serum IgG levels between MS and controls (Delmotte and Demonty 1976). However, about 40 years later it was demonstrated that high prevalence rates of reduced serum IgG concentrations were found in MS patients (Zoehner et al. 2019). The authors studied a total of 327 MS patients retrospectively and found that MS serum IgG concentrations were frequently below the lower limits of normal (< 700 mg/dl). Additionally, we showed that retrospective data of 90 MS patients revealed significantly higher levels of serum total IgG (Beseler et al. 2017) compared to patients with other inflammatory CNS disorders (n = 20), and elevated levels of glycosylated IgG are found in MS sera compared to other neurological disorders and healthy controls (Kennedy et al. 2021).
-
4)
There are contradicting results for IgG subclasses and the role of IgG3 in MS. Using single immunoassays, no significant differences of serum IgG1, IgG2, IgG3, and IgG4 were found between MS and controls (Kaschka et al. 1979; Lambin et al. 1991; Raknes et al. 2000; Greve et al. 2001; Di Pauli et al. 2010). However, recent studies showed higher IgG3 levels in sera may predict the development of MS from clinically isolated syndrome (Trend et al. 2018), and IgG3+ B cells are associated with the development of MS (Marsh-Wakefield et al. 2020). The roles of IgG3 in MS are further supported by the following studies: a) the IgG3 allotype G3m (11) was found to be MS-specific and present in the active brain plaques (Salier et al. 1983); b) the susceptibility to MS is associated with an IgG3 restriction fragment length polymorphism (Gaiser et al. 1987); c) intrathecal IgG synthesis in MS is associated with IgG3 heavy chain gene polymorphism (Buck et al. 2013).
-
5)
IgG3 has a unique structure and potent effector functions. The hinge region of IgG3 is about four times longer than that of IgG1, containing up to 62 amino acids with 11 hydrogen bonds (Vidarsson et al. 2014). This intra-protein feature may make it easier for the development of Fc-Fc interactions; however, it may prevent accurate detection in assays when Fc portions are required to be exposed. Furthermore, although a minor component in the blood, IgG3 has been shown to have superior effector functions compared to other IgG subclasses in neutralization, complement activation, opsonophagocytosis, and antibody-dependent cellular cytotoxicity (Damelang et al. 2019).
These findings suggest that MS IgG antibodies possess unique features, and their detections are assay dependent. Other evidence supporting the uniqueness of MS IgG is the presence of OCB in 95% MS CSF, and serum OCB is a common and persistent finding in MS and has a systemic source (Zeman et al. 1996). For decades it has been shown that antibodies play a direct role in MS pathology (Joseph et al. 2009; Farina et al. 2017; Yu et al. 2020; Karrenbauer et al. 2021), yet OCB were found to target random intercellular antigens, and MS antigens are patient-specific (Brandle et al. 2016; Graner et al. 2020).
We report here that higher levels of total IgG (H + L) and IgG3 antibodies in sera, but not in CSF, can distinguish MS patients from patients with other inflammatory and non-inflammatory neurological disorders.
Materials and Methods
Patients
With the approval of the Colorado Multiple Institutional Review Board (COMIRB protocols # 00–688, # 13–3007), CSF and serum from MS patients and controls were collected at the University of Colorado Hospital, Anschutz Medical Campus. Plasma samples were collected after centrifugation of blood samples at 2000 × g for 10 min; CSFs were immediately centrifuged at 500 × g for 10 min, and the supernatant was collected. Both CSF and sera were stored at − 80 °C until use. Additional sera samples (primary progressive MS – PPMS; secondary progressive MS – SPMS; relapsing–remitting MS – RRMS; healthy controls—HC) were from Accelerated Cure Project (https://www.acceleratedcure.org/).
We studied a total of 102 MS samples (67 RRMS, 8 SPMSS, and 19 PPMS) which contained 19 paired sera with CSF. We included 13 normal healthy controls (HC), 57 patients with non-inflammatory neurological disease (NIC), and 19 with other inflammatory CNS disorders (IC) (9 paired with CSF). All samples were from treatment-naive patients. Other neurological disorders include 19 IC patients, 5 patients with headaches, and 43 patients with meningioma, and 8 with glioblastoma (Table 1). The IC patients include: acute viral meningitis (1), B cell lymphoma (1), Behcet's disease (1), paraneoplastic syndrome (1), viral meningitis (1), chronic meningitis of unknown etiology (1), subacute sclerosing panencephalitis (2), acute disseminated encephalomyelitis (1), paraneoplastic encephalitis (1), neurosyphilis, chronic progressive meningoencephalitis (1), sarcoid (1), VZV myelopathy (1), VZV radiculomyelitis (1), VZV shingles (1), ischemic optic neuritis (1), retrobulbar optic neuritis (1), papillitis (1), and transverse myelitis (1) (Tables 1 and Table 2).
For all experiments, we used total IgG concentrations derived from patients’ clinical charts to determine the amounts of IgG used.
Western Blots
Mini-PROTEAN® TGX 4–15% Gels (BioRad) were used for SDS-PAGE analysis with 1 × Tris/Glycine/SDS running buffer (BioRad). CSF and paired plasma (1 µg total IgG antibody equivalent) in TBS were denatured and reduced by incubation with 1 × lane marker reducing sample buffer containing dithiothreitol (Thermo Scientific) at 95 °C for 10 min. Gels were electrophoresed for 40 min at a constant voltage of 150 V, and electro-blotted onto PVDF membranes (Bio-Rad) for 45 min at a constant 10 V using Trans-Blot® Semi-Dry Cell (Bio-Rad). Membranes were blocked overnight in 1 × casein/TBS/0.05% Tween 20 (Vector Labs). Triplicate blots were probed with corresponding antibodies for total IgG, IgG1, and IgG3 detection. HRP-conjugated goat anti-human IgG (H + L) (Vector Labs) was used for total IgG detection at a dilution of 1:2000. Monoclonal mouse anti-human IgG1 (1:2000 dilution, clone 8c/6–39, Sigma), and monoclonal mouse anti-human IgG3 (1:2000 dilution, clone HP-6050, Sigma) were incubated with membranes at 4ºC overnight. SuperSignal® West Pico was used for total IgG detection, and anti-mouse IgG (H + L) HRP/West Femto for detection of IgG1 & IgG3). Schematic structures of human IgG1 and IgG3 highlighting the difference between the hinge regions are shown in Fig. 1D.
Slot Blots
Total CSF and paired serum of two dilutions (total antibody equivalent of 0.8 ng & 4 ng for IgG1, 120 ng & 600 ng for IgG3) in duplicates were loaded into wells of Schleicher & Schuell Minifold II Slot Blotter (onto nitrocellulose membrane) followed by a brief vacuum. Membranes were blocked and detected with corresponding antibodies for total IgG, IgG1, and IgG3 as described above in the Western blots section.
Quantitative Western and Slot Blotting (Band Intensity Analysis)
Western blots and slot blots were imaged digitally on the FluorChem Q (Alpha Innotech/Protein Simple). The software AlphaView™Q was programmed to collect the images at different time exposures. Analysis of the images was conducted with AlphaView software provided by Alpha Innotech. The bands were designated by drawing boxes around them. The same-sized box was used for each band. For the FluorChem Q images, the background was measured from a single box adjacent to the bands and subtracted from the value for each band.
ELISA
ELISA with human IgG Subclass Kit. A commercial ELISA kit for human IgG subclasses (Invitrogen #991,000) was used for serum and CSF IgG 1–4 detection. The 1:100 pre-diluted MS and control sera samples were thawed on ice, further diluted to 1:3,000 of the original neat sera. For each well, 50 µl of the MAB-anti-subclass IgG antibodies were added to the wells of the ELISA stripes. Then 50 µl of diluted sera and standards were added to each well. After one hour of incubation at room temperature with shaking (25 rpm), the ELISA plates were washed 6 times with washing buffer. Wells were added with 100 µl of HRP-anti-human IgG (1:50) and incubated for one-hour at room temperature with shaking (25 rpm) followed by washing. The plates were developed with 100 µl/well TMB substrate for 20 min, and 100 µl of stopping buffer was added to each well. The color density was measured by a microplate reader (BioTek Synergy plate reader).
Direct ELISA for total IgG. ELISA plates (Thermal Scientific) were coated directly with diluted plasma (1:900 in TBS) at 4 °C overnight. Plates were blocked by 1 × casein (Vector Lab), and bound IgG was detected by goat anti-Human IgG (H + L) HRP (1:2000) at RT for 1 h, followed by the addition of TMB color substrate. The reaction was stopped by the addition of 100 µl of 1 N HCl. Plates were read at 450 nm.
Capture ELISA for detection of IgG3. MaxiSorp treated 96-well plates (Thermo #436,110) were used for in-house IgG3 ELISA. The plates were rinsed twice with 1 × TBST (Bio-Rad #1,706,435) (0.1% Tween-20). The plates were then coated with goat anti-human IgG (H + L) at 50 µg/ml (Vector Lab #AI-3000) in 0.1 M NaHCO3 (pH 9.4) (60 µl/well) at 4 °C overnight. The next day, the wells were blocked with 3% BSA in TBS for 5 h at room temperature with shaking (25 rpm). The plates were rinsed 3 times with 1 × TBST. Previously, the MS and control sera samples had been diluted at 1:100 in PBS and aliquoted and stored at -80 °C freezer. The pre-diluted sera samples were thawed on ice and diluted at 1:100 in 1 × TBS (total dilution of 1:10,000 from the original neat sera). For each well, 50 µl of the diluted sera (or 50 µl of standards) and 50 µl of TBS were added to the ELISA plates and incubated at 4 °C with shaking (25 rpm) overnight. The next day, the plates were washed 6 times with 1 × TBST, followed by the addition of 100 µl biotinylated mouse anti-human IgG3 antibody (Southern Biotech #9210–08, 1:3,000 in 1 × TBS), and incubated at room temperature for 1 h with shaking. The plates were washed 6 times with 1 × TBST, followed by incubation with NeutrAvidin-HRP (Thermo #31,030, 1:10,000 dilution in 1 × TBS, 100 µl/well) for 1 h at room temperature with shaking. Finally, the ELISA plates were washed 6 times with 1 × TBST, and developed with TMB (SeraCare two components substrate kit, #5120–0047, 100 µl/well) for 10 min. The reaction was stopped with 0.1 N HCl, and the color density was measured by a microplate reader (BioTek Synergy plate reader) at 450 nm.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism (version 8.3). One-way ANOVA followed by Tukey’s analysis for multiple comparisons was used to examine differences between groups. Mann–Whitney U test was used for paired samples.
Results
Justification for Use of Multiple Immunoassays
We used Western blots for the initial characterization of IgG antibodies, focusing on IgG1 and IgG3. Because of the semi-quantitative nature of the technique, we decided to apply relatively easy assays, Slot blots, for confirmation, and ELISA for testing larger samples. We used commercial ELISA kits for detection of IgG subclasses 1–4, and for validating our in-house ELISA method. We applied in-house ELISA to demonstrate higher levels of total IgG and IgG3 in MS.
SDS-PAGE Western Blots Showed That in MS the 75 kDa Band (combination of Heavy and Light Chains) and the 51 kDa Heavy Chain Band of IgG3 Are Significantly Elevated in the Ratio of Serum/CSF Compared to IgG1
Both IgG1 and IgG3 have been shown to be predominant IgG subclasses in MS CSF (Mehta 1988; Losy et al. 1992). However, high prevalence rates of reduced serum IgG concentrations were found in MS patients with and without disease-modifying treatments (Zoehner et al. 2019). To further characterize IgG1 and IgG3 in MS, we carried out Western blots of paired CSF and sera to detect levels of IgG1 and IgG3. IgG3 heavy chain is approximately 6.5 kDa larger than IgG1 heavy chain based on the molecular weight of 62 additional amino acids in the IgG3 hinge region. Although the accurate size of the IgG3 heavy and light chain band should be 81.5 kDa, we used the term of 75 kDa band for clarity. We observed that both subclasses can be detected in paired sera and CSF from MS patients. We also noticed that blots probed for total IgG show multiple bands of approximately150 kDa, 100 kDa, 75 kDa, 51 kDa, and 25 kDa. The 75 kDa band is the combination of one heavy chain (50 kDa) and one light chain (25 kDa). The 50 kDa is the heavy chain size, and the 25 kDa is the light chain size of IgG molecules. However, only in the blots probed for IgG1 and IgG3, but not in the total IgG blot, a 75 kDa band of heavy and light chains was detected. In total IgG blots, the failure to detect the 75 kDa bands may be due to oversaturation of detection with higher amounts of HRP anti-human IgG. Figure 1A shows representative Western blots probed for total IgG (left panel), IgG1 (middle panel), and IgG3 (right panel). Figure 1B are Western blots for IgG1 and IgG3 from all 7 MS patients. Note that SDS-PAGE was performed under reducing and denaturing conditions. We targeted heavy chains instead of full IgG3 because that the IgG subclasses are distinguished by the constant region of the heavy chains, and the amino acid sequences of the human IgG subclasses are > 95% homologous in the constant domains of their heavy chains (Damelang et al. 2019).
Western blots detecting IgG1 and IgG3 subclasses in paired CSF and serum from MS and controls. Total IgG concentrations were derived from the clinal data. (A) Representative Western blots show that the 75 kDa (heavy & light chains) band and the 51-kDa heavy chain band are detected by anti-IgG1 and anti-IgG3 antibodies in the CSF (C) and paired serum (S). Total CSF and serum (1 µg IgG equivalent/well) were separated, and blots were probed with anti-human IgG (H + L)-HRP (left), mouse anti-IgG1 (middle), and anti-IgG3 antibodies (right), followed by incubation with anti-mouse HRP secondary antibodies and chemiluminescent detection. Red squares highlight the bands of 51 kDa and 75 kDa. Notice that no 75 kDa heavy and light chain bands were detected by total IgG antibodies (left), suggesting an oversaturated detection. (B) Summary of Western blot data from 7 MS patients. Band intensity analysis of Western blots revealed that both bands of IgG3 are significantly elevated in the ratio of serum/CSF compared to IgG1 levels. We measured the intensity of individual bands of 75 kDa and 51 kDa in the highlighted area in Fig. 1 A and compared the ratios of serum to CSF of both heavy chains between IgG3 and IgG1. Both the 75 kDa and the 51 kDa IgG3 bands are significantly higher in the ratio of serum/CSF compared to that of the IgG1 heavy chains (p = 0.0017, n = 7; two-way ANOVA). (C) Western blots for total IgG, IgG1, and IgG3 from patients with non-inflammatory neurological diseases (NIC). No IgG3 75 kDa and 51 kDa bands were detected in the CSF of NIC patients. (D). Schematic structures of human IgG1 and IgG3 show differences between the hinge regions. IgG molecules consist of four polypeptide chains, composed of two identical γ heavy (grey) chains of 50 kDa and two identical light (green) chains of 25 KDa, linked together by interchain disulfide bonds. IgG molecules comprise similarly sized globular portions joined by a flexible stretch of polypeptide chain between CH1 and CH2, known as the hinge region. Notice that IgG3 has 4 times longer hinge region compared to IgG1 (based on Demelang et al. 2019)
Band intensity analyses of the 51 kDa and 75 kDa bands of all 7 paired CSF and sera IgG1 and IgG3 blots were carried out, and the ratios of both bands were calculated between sera and paired CSF. We calculated the heavy chain ratios of serum to CSF for both IgG1 and IgG3 antibodies. We showed that in MS patients, both the 75 kDa band and the 51 kDa band of IgG3 were significantly elevated in the sera, relative to the CSF, compared to IgG1 levels (p = 0.0017, n = 7; two-way ANOVA); summary of the data are shown in Fig. 1C. To demonstrate that the higher serum levels of IgG3 are unique to MS, we carried out similar Western blot analyses of paired CSF and sera in patients with non-inflammatory neurological diseases (NIC, n = 5) for detection of total IgG, IgG1, and IgG3. No IgG3 75 kDa and 51 kDa bands were detected in the CSF of NIC patients (Fig. 1D), suggesting that the increased IgG3 heavy chain bands are unique in MS. It is of interest that IgG3 molecules have extended hinge regions replete with disulfide bonds (Fig. 1E); even under reducing conditions, there may be aberrant disulfide scrambling with potential binding of other components.
Slot Blots Native Sample Analysis Demonstrates the Presence of Significantly Higher Levels of IgG3 Antibodies in MS Sera Compared to Patients With Other CNS Inflammatory Disorders
To confirm the Western blots results that MS sera have higher levels of IgG3 antibodies, we carried out slot blot analysis with larger numbers of MS and IC samples. We determined the relative levels of IgG3, total IgG, and IgG1 in MS CSF and paired sera in comparison with individuals with other CNS inflammatory disorders using a slot blot technique where the proteins are in a non-reduced, non-denatured state. Total CSF and sera of two fivefold dilutions in duplicates were used followed by antibody detections as described above. We used higher amounts of total IgG equivalent for detection of IgG3 and lower total IgG for detection of IgG1 to make sure the bands are in the linear range of detection. Further, we used a dilution series to normalize the quantification. A representative blot was shown in Fig. 2A. The experiments were repeated at least once. We analysed 38 MS samples (19 paired CSF and serum samples) using this method together with band intensity analysis. The results were compared with those obtained in 18 samples from IC patients (9 paired CSF and serum samples). The ratio of serum/CSF of IgG3 was higher in MS patients than other IC patients, and this is due to higher mean levels of IgG3 in the serum of MS patients (Fig. 2B, p = 0.0061). Further, no significant differences were detected in IgG1 and total IgG levels when MS samples were compared with those from IC patients (Fig. 2C and 2D).
Slot blot analysis demonstrates that IgG3 levels are significantly higher in MS sera /CSF ratio compared to sera/CSF ratio from patients with other CNS inflammatory disorders. We analyzed 38 MS samples (19 pairs of CSF & serum) and 18 samples (9 pairs of CSF and serum) from IC patients to determine the levels of total IgG, IgG1, and IgG3 using slot blots. A Representative slot blots from MS and IC patients with duplicate samples. Total CSF and sera of two fivefold dilutions (in duplicates) were loaded into wells of Schleicher & Schuell Minifold II Slot Blotter (onto nitrocellulose membrane). Membranes were blocked and detected with corresponding antibodies for total IgG, IgG1, and IgG3. (B-D) Summaries of slot blots for IgG3 B, IgG1 C, and total IgG D. Band intensities analyses were performed and ratios of serum to paired CSF were calculated for all antibodies. Levels of IgG3 in serum/CSF are significantly higher in MS compared to other inflammatory CNS disorders, p = 0.0061 B, and no differences were detected in IgG1 and total IgG between MS and IC C & D. Unpaired t-test (nonparametric) Mann–Whitney test. ** = p < 0.01
IN CSF, There Are No Differences in the Levels of IgG3 and IgG1 Between MS Patients and Individuals With Other Neurological Disorders
When Western blots were used to compare levels of IgG3 and IgG1 in MS and IC CSF, no differences for 75 kDa and 51 kDa bands in both IgG1 and IgG3 blots were observed. This is shown in Fig. 3A. These studies were then extended by using CSF capture ELISA with larger numbers of MS CSF (n = 12) and OND CSF (n = 8) using a commercial human IgG subclass ELISA kit (Thermal Scientific) to quantify levels of IgG1 and IgG3 in the CSF. We demonstrated that there was no difference in IgG1 and IgG3 levels in the CSF between MS patients and individuals with other neurological diseases (inflammatory and non-inflammatory). These data are shown in Fig. 3B.
In CSF there are no differences in IgG3 and IgG1 levels between MS and other neurological disorders. A We carried out Western blots to compare levels of IgG3 and IgG1 in MS and IC CSF, and no differences were observed. B CSF capture ELISAs using commercial kits for IgG1 and IgG3 do not show differences between MS and OND. Pre-coated ELISA wells were added in duplicates of CSF (MS = 12; OND = 8) (1:1dilution) together with anti-IgG1 or anti-IgG3 antibodies, followed by the addition of HRP secondary antibodies and TMB detection. IgG1 and IgG3 standards were included for the determination of the subclass concentration
Using Commercial Human IgG Subclass ELISA Kits, No Differences in Any IgG Subclasses Were Detected Between MS and Control Sera
We are aware that Western blots for quantification can only provide semi-quantitative results. We therefore extended our investigation using ELISA to determine levels of IgG subclasses using a commercial human IgG subclass ELISA kit. The results of these experiments are shown in Fig. 4. We detected no differences between MS and control sera in all IgG subclasses (IgG1, IgG2, IgG3, IgG4) (Fig. 4A). We expanded MS samples (24 RRMS, 16 SPMS, 11 PPMS) and controls (16 healthy controls, and 24 OND) for detection of IgG3, and we found that there was no difference in IgG3 levels between MS and control sera (Fig. 4B). We also developed an in-house ELISA using goat anti-human IgG (H + L) as capture antibodies and Biotin-anti-IgG3 as detection antibodies. We carried out a comparison between our in-house ELISA protocol and the commercial IgG subclass kit using the IgG3 standard. We obtained near identical results of both methods which validated the in-house ELISA protocol (Fig. 4C).
Using commercial human IgG subclass kits, no differences in any IgG subclasses were detected between MS and control sera. Human IgG subclass ELISA kit (Invitrogen) was used to determine the levels of each IgG subclass (IgG1, IgG2, IgG3, IgG4) in sera (12 MS and 7 HC) following the recommended procedures by the manufacturer, with human IgG subclass standards included for quantification. No differences were detected between MS and HC sera in every IgG subclass A. We further determined the levels of IgG3 subclass with increased numbers of samples including 51 MS (24 RRMS, 16 SPMS, 11 PPMS), 16 healthy controls, and 24 sera from patients with other neurological disorders (8 IC and 16 brain tumor samples). No significant differences between MS and controls were detected B. C. Comparison of IgG3 standard using the commercial kit and in-house capture ELISA protocols. Similar linear regressions were obtained by both methods
Direct and Capture ELISA Show Significantly Higher Levels of Total IgG (H + L) and IgG3 in MS Sera Which Distinguish MS From Other Neurological Disorders
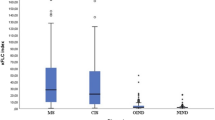
We applied in-house ELISA to determine levels of total IgG and IgG3 in MS sera. A total of 102 MS, 76 OND, and 13 HC sera were used for both ELISA studies. We first carried direct ELISA by coating diluted sera directly onto wells of ELISA plates. After the addition of sera dilutions directly onto wells of ELISA plates at 4 °C overnight, bound IgG was detected by HRP goat-anti-human IgG. While we demonstrated that sera from MS patients had significantly higher levels of total IgG compared to OND patients (p < 0.0001), we did not detect a significant difference between MS and HC (Fig. 5A). Furthermore, we carried out a capture ELISA to detect serum IgG3. We expanded MS samples including 24 RRMS, 24 SPMS, and 19 PPMS, as well as OND and HC. The results of capture IgG3 ELISA are shown in Fig. 5C. MS sera had significantly higher levels of IgG3 compared to OND sera (p < 0.0001), and a mild increase of IgG3 in MS patients compared to normal healthy controls was also detected (p = 0.015). We carried out Receiver Operating Characteristic (ROC) curves analysis to determine whether serum total IgG and IgG3 in MS can be used as potential biomarkers, and the results are shown in Fig. 5B, D. For relatively small sample sizes (MS = 67, OND = 55), serum total IgG showed a moderate power to distinguish MS from other neurological disorders, with an AUC of 0.79 (p < 0.0001; 95% CI, 0.7066 to 0.879). The ROC curve for serum IgG3 in MS (n = 43) compared to OND (n = 32) showed that the AUC was 0.7911 (p < 0.0001; 95% CI, 0.6903 to 0.8918).
Serum total IgG and IgG3 distinguish MS from other neurological disorders, with 79% sensitivity and specificity. A Direct ELISA shows that MS sera have significantly higher levels of total IgG compared to OND. Sera from MS (n = 67), OND (n = 55), and HC (n = 13) diluted in TBS were coated directly in wells of ELISA plates at 4ºC overnight. Coated wells were blocked with casein and detected with goat anti-Human IgG (H + L) HRP followed by TMB color reaction. MS sera have significantly higher levels of total IgG compared to sera of patients with other neurological diseases (p < 0.0001), but no difference was detected between MS and HC. B ROC curve for serum total IgG in MS (n = 67) compared to OND (n = 55). The area under the curve is 0.79 (p < 0.0001; 95% CI, 0.7066 to 0.879). C Capture ELISA shows serum IgG3 levels in MS are significantly higher compared to OND (p < 0.0001) and HC (p = 0.015). MS (n = 43), OND (n = 24), and HC (n = 16) sera (1;10,000 dilution in TBS) were added to wells pre-coated with goat anti-human IgG (H + L) at 4ºC overnight. Wells were blocked with 3% BSA and detected with mouse anti-human IgG3 antibody followed by NeutrAvidin-HRP. Notice the higher levels of IgG3 in MS compared to OND (p < 0.0001) and HC (p = 0.015), with no difference of IgG3 between OND and HC. D ROC curve for serum IgG3 in MS (n = 43) compared to OND (n = 32). The area under the curve is 0.7911 (p < 0.0001; 95% CI, 0.6903 to 0.8918)
Discussion
We previously compared total IgG concentrations between 90 MS and 24 inflammatory CNS diseases (IC) patients based on the data from clinical laboratory measurements. We showed that there was no significant difference in CSF IgG levels between MS and IC. However, significantly elevated levels of serum IgG were found in MS compared to IC (Beseler et al. 2017). Similarly, using capture ELISA, we showed that there is no difference in CSF IgG between MS and IC, but higher serum IgG levels in MS were detected in our recent publication (Kennedy et al. 2021). Our current findings are consistent with previous studies demonstrating the higher IgG levels in MS sera. Furthermore, there is no correlation between the number of oligoclonal bands and CSF IgG concentration (Beseler et al. 2017), and those results are consistent with studies showing that there is no correlation between the level of intrathecal synthesis obtained by Reibergrams and the number of oligoclonal bands on gels (Delic-Sarac et al. 2013).
In the current study, we focussed on MS serum IgG1 and IgG3 because of the following evidence:
-
1)
In MS CSF, the predominant subclasses are IgG1 and IgG3 (Keir et al. 1982; Vartdal and Vandvik 1982; Grimaldi et al. 1986; Mehta 1988; Losy et al. 1992).
-
2)
There is a strong correlation between MS serum IgG and CSF IgG (Beseler et al. 2017).
-
3)
Studies that had shown the potential pathological role of MS serum IgG antibodies (Yu et al. 2020).
-
4)
Higher IgG3 levels in sera may predict the development of MS from a clinically isolated syndrome (Trend et al. 2018), and IgG3 + B cells are associated with the development of MS (Marsh-Wakefield et al. 2020).
The main finding of this study was that higher levels of IgG3 in the serum, but not in the CSF, were detected in MS compared with individuals with inflammatory and non-inflammatory disorders. The commercial kit for the IgG subclass works well for standards. However, only the in-house ELISA was able to detect the differences for MS IgG which suggests that MS antibodies have unique features, and this needs further investigation. We showed that by in-house direct sera plating, and by capture ELISA, both total IgG and IgG3 are significantly higher in MS compared to controls. These results showed a moderate capability to distinguish MS from other CNS disorders, with an AUC of 0.79 for both total IgG and IgG3.
Because of the unique feature of IgG3 possessing 4 times longer hinge region and 11 hydrogen bonds, compared to IgG1, accurate detection may depend on the methods used. Indeed, contradictory results of IgG3 in MS have been reported. For example, using commercial IgG subclass kits, it’s been shown that no differences in serum IgG3 levels between MS and controls (Di Pauli et al. 2010). However, higher serum IgG3 levels in MS were detected using cytometric bead arrays (BD Biosciences), with data captured using the BD LSRFortessa flow cytometer, and may predict the development of MS from a clinically isolated syndrome (Trend et al. 2018). The longer hinge regions and 11 hydrogen bonds of IgG3 may make it easier to promote or maintain Fc-Fc interactions; however, it may prevent accurate detection in assays when Fc portions are required to be exposed. Thus, the extended region may be detectable in other assays such as our in-house ELISA using direct serum coating or using anti-human IgG (H + L) as capture antibodies. Our data were generated with three independent immunoassays and repeated at least once by several different investigators. The fact that our in-house custom ELISA detection of IgG3 standard showed similar sensitivity and specificity to that of a commercial human IgG ELISA kit (Fig. 4C) provides confidence in our custom ELISA methodology and our results.
We applied varied sample sizes for different methods for the following reasons:
1) Sample size for Western blots: We used 7 paired MS CSF and sera for Western blots for initial characterization of total IgG, IgG1, and IgG3. These are non-trivial experimental procedures that may utilize precious samples such as matched CSF along with the patient sera.
2) Slot blots: We increased the sample numbers to 19 paired MS CSF and sera and 9 IC with paired CSF and sera. We chose IC samples because they have been considered the best controls for MS since both MS and IC have oligoclonal bands (Gilden 2005). However, matched sera/CSF pairs are limited by CSF availability.
3) Sample size for IgG3 using commercial IgG ELISA kit: We showed no differences for IgG1-IgG4 between MS and HC using ELISA kits (Fig. 4A) which is consistent with previous reports (Kaschka et al. 1979; Lambin et al. 1991; Raknes et al. 2000; Greve et al. 2001; Di Pauli et al. 2010); we also found no differences in sera IgG3 levels between MS and controls. We used 51 serum samples for ELISA kits because all these samples are from Accelerated Cure Project and included 3 subtypes of MS (24 RRMS, 16 SPMS, 11 PPMS), 16 HC, and 24 sera from patients with other neurological disorders (8 IC and 16 brain tumor samples) to eliminate sample collection variability. Using commercial ELISA kits with samples collected from 2 MS centers, we showed consistent results that the higher IgG3 in MS were not detected by the commercial kits, and there was no difference of IgG3 among MS subtypes (Fig. 4B).
4) Sample size for in-house ELISA: We compared detection of IgG3 standard using the commercial kit and in-house capture ELISA protocol. We showed similar linear regressions obtained by both methods (Fig. 4C). For larger sample evaluation, we included all available MS samples (n = 102) from both our University and ACP for in-house ELISA to demonstrate the presence of higher IgG3 levels in MS sera.
We previously reported that MS IgG antibodies target patient-specific peptides (Graner et al. 2020). The higher levels of total IgG and IgG3 are possibly stimulated by infectious antigens or self-antigens (Brandle et al. 2016), which may not be MS-specific. Kwon et al. (Kwon et al. 2022) reported that MOG-IgG CSF, but not MOG-IgG serum titers are associated with disease severity; they also demonstrated that there is a strong correlation between CSF/serum MOG-IgG index and disease severity. Early studies revealed that MS patients who suffered from relapse were significantly more frequently seropositive for anti-MOG IgG3 than were those in remission, and patients treated with intravenous Ig or interferon-beta showed a significant reduction of anti-MOG IgG3 antibodies (García-Merino et al. 1986). These studies demonstrated the importance of CSF/serum MOG-IgG antibodies and IgG3 in MS disease pathogenesis, which are consistent with our findings. It is also conceivable that the IgG reactivity with MOG may maintain higher antibody concentrations within the CSF versus the plasma, and/or that while an important antibody in MS pathology, the overall concentration of the MOG antibody may not be that high in the larger antibody population in the plasma.
The novelty of our study includes the following: 1) utilization of multiple immunoassays detecting denatured and native IgG; 2) validation of in-house ELISA (direct sera coating ELISA and anti-human IgG H + L coating as capture antibody); 3) utilizing samples collected from two centers (our University and ACP); 4) demonstration of higher levels of serum total IgG and IgG3 in MS. Although limited by small sample size, the results of higher levels of total IgG and IgG3 in MS serum are consistent with our recent report of increased serum glycosylated IgG antibodies in MS (Kennedy et al. 2021). The assay-dependent feature of MS serum antibodies may suggest a complex nature of these antibodies which needs further investigations. Our findings are significant and consistent with previous studies supporting the significance of serum IgG antibodies in MS disease pathogenesis: 1) Serum antibodies of MS target microvessels in the brain tissues (Long et al. 2013), and correlate with brain MRI measures of disease severity (Bakshi et al. 2016); 2) Sera from RRMS and SPMS disrupt the blood–brain barrier (Shimizu et al. 2014); 3) B-cell clusters corresponding to OCBs in MS are observed only in the blood (Bankoti et al. 2014a, b) There is a strong correlation between disease activity and demyelinating activity of the MS serum (Lumsden 1971). Although we did not necessarily reveal mechanisms of MS, we provide novel findings that MS serum IgG antibodies are unique, which may provide a steppingstone for future investigations for mechanisms.
References
Bankoti J, Apeltsin L, Hauser SL, Allen S, Albertolle ME, Witkowska HE, Von Büdingen HC (2014) In multiple sclerosis, oligoclonal bands connect to peripheral B-cell responses. Ann Neurol 75:266–276
Bakshi R, Yeste A, Patel B, Tauhid S, Tummala S, Rahbari R, Chu R, Regev K, Kivisäkk P, Weiner HL, Quintana FJ (2016) Serum lipid antibodies are associated with cerebral tissue damage in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 3:e200
Bankoti J, Apeltsin L, Hauser SL, Allen S, Albertolle ME, Witkowska HE, Von Büdingen HC (2014) In multiple sclerosis, oligoclonal bands connect to peripheral B-cell responses. Ann Neurol 75:266–276
Beseler C, Vollmer T, Graner M, Yu X (2017) The complex relationship between oligoclonal bands, lymphocytes in the cerebrospinal fluid, and immunoglobulin G antibodies in multiple sclerosis: Indication of serum contribution PLoS One 12: e0186842
Brandle SM, Obermeier B, Senel M, Bruder J, Mentele R, Khademi M, Olsson T, Tumani H, Kristoferitsch W, Lottspeich F, Wekerle H, Hohlfeld R, Dornmair K (2016) Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proc Natl Acad Sci U S A 113:7864–7869
Buck, D., Albrecht, E., Aslam, M., Goris, A., Hauenstein, N., Jochim, A., International Multiple Sclerosis Genetics, C., Wellcome Trust Case Control, C., Cepok, S., Grummel, V., Dubois, B., Berthele, A., Lichtner, P., Gieger, C., Winkelmann, J., and Hemmer, B (2013) Genetic variants in the immunoglobulin heavy chain locus are associated with the IgG index in multiple sclerosis. Ann Neurol 73:86–94
Damelang T, Rogerson SJ, Kent SJ, Chung AW (2019) Role of IgG3 in Infectious Diseases. Trends Immunol 40:197–211
Delic-Sarac M, Izetbegovic S, Karamehic J, Subasic D, Saban A, Jukic T, Coric J, Panjeta M (2013) Detection methods of immunoglobulin IgG in CSF and serum. Med Arch 67:368–371
Delmotte P, Demonty L (1976) Biochemical findings in multiple sclerosis. II. A detailed study of the serum IgA, IgG and IgM levels of 772 MS patients compared with 226 neurological controls. J Neurol 211:135–142
Di Pauli F, Gredler V, Kuenz B, Lutterotti A, Ehling R, Gneiss C, Schocke M, Deisenhammer F, Reindl M, Berger T (2010) Features of intrathecal immunoglobulins in patients with multiple sclerosis. J Neurol Sci 288:147–150
Farina G, Magliozzi R, Pitteri M, Reynolds R, Rossi S, Gajofatto A, Benedetti MD, Facchiano F, Monaco S, Calabrese M (2017) Increased cortical lesion load and intrathecal inflammation is associated with oligoclonal bands in multiple sclerosis patients: a combined CSF and MRI study. J Neuroinflammation 14:40
Ferreira D, Voevodskaya O, Imrell K, Stawiarz L, Spulber G, Wahlund LO, Hillert J, Westman E, Karrenbauer VD (2014) Multiple sclerosis patients lacking oligoclonal bands in the cerebrospinal fluid have less global and regional brain atrophy. J Neuroimmunol 274:149–154
Fischer-Williams M, Roberts RC (1971) Cerebrospinal fluid proteins and serum immunoglobulins. Occurrence in multiple sclerosis and other neurological diseases: comparative measurement of gamma-globulin and the IgG class. Arch Neurol 25:526–534
Gaiser CN, Johnson MJ, De Lange G, Rassenti L, Cavalli-Sforza LL, Steinman L (1987) Susceptibility to multiple sclerosis associated with an immunoglobulin gamma 3 restriction fragment length polymorphism. J Clin Invest 79:309–313
García-Merino A, Persson MA, Ernerudh J, Díaz-Gil JJ, Olsson T (1986) Serum and cerebrospinal fluid antibodies against myelin basic protein and their IgG subclass distribution in multiple sclerosis. J Neurol Neurosurg Psychiatry 49:1066–1070
Gilden DH (2005) Infectious causes of multiple sclerosis. Lancet Neurol 4:195–202
Glynn P, Gilbert HM, Newcombe J, Cuzner ML (1982) Analysis of immunoglobulin G in multiple sclerosis brain: quantitative and isoelectric focusing studies. Clin Exp Immunol 48:102–110
Graner M, Pointon T, Manton S, Green M, Dennison K, Davis M, Braiotta G, Craft J, Edwards T, Polonsky B, Fringuello A, Vollmer T, Yu X (2020). Oligoclonal IgG antibodies in multiple sclerosis target patient-specific peptides. PLoS One 15:e0228883
Greve B, Magnusson CG, Melms A, Weissert R (2001) Immunoglobulin isotypes reveal a predominant role of type 1 immunity in multiple sclerosis. J Neuroimmunol 121:120–125
Grimaldi LM, Maimone D, Reggio A, Raffaele R (1986) IgG1,3 and 4 oligoclonal bands in multiple sclerosis and other neurological diseases. Ital J Neurol Sci 7:507–513
Joseph FG, Hirst CL, Pickersgill TP, Ben-Shlomo Y, Robertson NP, Scolding NJ (2009) CSF oligoclonal band status informs prognosis in multiple sclerosis: a case control study of 100 patients. J Neurol Neurosurg Psychiatry 80:292–296
Karrenbauer VD, Bedri SK, Hillert J, Manouchehrinia A (2021) Cerebrospinal fluid oligoclonal immunoglobulin gamma bands and long-term disability progression in multiple sclerosis: a retrospective cohort study. Sci Rep 11:14987
Kaschka WP, Theilkaes L, Eickhoff K, Skvaril F (1979) Disproportionate elevation of the immunoglobulin G1 concentration in cerebrospinal fluids of patients with multiple sclerosis. Infect Immun 26:933–941
Keir G, Walker RW, Johnson MH, Thompson EJ (1982) Nitrocellulose immunofixation following agarose electrophoresis in the study of immunoglobulin G subgroups in unconcentrated cerebrospinal fluid. Clin Chim Acta 121:231–236
Kennedy PGE, Graner M, Pointon T, Li X, Tanimoto K, Dennison K, Im G, Fringuello A, Zhou W, Graner A, Sillau S, Vollmer T, Yu X (2021) Aberrant Immunoglobulin G Glycosylation in Multiple Sclerosis. J Neuroimmune Pharmacol
Kwon YN, Kim B, Kim JS, Mo H, Choi K, Oh SI, Kim JE, Nam TS, Sohn EH, Heo SH, Kim SB, Park KC, Yoon SS, Oh J, Baek SH, Kim BJ, Park KS, Sung JJ, Jung JH, Kim SJ, Park SH, Waters P, Kim SM (2022) Myelin Oligodendrocyte Glycoprotein-Immunoglobulin G in the CSF: Clinical Implication of Testing and Association With Disability. Neurol Neuroimmunol Neuroinflamm 9
Lambin P, Gervais A, Levy M, Defendini E, Dubarry M, Lebon P, Rouger P, Schuller E (1991) Intrathecal synthesis of IgG subclasses in multiple sclerosis and in acquired immunodeficiency syndrome (AIDS). J Neuroimmunol 35:179–189
Long Y, Gao C, Qiu W, Hu X, Peng F, Lu Z (2013) Antibodies target microvessels in neuromyelitis optica and multiple sclerosis patients. Neurol Res 35:922–929
Losy J, Michałowska-Wender G, Wender M (1992) IgG1-IgG4 subclasses in the cerebrospinal fluid and blood serum and their synthesis in the central nervous system in multiple sclerosis. Neurol Neurochir Pol 26:297–303
Lumsden CE (1971) The immunogenesis of the multiple sclerosis plaque. Brain Res 28:365–390
Marsh-Wakefield F, Ashhurst T, Trend S, Mcguire HM, Juillard P, Zinger A, Jones AP, Kermode AG, Hawke S, Grau GE, Hart PH, Byrne SN (2020) IgG3 (+) B cells are associated with the development of multiple sclerosis. Clin Transl Immunology 9:e01133.
Mehta PD (1988) Quantitation of IgG subclasses in cerebrospinal fluid of patients with multiple sclerosis. Ann N Y Acad Sci 540:261–263
Raknes G, Fernandes Filho JA, Pandey JP, Myhr KM, Ulvestad E, Nyland H, Vedeler CA (2000) IgG allotypes and subclasses in Norwegian patients with multiple sclerosis. J Neurol Sci 175:111–115
Salier JP, Glynn P, Goust JM, Cuzner ML (1983) Distribution of nominal and latent IgG (Gm) allotypes in plaques of multiple sclerosis brain. Clin Exp Immunol 54:634–640
Shimizu F, Tasaki A, Sano Y, Ju M, Nishihara H, Oishi M, Koga M. Kawai M, Kanda T (2014) Sera from remitting and secondary progressive multiple sclerosis patients disrupt the blood-brain barrier. PLoS One 9:e92872
Trend S, Jones AP, Cha L, Byrne SN, Geldenhuys S, Fabis-Pedrini MJ, Carroll WM, Cole JM, Booth DR, Lucas RM, Kermode AG, French MA, Hart PH (2018) Higher Serum Immunoglobulin G3 Levels May Predict the Development of Multiple Sclerosis in Individuals With Clinically Isolated Syndrome. Front Immunol 9:1590
Vartdal F, Vandvik B (1982) Multiple sclerosis. Electrofocused “bands” of oligoclonal CSF IgG do not carry antibody activity against measles, varicella-zoster or rotaviruses. J Neurol Sci 54:99–107
Vidarsson G, Dekkers G, Rispens T (2014) IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5:520
Yu X, Graner M, Kennedy PGE, Liu Y (2020) The Role of Antibodies in the Pathogenesis of Multiple Sclerosis. Front Neurol 11:533388
Zeman AZ, Keir G, Luxton R, Thompson EJ (1996) Serum oligoclonal IgG is a common and persistent finding in multiple sclerosis, and has a systemic source. QJM 89:187–193
Zoehner G, Miclea A, Salmen A, Kamber N, Diem L, Friedli C, Bagnoud M, Ahmadi F, Briner M, Sédille-Mostafaie N, Kilidireas C, Stefanis L, Chan A, Hoepner R, Evangelopoulos ME (2019) Reduced serum immunoglobulin G concentrations in multiple sclerosis: prevalence and association with disease-modifying therapy and disease course. Ther Adv Neurol Disord 12:1756286419878340
Acknowledgements
We would like to thank the Neurosurgery Nervous System Biorepository in the Department of Neurosurgery of the University of Colorado Anschutz Medical Campus (https://medschool.cuanschutz.edu/neurosurgery/research-and-innovation) for providing control samples from healthy donors and patients with brain tumors and other non-inflammatory neurological disorders. We thank Accelerated Cure Project (https://www.acceleratedcure.org/) for providing MS and HC samples.
Funding
This work was supported by the Department of Neurosurgery Research Funds, University of Colorado Anschutz Medical Campus. Additional support was from NIH NIMH (5R21MH118174-02) for MWG & XY.
Author information
Authors and Affiliations
Contributions
PGEK, MWG, and XY analyzed data and wrote the manuscript. AF, TP, KA, SB, TC performed experiments. DL provided samples and the revision of the manuscript. XY designed the studies, did data analysis, prepared figures, and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Financial Interests
The authors declare they have no financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kennedy, P.G.E., Graner, M.W., Fringuello, A. et al. Higher Levels of IgG3 Antibodies in Serum, But Not in CSF, Distinguish Multiple Sclerosis From Other Neurological Disorders. J Neuroimmune Pharmacol 17, 526–537 (2022). https://doi.org/10.1007/s11481-021-10048-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-021-10048-x