Abstract
Histamine is a major peripheral inflammatory mediator and a neurotransmitter in the central nervous system. We have reported that histamine induces microglia activation and releases proinflammatory factors in primary cultured microglia. Whether histamine has similar effects in vivo is unknown. In the present study, we aimed to investigate the role of histamine and its receptors in the release of inflammatory mediators and activation of microglia in rat brain. We site-directed injected histamine, histamine receptor agonists or histamine receptor antagonists in the rat lateral ventricle using stereotaxic techniques. Flow cytometry was employed to determine histamine receptor expression in rat microglia. Microglia activation was assessed by Iba1 immunohistochemistry. The levels of tumour necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β) and interleukin-10 (IL-10) were measured with commercial enzyme-linked immunosorbent assay (ELISA) kits, TNF-α, IL-1β and IL-10 mRNA expressions were determined with Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR). We found that all four types of histamine receptors were expressed in rat brain microglia. Histamine was able to induce microglia activation and subsequent production of the inflammatory factors TNF-α, IL-1β and IL-10, and these effects were partially abolished by H1R and H4R antagonists. However, H2R and H3R antagonists significantly increased production of TNF-α and IL-1β, and decreased IL-10 levels. The H1R or H4R agonists stimulated the production of TNF-α and IL-1β, while the H2R or H3R agonists increased IL-10 release. Our results demonstrate that histamine induces microglia activation and the release of both proinflammatory and anti-inflammatory factors in rat brain, thus contributing to the development of inflammation in the brain.

Histamine induces microglia activation and the release of both proinflammatory (TNF-α and IL-1β) and anti-inflammatory factors (IL-10) in rat brain, thus contributing to the development of inflammation in the brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The resident immune cells of CNS, microglia, play a pivotal role in the immune surveillance by avidly probing the brain in search of pathological sources such as infection, injury, and so on (Casano and Peri 2015; Graeber and Streit 2010; Kierdorf and Prinz 2017; Mrdjen et al. 2018). Increasing evidence has demonstrated that the microglia-mediated neuroinflammatory process is critically involved in the initiation and development of neurodegenerative disorders such as Parkinson’s disease (PD) (Joers et al. 2017), Alzheimer’s disease (AD) (Block et al. 2007; Katoh et al. 2001) and multiple sclerosis (Zrzavy et al. 2017). Neurotoxins, neuronal debris, injury, or other abnormal stimulation induces microglia activation and produces a host of inflammatory cytokines. Accumulation of these inflammatory cytokines is deleterious directly to neurons and subsequently induces further activation of microglia, which leads to a vicious cycle (Lenz and Nelson 2018; Ma et al. 2017). Therefore, inhibition of microglia M1 phenotype activation and the subsequent release of pro- inflammatory cytokines may eliminate deleterious effects of microglia and may potentially provide novel therapeutic strategies. However, the regulators and related mechanisms involved in microglia activation are not completely known.
As an endogenous biogenic amine, histamine is synthesized from L-histidine through the catalytic action of histidine decarboxylase. Mast cells and basophils are considered to be the main sources of histamine in peripheral tissues (Rocha et al. 2014). In the brain, histamine is produced mainly by histaminergic neurons, mast cells, and microglia (Chikahisa et al. 2013; Hu and Chen 2017; Rocha et al. 2016). Histamine is a ubiquitous mediator involved in a variety of physiological processes, such as regulation of pituitary hormones secretion, regulation of gastrointestinal and circulatory functions (Parsons and Ganellin 2006). Histamine also plays an important role in the CNS. Histamine can affect neurotransmission release and brain function (Shan et al. 2013, 2015). Histamine was dysregulated in neurodegenerative diseases such AD (Shan et al. 2012a, b, c) and PD (Shan et al. 2012a, b, c). In addition, histamine is a potent mediator of inflammation and a regulator of innate and adaptive immune responses. Four histamine receptors have been identified: H1R, H2R, H3R and H4R (Haas and Panula 2016). Histamine receptors are prominent in many brain regions. It has been discovered that brain histamine regulates satiety signalling, nociception, the sleep–wake cycle, motor circuits, and neuroimmune functions mainly through the four subtypes of receptors (Panula and Nuutinen 2013; Provensi et al. 2014). Increasing studies have shown that histamine played an important role in microglia activation in neurological diseases. For example, histamine could regulate activation of microglia by H1R or H4R in PD (Rocha et al. 2016; Zhou et al. 2019), resulting in the release of proinflammatory factors. Histamine released from mast cells (MCs) further stimulating microglia activation in AD (Theoharides et al. 2015). Histamine H1 receptor blockade prevents early microglia function, resulting in subsequent reduction in immune cell accumulation in MS (Barkauskas et al. 2015).
We have reported that histamine induce microglia activation and subsequently the release of proinflammatory factors in primary cultured microglia (Dong et al. 2014). The results suggested that histamine might participate in microglia activation and play an important role in neuroinflammation-related diseases. In the present study, we further investigated the effects of histamine and its receptors on microglia activation in vivo, and we also explored the effect of histamine on the release of inflammatory mediators in rat brain.
Materials and Methods
Reagents
Histamine was purchased from Sigma–Aldrich (St. Louis, MO, USA). H1R agonist 2-pyridylethlamine dihydrochloride (2-pyridylethlamine), H1R antagonist cetirizine dihydrochloride (cetirizine), H2R agonist amthamine dihydrobromide (amthamine), H2R antagonist ranitidine hydrochloride (ranitidine), H3R agonist (R)-(−)-α-methylhistamine dihydrobromide ((R)-(−)-α-methylhistamine), H3R antagonist carcinine ditrifluoroacetate (carcinine), H4R agonist 4-methylhistamine dihydrochloride (4-methylhistamine) and H4R antagonist A943931 dihydrochloride (A943931) were purchased from Tocris Bioscience (Bristol, UK). All the compounds were dissolved in sterile 0.9% sodium chloride. Rat TNF-α, IL-1β and IL-10 Immunoassay Kit were obtained from R&D Systems, Inc. (Minneapolis, MN, USA). Rabbit anti-Iba1 antibody was purchased from Wako Chemicals USA, Inc. Rabbit anti-H1R and anti-H2R antibodies were purchased from Alomone Labs (Jerusalem, Israel). Rabbit anti-H3R antibody was purchased from Abcam (Hong Kong, China). And rabbit anti-H4R was purchased from Santa Cruz (CA, USA). Fluorescein isothiocyanate (FITC) - conjugated mouse anti-OX-42 antibody and isotype control, phycoerythrin (PE)-conjugated goat anti-rabbit secondary antibody were purchased from BD (BD Biosciences, USA).
Animals
Male Sprague-Dawley (200–250 g) rats were purchased from Model Animal Research Center of Nanjing University (Nanjing, China). All animals were housed in groups of five animals per cage under standard laboratory conditions with free access to food and water, a constant room temperature of 22 °C, 50–60% humidity, and a 12:12 day-night cycle. All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication no. 85–23, revised 1985) and the Guidelines for the Care and Use of Animals in Neuroscience Research by the Society for Neuroscience and approved by IACUC (Institutional Animal Care and Use Committee of Nanjing Medical University, NO: 14030126).
Flow Cytometry Analysis
Flow cytometry was employed to determine histamine receptor expression in rat microglia. As previously described (Dong et al. 2019), the dissociated cells from cerebral cortex tissues were incubated with rabbit anti-H1R, anti-H2R, anti-H3R, anti-H4R primary antibody or normal rabbit IgG overnight at 4 °C, then incubated with 1 μg/ml of FITC-conjugated goat anti-rabbit secondary antibody with PE-conjugated mouse anti-OX-42 antibody or isotype control (1:200) for 1 h at 37 °C. FACS Calibur flow cytometer (BD Biosciences, USA) was used to analyze the cells.
qRT-PCR
Unfixed cerebral cortex tissue was homogenized in 1 ml of Trizol reagent (Invitrogen), and RNA was isolated following the manufacturer’s instructions. Reverse transcription was performed from 1 μg of total RNA for each sample using the Transcription First Strand cDNA Synthesis Kits (Roche) according to the manufacturer’s instructions. Real-time PCR amplification was performed using the STEP ONE Real-time PCR Detection System (Foster City, CA) with the SYBR Green master mix (Applied Biosystems, Foster City, CA) in a final volume of 10 μl that contained 1 μl cDNA template from each sample. Primers are listed as the following: rat TNF-α forward TGCCTCAGCCTCTTCTCATT, reverse CCCATTTGGGAACTTCTCCT; rat IL-1β forward ACTATGGCAACTGTCCCTGAAC, reverse GTGCTTGGGTCCTCATCCTG; rat IL-10 forward ACTGCTATGTTGCCTGCTCTT, reverse TCATTCTTCACCTGCTCCACT; rat GAPGH forward GGGTGTGAACCACGAGAAAT, reverse CCACAGTCTTCTGAGTGGCA. The cycling conditions were 95 °C for 30 s followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The relative mRNA values were normalized to the GAPDH gene control values and calculated using the comparative cycle threshold (ΔΔCt) method.
Surgery and Compounds Administration
Following anaesthesia, rats were placed in the stereotaxic apparatus (Stoelting Instruments, USA). Guide cannulas (Plastic One) were inserted into the right lateral ventricle at the following coordinates: 1.5 mm lateral to the midline, 1.0 mm posterior to the bregma, and 3.8 mm deep (Zhou et al. 2019). The animals were allowed to recover in the animal facility for 14 days before use. Animals were handled daily to check the guide cannula and to familiarize the rats with the investigators.
To determine whether histamine can activate microglia, we administered histamine centrally by a unilateral injection in the lateral ventricle. The rats were randomly allocated to 4 groups (i.e., groups A-D) with 32 rats in each group, and investigators were blinded to the experimental treatment. Rats in groups B-D were intracerebroventricularly (i.c.v.) injected with 1 μl of histamine 20 μg/μl, 40 μg/μl and 80 μg/μl, respectively, and rats in group A (control rats) were injected with 1 μl 0.9% NaCl. Eight rats were randomly selected from each group and were sacrificed 0.5, 8, 24 and 72 h after compounds administration. To determine which histamine receptor mediated the effect of histamine on microglia activation, we intracerebroventricularly injected different H1-H4 antagonists 30 min prior to histamine. The rats were randomly allocated to six groups (group A-F) with 8 rats in each group. The rats in groups C-F were intracerebroventricularly injected with the H1R antagonist cetirizine (10 μM), the H2R antagonist ranitidine (10 μM), the H3R antagonist carcinine ditrifluoroacetate (10 μM) and the H4R antagonist A943931 (10 μM), respectively, and the rats of group A and B were injected with 0.9% NaCl. Thirty min later, the rats in groups B-F were intracerebroventricularly injected with 1 μl histamine (80 μg), and the rats in group A (control rats) were injected with 1 μl 0.9% NaCl. To determine the effect of H1-H4 agonists or antagonists on inflammatory mediator release, the H1R agonist 2-pyridylethlamine, the H2R agonist amthamine (100 μM), the H3R agonist (R)-(−)-α-methylhistamine (100 μM) and the H4R agonist 4-methylhistamine (100 μM) or the H1-H4 antagonists (10 μM) were intracerebroventricularly injected. Compounds were infused in a total volume of 1 μl using an UltraMicroPump III (World Precision Instruments Inc.) at a rate of 100 nl/min. After administration, the rat brain were collected for morphological or biochemical analyses.
Immunochemistry
Rats were anaesthetized by chloral hydrate and perfused first with 0.9% saline and then with cold 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were removed and maintained in 4% paraformaldehyde overnight. The brains were cryopreserved in 30% sucrose in phosphate-buffered saline (PBS) and then stored at −70 °C until used. Free-floating sections (30 μm) encompassing the cerebral cortex were prepared using a cryostat. Tissue sections were incubated for one hour in 10% bovine serum albumin (BSA) with 0.3% Triton X-100 in 0.01 M phosphate-buffered saline, then overnight with rabbit anti-Iba1 primary antibody (1:200) at 4 °C. Tissue sections were washed and incubated in the following day with goat anti-rabbit secondary antibodies for one hour at room temperature. Immunostaining was visualized with 3, 3′- diaminobenzidine, after which sections were counterstained with hematoxylin. The slides were scanned using a Leika 2500 (Leica Microsystems, Wetzlar, Germany) at 200 × magnification.
TNF-α, IL-1β and IL-10 Assay
The content of TNF-α, IL-1βand IL-10 in the rat brain tissue extracts was measured with a commercial ELISA kit from R&D Systems.
Statistical Analysis
All values are the means ± SEM. The significance of the difference between control and samples treated with various compounds was determined by one-way analysis of variance (ANOVA) followed by the post hoc least significant difference test. Differences were considered significant at P < 0.05.
Results
Rat Brain Microglia Express Histamine H1R, H2R, H3R, and H4R
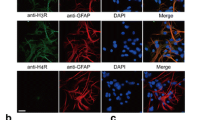
Ferreira R et al. have reported that the N9 microglia cell line expressed low levels of H1R, H2R, and H3R at the mRNA level but a high level of H4R (Ferreira et al. 2012). We previously reported that primary cultured microglia express all these histamine receptors, including H1R, H2R, H3R and H4R (Dong et al. 2014). In the present study, flow cytometry analysis showed that approximately 60, 22, 46 and 58% of microglia in cerebral cortex expressed H1R, H2R, H3R, and H4R, respectively (Fig. 1). The results indicate that H1R, H2R, H3R, and H4R are expressed in rat brain microglia.
Histamine Induces Rat Brain Microglia Activation and Inflammatory Cytokine Production
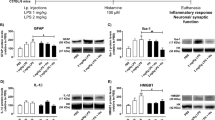
Activated microglia was detected with Iba1 immunohistochemistry. Immunohistochemical analysis at 0.5 h, 8 h, 24 h and 72 h showed that 20 μg histamine induced approximately 23, 27, 45 and 28% microglia activation, respectively; 40 μg histamine induced approximately 47, 61, 83 and 53% microglia activation, respectively; and 80 μg histamine induced 67, 82, 100 and 66% microglia activation, respectively. The study indicated that histamine at 40 and 80 μg induced activation of rat brain microglia at 0.5 h, peaked at 24 h, and decreased by 72 h (Fig. 2a). As neuroinflammation is mainly due to inflammatory factors from brain and their downstream signalling cascades, the levels of inflammatory factors were determined by ELISA, mRNA expressions were detected by qRT-PCR. As shown in Fig. 2b, histamine at 40 and 80 μg for 0.5 h increased TNF-α up to 404% and 529% that of the control, respectively, which peaked at 24 h and decreased at 72 h. IL-1β was increased by up to approximately 205% and 271% after administration of 40 and 80 μg histamine for 8 h. Likewise, the IL-10 level was significantly increased by up to approximately 589% and 845% after exposure to 40 and 80 μg histamine for 72 h, respectively. The mRNA expression levels are consistent with ELISA results (Fig. 2c). These results suggest that histamine could induce microglia activation and the release of the inflammatory factors TNF-α, IL-1β and IL-10 from rat brain.
Activation of rat brain microglia and TNF-α, IL-1β and IL-10 production by histamine. Rats were intracerebroventricularly injected with 0.9% NaCl or 20 μg, 40 μg or 80 μg histamine and were sacrificed 0.5, 8, 24 and 72 h after compounds administration. a Immunohistochemistry analysis of the activation of microglia. b Release of TNF-α, IL-1β and IL-10 induced by histamine. c TNF-α, IL-1β and IL-10 mRNA expression induced by histamine. *P < 0.05, **P < 0.01 vs. the control group. Data are presented as the mean ± SEM
Inhibition of Histamine-Induced TNF-α and IL-1β Production by H1R or H4R Antagonists
Then, antagonists for each of the four histamine receptor subtypes were used to determine which receptor subtype had an effect on histamine-induced microglia activation and proinflammatory factor release in the brain. As shown in Fig. 3, the H1R antagonist cetirizine (10 μM) and the H4R antagonist A943931 (10 μM) partially abolished histamine (80 μg, 24 h)-induced microglia activation (Fig. 3a) and release of the proinflammatory factors TNF-α and IL-1β (Fig. 3b). However, the H2R antagonist ranitidine (10 μM) and the H3R antagonist Carcinine ditrifluoroacetate (10 μM) increased histamine-induced microglia activation (differences are not statistically significant) but failed to effect histamine-induced TNF-α and IL-1β production. These results showed that the H1R or H4R antagonists inhibited histamine-induced microglia activation and TNF-α and IL-1β release in rat brain, suggesting that histamine may induce microglia activation and TNF-α and IL-1β release via H1R and H4R.
Effects of HR antagonists on histamine-induced microglia activation and TNF-α and IL-1β release. a Immunohistochemistry analysis of the activation of microglia. H1R antagonist cetirizine (10 μM), H2R antagonist ranitidine (10 μM), H3R antagonist Carcinine ditrifluoroacetate (10 μM), and H4R antagonist A943931 (10 μM) were intracerebroventricularly injected into rats 30 min before addition of histamine (80 μg) for 24 h. b Release of TNF-α and IL-1β from rat brain. **P < 0.01 vs. the control group. ##P < 0.01 vs. histamine (80 μg) treatment group. Data are presented as the mean ± SEM
Effect of Histamine Receptor Agonists on Production of Inflammatory Cytokines in Rat Brain
To ascertain which histamine receptor subtype caused the release of inflammatory cytokines, agonists for the histamine receptor subtypes were used. The H1R agonist 2-pyridylethlamine used at 100 μM for 8 h caused a significant increase in TNF-α and IL-1β production in the brain (Fig. 4a). The H1R antagonist cetirizine (10 μM) partly abolished 2-pyridylethlamine (100 μM)-induced TNF-α and IL-1β release. However, 2-pyridylethlamine did not stimulate IL-10 release. In contrast, the H2R agonist amthamine (100 μM) and the H3R agonist (R)-(−)-α-methylhistamine (100 μM) used for 8 h failed to affect the production of TNF-α and IL-1β in rat brain (Fig. 4b,c), but after 24 h, they increased IL-10 by up to 609% and 751%, respectively. The corresponding antagonists blocked the effects. 4-methylhistamine, which actives the H4R, was also used at 100 μM for 8 h. It increased the production of TNF-α and IL-1β. In addition, the H4R antagonist A943931 (10 μM) abolished 4-methylhistamine (100 μM) induced TNF-α and IL-1β release (Fig. 4d). Similarly, it had no effect on IL-10 production. qRT-PCR confirmed the ELISA results (Fig. 4e–h). These results showed that the agonists of H1R or H4R stimulated TNF-α and IL-1β release, while the agonist of H2R or H3R affected IL-10 release, suggesting that all histamine receptors are involved in the production of inflammatory factors in rat brain.
Effect of agonists specific to histamine receptor subtypes (H1R [a], H2R [b], H3R [c], and H4R [d]) on TNF-α, IL-1β and IL-10 release and effect of agonists specific to histamine receptor subtypes (H1R [e], H2R [f], H3R [g], and H4R [h]) on TNF-α, IL-1β and IL-10 mRNA expression. Rats were intracerebroventricularly injected with 0.9% NaCl, H1R agonist 2-pyridylethlamine (100 μM), H2R agonist amthamine (100 μM), H3R agonist(R)-(−)-α-methylhistamine (100 μM), and H4R agonist 4-methylhistamine (100 μM). **P < 0.01 vs. the control group. ##P < 0.01 vs. the response to HR antagonist treatment groups. Data are presented as the mean ± SEM
Effect of Histamine Receptor Antagonists on Production of Inflammatory Cytokines in Rat Brain
The antagonists of H1R or H4R inhibited histamine-induced microglia activation and TNF-α and IL-1β release from rat brain, and the agonist of H1R or H4R stimulated the production of TNF-α and IL-1β. Thus, we used the histamine receptor antagonists to investigate whether antagonists alone influenced production of inflammatory cytokines in the brain. As shown in Fig. 5, the H1R antagonist cetirizine (10 μM) and the H4R antagonist A943931 (10 μM) alone had no effect on inflammatory cytokine (i.e., TNF-α, IL-1β and IL-10) release and mRNA expressions. However, the H2R antagonist ranitidine (10 μM) and the H3R antagonist carcinine ditrifluoroacetate (10 μM) used alone for 8 h significantly increased protein and mRNA expressions of TNF-α and IL-1β but decreased IL-10 protein and mRNA level after 24 h. The H1R and H4R antagonists have a greater effect on TNF-α and IL-1β release and mRNA expressions than the H2R and H3R antagonists. These results further indicated that histamine was able to induce TNF-α and IL-1β release in rat brains mainly via H1R and H4R and induce IL-10 release via H2R and H3R. Activation of H1R and H4R has proinflammatory effects and activation of H2R and H3R has anti-inflammatory effects.
Effects of HR antagonists on TNF-α, IL-1β and IL-10 release (a), and effects of HR antagonists on TNF-α, IL-1β and IL-10 mRNA expression (b). Rats were intracerebroventricularly injected with 0.9% NaCl, H1R antagonist cetirizine (10 μM), H2R antagonist ranitidine (10 μM), H3R antagonist carcinine ditrifluoroacetate (10 μM), and H4R antagonist A943931 (10 μM). *P < 0.05, **P < 0.01 vs. the control group. Data are presented as the mean ± SEM
Discussion
Histamine is now commonly acknowledged not only as an inflammatory mediator in peripheral tissues but also as a neurotransmitter in CNS. It has become an important factor in the pathogenesis of allergic and autoimmune diseases because of its proinflammatory features. Histamine exerts various effects on different cells by reason of the differential expression of histamine receptors. The discovery of new histamine sources in the brain and new histamine receptors has made it clear that histamine has an increasingly defined role in the CNS. Our in vitro study showed for the first time that all four histamine receptors are expressed in primary cultured microglia, histamine can upregulate expression of H1R and H4R, and histamine induces microglia activation and the subsequent release of TNF-α and IL-6 from microglia (Dong et al. 2014). Frick et al. reported that histamine can regulate microglia in vivo via the H4 receptor (Frick et al. 2016). Microglia are the residential macrophages of the CNS, which associates them with the immune system. From the early stages of development to the homeostasis of the CNS, they are involved in the biology and pathology of the CNS (Lannes et al. 2017). It has been shown that activation of microglia is an early sign that usually precedes and causes neuronal death in chronic neurodegenerative diseases (Gao et al. 2003; Minghetti 2005; Block and Hong 2005). Therefore, inhibition of microglia M1 phenotype activation and subsequent neuroinflammation may provide a prospective clinical therapy for neuroinflammation-related neurodegenerative disorders. Histamine was thought to have a dual role in neurological diseases, evidences have shown its crucial involvement in the modulation of microglia-mediated neuroinflammation (Barata-Antunes et al. 2017). Histamine modulated microglial motility and cytokine release, and it was an important modulator of microglial phagocytosis and ROS production, both components involved in the vulnerability and cell death of DA neurons in the SN in vivo (Rocha et al. 2016). A decrease in NF-κB and NADPH oxidase 2 with an increase in arginase 1 and P2Y12 receptor was induced by histamine only in the ALS in-ammatory environment, but not in the healthy microglia, together with an increase in IL-6, IL-10, CD163, and CD206 phenotypic markers in SOD1-G93A cells (Apolloni et al. 2017). Here, we aimed to reveal the role of histamine and its receptors in microglia activation and the production of inflammatory cytokines in the rat brain. A previous study has reported that injection of 100 and 250 nmol of histamine into the substantia nigra could induce microglia activation (Vizuete et al. 2000), which may be consistent with the present findings. Proinflammatory cytokines, such as TNF-α and IL-1β, are detrimental and associated with brain diseases, especially when present at elevated concentrations (Pozzi et al. 2018). Upregulated TNF-α has been demonstrated in various neurodegenerative diseases, including acute (e.g., stroke and head trauma) and chronic (e.g., AD, Parkinson’s disease, and amyotrophic lateral sclerosis) diseases (Tweedie et al. 2007). IL-1β, as an important mediator of neuronal injury, also plays diverse roles in the CNS. A large number of reports have indeed shown that IL-1β is involved in brain diseases (Girard et al. 2010; Griffin et al. 2006; Iori et al. 2016; Krakowiak et al. 2017; Lin and Edelson 2017; Murray et al. 2015), such as Alzheimer disease, multiple sclerosis, epilepsy, stroke, and even neurodevelopmental disorders, including schizophrenia and autism. Recent research has indicated that IL-1β could selectively affect cell-to-cell communication in the brain through targeting specific synaptic pathways (Han et al. 2017; Mishra et al. 2012). IL-10 plays a critical role in preventing inflammatory and autoimmune pathologies by limiting the immune response to pathogens and microbial flora (O’Garra and Vieira 2007). However, the regulation of IL-10 production by central nervous cells remains unknown. The concentration course study demonstrated as little as 40 μg of histamine could stimulate approximately half maximal microglia activation, suggesting that histamine is a potent stimulus for microglia activation.
Cytokines are a broad class of small proteins secreted by various cell types including microglia. They also have pro-inflammatory and anti-inflammatory properties. Microglia share comparative properties in reaction to acute or prolonged stimuli, and are classified into two extreme phenotypes: the classically activated M1 phenotype and the alternatively activated M2 phenotype (Yang et al. 2017). Differences between these two phenotypes range from morphological changes to alteration of representative cytokines (Hu et al. 2015). Classically activated M1 microglia release pro-inflammatory cytokines, such as IL-1β, IL-6, IL-8, and TNF-α et al. (Orihuela et al. 2016). M2 microglia are characterized by producing anti-inflammatory cytokines such as IL-10. IL-10 played a key role in phagocytic microglia to engulf apoptotic cells (Cianciulli et al. 2015). M1 and M2 microglia are defined by their contradictory functions and distinctive biomarkers. And co-localization of representative biomarkers of both activation states in the same microglia indicated concurrence of M1 and M2 phenotype, which was found in traumatic brain injuries (TBI) (Morganti et al. 2016), amyotrophic lateral sclerosis (ALS) (Chiu et al. 2013) and spinal cord injury (SCI) (Shechter et al. 2013). In this study, we found that histamine-induced TNF-α release occurred first, followed by IL-1β release, and that IL-10 release occurred last, suggesting that TNF-α, IL-1β and IL-10 release may by regulated by different signalling pathways in the brain.
Our additional experiments demonstrated that the antagonists of H1R or H4R alone failed to affect TNF-α, IL-1β and IL-10 production, and they partly blocked histamine-induced TNF-α and IL-1β release. However, H2R or H3R antagonists by themselves induced a significant increase in TNF-α and IL-1β release and inhibited IL-10 production but had no effect on histamine-induced TNF-α and IL-1β production. Meanwhile, the agonists of H1R or H4R stimulated the rat brain to produce TNF-α and IL-1β in vivo, while the H2R or H3R agonist affected IL-10 levels. Therefore, these data suggest that histamine appears to induce microglia activation and TNF-α and IL-1β release via a similar mechanism, which depends at least partially on activation of H1R and H4R, but IL-10 release is through activation of H2R and H3R. It is widely accepted that histamine exerts the stimulatory effects on the immune system through H1R and exerts the inhibitory effects through H2R (Medina et al. 1999). This concept has also been supported by a multiple sclerosis model, which showed that histamine’s dual effects are due to the different pathophysiological features of the four histamine receptors. Therefore, H1R and H4R may be involved in exacerbating the disease, while H2R and H3R may be effective in ameliorating the disease (Saligrama et al. 2012). This finding may help explain the relationships between the production of inflammatory factors and histamine receptors. However, the exact mechanism requires further detailed study. The mitochondrial membrane potential and the downstream MAPKs have been demonstrated to regulate microglia activation and the release of proinflammatory factors (Akundi et al. 2005; Ciallella et al. 2005; Lund et al. 2005; Waetzig et al. 2005). Our previous results have also suggested that histamine could induce microglia activation by reducing the mitochondrial membrane potential and regulating the downstream MAPKs. Our present results suggest that effects of histamine on microglia are likely not only proinflammatory through H1R and H4R but also anti-inflammatory through H2R and H3R.
Shan (Shan et al. 2019) reported that H4R agonist 4-methylhistamine inhibited LPS-induced secretion of IL-1β and TNF-α at concentrations of 0.1 μM and 1 μM. The H4R antagonist JNJ77777120 significantly blocked the anti-inflammatory effects of 4-methylhistamine. All of these results come from microglia HAPI cells. We have reported that H4R agonist 4-methylhistamine, used at 0.1 to 100 μM for 24 h, produced a concentration dependent increase in TNF-α and IL-6 release from microglia. However, H4R antagonist A943931 (10 μM) alone failed to affect the productions of TNF-α and IL-6 in microglia (Dong et al. 2014). We also found that H4R agonist 4- methylhistamine had no effect on LPS-induced inflammatory cytokines releases from microglia (data not shown). This discrepancy may be due to the fact that primary cultured microglia are more sensitive than cell lines. Activation of H4 receptor alone can induce inflammatory cytokines releases from primary cultured microglia. However, it’s known that LPS has a strong pro-inflammatory effect on microglia. Activation of H4 receptor on primary cultured microglia is not enough to synergize the inflammatory effect caused by LPS on primary cultured microglia. In the present study, we found that H4R agonist 4-methylhistamine increased the production of TNF-α and IL-1β release in rat cerebral cortex. The pro-inflammatory effect of H4R agonists under normal condition in vivo study is consistent with our in vitro study. The role of H4R in LPS-induced neuroinflammation in vivo need to be further invested. The Santa Cruz H4R antibody sc M120 was used to evaluate H4R expression on microglia in this study and our previous study (Dong et al. 2014). However, Silke reported that the H4R antibodies (Santa Cruz sc M120 and sc H110) have no specificity on H4R protein in flow cytometric analysis and Western blot analysis. Commercially available H4R antibodies that have been validated are needed to further confirm H4R expression in microglia.
A large amount of brain histamine is contained not only in neurons but also in brain mast cells (Borriello et al. 2017; Dong et al. 2017). Mast cells, as a main source of histamine in the CNS, have been proved to play a role in the pathogenesis of the experimental autoimmune encephalomyelitis (EAE), experimental allergic neuritis, and experimental autoimmune demyelinating diseases (Elieh-Ali-Komi and Cao 2017; Yin et al. 2017). A previous study suggested that the “first responders” in brain injury are activated mast cells rather than microglia (Jin et al. 2009). Recent studies indicate that mast cells actively participated in the pathogenesis of inflammation through the release of proinflammatory mediators in vivo. We have previously reported that corticotropin-releasing hormone (CRH)-stimulated human mast cells (HMC-1) activate microglia and induce TNF-α and IL-6 release (Zhang et al. 2016), suggesting the importance of mast cells in microglia activation and the release of proinflammatory factors in the CNS. It has been reported that mast cell activation leads to accumulation of cerebral histamine (Biran et al. 2008), which suggests that local histamine concentration may be sufficient for microglia activation. We also reported that the released histamine from mast cells acts on microglia activation and that mast cell-microglia crosstalk contributes to neuroinflammatory disease (Dong et al. 2017). The functions of histamine receptors may be more interesting and important in neuroinflammatory-related diseases. It is technically challenging to use in vivo experiments, such as those presented here, to certify the effect of microglia in histamine-induced inflammatory mediator release. Therefore, a better understanding of the roles and the molecular mechanisms of histamine receptors, especially microglial histamine receptors, in activating microglia and regulating inflammatory processes in the CNS is required, and more research is needed for these areas of neuroimmunology.
Conclusion
We demonstrate that histamine induces activation of microglia in vivo, and prompts proinflammatory cytokine release via H1R and H4R but stimulates IL-10 release via H2R and H3R in the rat brain. These results suggest that histamine plays an important role in microglia activation and neuroinflammation-related diseases. Histamine H1 and H4 antagonists may be used as prospective clinical therapeutic tools to inhibit histamine-induced microglia activation and subsequent neuroinflammation, as well as neuroinflammation-related neurodegenerative disorders.
References
Akundi RS, Candelario-Jalil E, Hess S, Hull M, Lieb K, Gebicke-Haerter PJ, Fiebich BL (2005) Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia 51(3):199–208
Apolloni S, Fabbrizio P, Amadio S, Napoli G, Verdile V, Morello G, Iemmolo R, Aronica E, Cavallaro S, Volonte C (2017) Histamine regulates the inflammatory profile of SOD1-G93A microglia and the Histaminergic system is dysregulated in amyotrophic lateral sclerosis. Front Immunol 8:1689
Barata-Antunes S, Cristovao AC, Pires J, Rocha SM, Bernardino L (2017) Dual role of histamine on microglia-induced neurodegeneration. Biochim Biophys Acta Mol basis Dis 1863(3):764–769
Barkauskas DS, Dixon Dorand R, Myers JT, Evans TA, Barkauskas KJ, Askew D, Purgert R, Huang AY (2015) Focal transient CNS vessel leak provides a tissue niche for sequential immune cell accumulation during the asymptomatic phase of EAE induction. Exp Neurol 266:74–85
Biran V, Cochois V, Karroubi A, Arrang JM, Charriaut-Marlangue C, Heron A (2008) Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol 18(1):1–9
Block ML, Hong JS (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76(2):77–98
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69
Borriello F, Iannone R, Marone G (2017) Histamine release from mast cells and basophils. Handb Exp Pharmacol 241:121–139
Casano AM, Peri F (2015) Microglia: multitasking specialists of the brain. Dev Cell 32(4):469–477
Chikahisa S, Kodama T, Soya A, Sagawa Y, Ishimaru Y, Sei H, Nishino S (2013) Histamine from brain resident MAST cells promotes wakefulness and modulates behavioral states. PLoS One 8(10):e78434
Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O'Keeffe S, Phatnani HP, Muratet M, Carroll MC, Levy S, Tavazoie S, Myers RM, Maniatis T (2013) A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 4(2):385–401
Ciallella JR, Saporito M, Lund S, Leist M, Hasseldam H, McGann N, Smith CS, Bozyczko-Coyne D, Flood DG (2005) CEP-11004, an inhibitor of the SAPK/JNK pathway, reduces TNF-alpha release from lipopolysaccharide-treated cells and mice. Eur J Pharmacol 515(1–3):179–187
Cianciulli A, Dragone T, Calvello R, Porro C, Trotta T, Lofrumento DD, Panaro MA (2015) IL-10 plays a pivotal role in anti-inflammatory effects of resveratrol in activated microglia cells. Int Immunopharmacol 24(2):369–376
Dong H, Zhang W, Zeng X, Hu G, Zhang H, He S, Zhang S (2014) Histamine induces upregulated expression of histamine receptors and increases release of inflammatory mediators from microglia. Mol Neurobiol 49(3):1487–1500
Dong H, Zhang X, Wang Y, Zhou X, Qian Y, Zhang S (2017) Suppression of brain mast cells degranulation inhibits microglial activation and central nervous system inflammation. Mol Neurobiol 54(2):997–1007
Dong H, Wang Y, Zhang X, Zhang X, Qian Y, Ding H, Zhang S (2019) Stabilization of brain mast cells alleviates LPS-induced neuroinflammation by inhibiting microglia activation. Front Cell Neurosci 13:191
Elieh-Ali-Komi D, Cao Y (2017) Role of mast cells in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Rev Allergy Immunol 52(3):436–445
Ferreira R, Santos T, Goncalves J, Baltazar G, Ferreira L, Agasse F, Bernardino L (2012) Histamine modulates microglia function. J Neuroinflammation 9:90
Frick L, Rapanelli M, Abbasi E, Ohtsu H, Pittenger C (2016) Histamine regulation of microglia: gene-environment interaction in the regulation of central nervous system inflammation. Brain Behav Immun 57:326–337
Gao HM, Liu B, Zhang W, Hong JS (2003) Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol Sci 24(8):395–401
Girard S, Tremblay L, Lepage M, Sebire G (2010) IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J Immunol 184(7):3997–4005
Graeber MB, Streit WJ (2010) Microglia: biology and pathology. Acta Neuropathol 119(1):89–105
Griffin WS, Liu L, Li Y, Mrak RE, Barger SW (2006) Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation 3:5
Haas HL, Panula P (2016) Histamine receptors. Neuropharmacology 106:1–2
Han Q, Lin Q, Huang P, Chen M, Hu X, Fu H, He S, Shen F, Zeng H, Deng Y (2017) Microglia-derived IL-1beta contributes to axon development disorders and synaptic deficit through p38-MAPK signal pathway in septic neonatal rats. J Neuroinflammation 14(1):52
Hu W, Chen Z (2017) The roles of histamine and its receptor ligands in central nervous system disorders: an update. Pharmacol Ther 175:116–132
Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J (2015) Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 11(1):56–64
Iori V, Frigerio F, Vezzani A (2016) Modulation of neuronal excitability by immune mediators in epilepsy. Curr Opin Pharmacol 26:118–123
Jin Y, Silverman AJ, Vannucci SJ (2009) Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke 40(9):3107–3112
Joers V, Tansey MG, Mulas G, Carta AR (2017) Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog Neurobiol 155:57–75
Katoh Y, Niimi M, Yamamoto Y, Kawamura T, Morimoto-Ishizuka T, Sawada M, Takemori H, Yamatodani A (2001) Histamine production by cultured microglial cells of the mouse. Neurosci Lett 305(3):181–184
Kierdorf K, Prinz M (2017) Microglia in steady state. J Clin Invest 127(9):3201–3209
Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I, Van de Water J (2017) Neonatal cytokine profiles associated with autism spectrum disorder. Biol Psychiatry 81(5):442–451
Lannes N, Eppler E, Etemad S, Yotovski P, Filgueira L (2017) Microglia at center stage: a comprehensive review about the versatile and unique residential macrophages of the central nervous system. Oncotarget 8(69):114393–114413
Lenz KM, Nelson LH (2018) Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front Immunol 9:698
Lin CC, Edelson BT (2017) New insights into the role of IL-1beta in experimental autoimmune encephalomyelitis and multiple sclerosis. J Immunol 198(12):4553–4560
Lund S, Porzgen P, Mortensen AL, Hasseldam H, Bozyczko-Coyne D, Morath S, Hartung T, Bianchi M, Ghezzi P, Bsibsi M, Dijkstra S, Leist M (2005) Inhibition of microglial inflammation by the MLK inhibitor CEP-1347. J Neurochem 92(6):1439–1451
Ma Y, Wang J, Wang Y, Yang GY (2017) The biphasic function of microglia in ischemic stroke. Prog Neurobiol 157:247–272
Medina MA, Quesada AR, Nunez de Castro I, Sanchez-Jimenez F (1999) Histamine, polyamines, and cancer. Biochem Pharmacol 57(12):1341–1344
Minghetti L (2005) Role of inflammation in neurodegenerative diseases. Curr Opin Neurol 18(3):315–321
Mishra A, Kim HJ, Shin AH, Thayer SA (2012) Synapse loss induced by interleukin-1beta requires pre- and post-synaptic mechanisms. J NeuroImmune Pharmacol 7(3):571–578
Morganti JM, Riparip LK, Rosi S (2016) Call off the dog(ma): M1/M2 polarization is concurrent following traumatic brain injury. PLoS One 11(1):e0148001
Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, Greter M, Becher B (2018) High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48(2):380–395
Murray KN, Parry-Jones AR, Allan SM (2015) Interleukin-1 and acute brain injury. Front Cell Neurosci 9:18
O’Garra A, Vieira P (2007) T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol 7(6):425–428
Orihuela R, McPherson CA, Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173(4):649–665
Panula P, Nuutinen S (2013) The histaminergic network in the brain: basic organization and role in disease. Nat Rev Neurosci 14(7):472–487
Parsons ME, Ganellin CR (2006) Histamine and its receptors. Br J Pharmacol 147(Suppl 1):S127–S135
Pozzi D, Menna E, Canzi A, Desiato G, Mantovani C, Matteoli M (2018) The communication between the immune and nervous systems: the role of IL-1beta in synaptopathies. Front Mol Neurosci 11:111
Provensi G, Coccurello R, Umehara H, Munari L, Giacovazzo G, Galeotti N, Nosi D, Gaetani S, Romano A, Moles A, Blandina P, Passani MB (2014) Satiety factor oleoylethanolamide recruits the brain histaminergic system to inhibit food intake. Proc Natl Acad Sci U S A 111(31):11527–11532
Rocha SM, Pires J, Esteves M, Graca B, Bernardino L (2014) Histamine: a new immunomodulatory player in the neuron-glia crosstalk. Front Cell Neurosci 8:120
Rocha SM, Saraiva T, Cristovao AC, Ferreira R, Santos T, Esteves M, Saraiva C, Je G, Cortes L, Valero J, Alves G, Klibanov A, Kim YS, Bernardino L (2016) Histamine induces microglia activation and dopaminergic neuronal toxicity via H1 receptor activation. J Neuroinflammation 13(1):137
Saligrama N, Noubade R, Case LK, del Rio R, Teuscher C (2012) Combinatorial roles for histamine H1-H2 and H3-H4 receptors in autoimmune inflammatory disease of the central nervous system. Eur J Immunol 42(6):1536–1546
Shan L, Bossers K, Luchetti S, Balesar R, Lethbridge N, Chazot PL, Bao AM, Swaab DF (2012a) Alterations in the histaminergic system in the substantia nigra and striatum of Parkinson’s patients: a postmortem study. Neurobiol Aging 33(7):1488.e1–1488.e13
Shan L, Bossers K, Unmehopa U, Bao AM, Swaab DF (2012b) Alterations in the histaminergic system in Alzheimer’s disease: a postmortem study. Neurobiol Aging 33(11):2585–2598
Shan L, Liu CQ, Balesar R, Hofman MA, Bao AM, Swaab DF (2012c) Neuronal histamine production remains unaltered in Parkinson’s disease despite the accumulation of Lewy bodies and Lewy neurites in the tuberomamillary nucleus. Neurobiol Aging 33(7):1343–1344
Shan L, Swaab DF, Bao AM (2013) Neuronal histaminergic system in aging and age-related neurodegenerative disorders. Exp Gerontol 48(7):603–607
Shan L, Bao AM, Swaab DF (2015) The human histaminergic system in neuropsychiatric disorders. Trends Neurosci 38(3):167–177
Shan Y, Gao Y, Zhang L, Ma L, Shi Y, Liu X (2019) H4 receptor inhibits lipopolysaccharide-induced NF-kappaB activation by interacting with tumor necrosis factor receptor-associated factor 6. Neuroscience 398:113–125
Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M (2013) Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 38(3):555–569
Theoharides TC, Stewart JM, Hatziagelaki E, Kolaitis G (2015) Brain “fog,” inflammation and obesity: key aspects of neuropsychiatric disorders improved by luteolin. Front Neurosci 9:225
Tweedie D, Sambamurti K, Greig NH (2007) TNF-alpha inhibition as a treatment strategy for neurodegenerative disorders: new drug candidates and targets. Curr Alzheimer Res 4(4):378–385
Vizuete ML, Merino M, Venero JL, Santiago M, Cano J, Machado A (2000) Histamine infusion induces a selective dopaminergic neuronal death along with an inflammatory reaction in rat substantia nigra. J Neurochem 75(2):540–552
Waetzig V, Czeloth K, Hidding U, Mielke K, Kanzow M, Brecht S, Goetz M, Lucius R, Herdegen T, Hanisch UK (2005) c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 50(3):235–246
Yang HM, Yang S, Huang SS, Tang BS, Guo JF (2017) Microglial activation in the pathogenesis of Huntington’s disease. Front Aging Neurosci 9:193
Yin JJ, Hu XQ, Mao ZF, Bao J, Qiu W, Lu ZQ, Wu HT, Zhong XN (2017) Neutralization of Interleukin-9 decreasing mast cells infiltration in experimental autoimmune encephalomyelitis. Chin Med J 130(8):964–971
Zhang X, Wang Y, Dong H, Xu Y, Zhang S (2016) Induction of microglial activation by mediators released from mast cells. Cell Physiol Biochem 38(4):1520–1531
Zhou P, Homberg JR, Fang Q, Wang J, Li W, Meng X, Shen J, Luan Y, Liao P, Swaab DF, Shan L, Liu C (2019) Histamine-4 receptor antagonist JNJ7777120 inhibits pro-inflammatory microglia and prevents the progression of Parkinson-like pathology and behaviour in a rat model. Brain Behav Immun 76:61–73
Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H (2017) Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140(7):1900–1913
Acknowledgements
This project was sponsored by the National Natural Science Foundation of China (No. 81102422, 81373398, 81570522 and 81501202), Hubei natural science foundation (2018CFB301), but they had no role in the design of the study collection, analysis, or interpretation of the data; or writing of the manuscript. We would like to thank the Core Facility of Jiangsu Provincial People’s Hospital for its help in the detection of experimental samples.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure Statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Statement on the Welfare of Animals
This article does not contain any studies with human participants performed by any of the authors.
All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication no. 85–23, revised 1985) and the Guidelines for the Care and Use of Animals in Neuroscience Research by the Society for Neuroscience and approved by IACUC (Institutional Animal Care and Use Committee of Nanjing Medical University, NO: 14030126).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, W., Zhang, X., Zhang, Y. et al. Histamine Induces Microglia Activation and the Release of Proinflammatory Mediators in Rat Brain Via H1R or H4R. J Neuroimmune Pharmacol 15, 280–291 (2020). https://doi.org/10.1007/s11481-019-09887-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-019-09887-6