Abstract
Dopamine has emerged as a fundamental regulator of inflammation. In this regard, it has been shown that dopaminergic signalling pathways are key players promoting homeostasis between the central nervous system and the immune system. Dysregulation in the dopaminergic system affects both innate and adaptive immunity, contributing to the development of numerous autoimmune and inflammatory pathologies. This makes dopamine receptors interesting therapeutic targets for either the development of new treatments or repurposing of already available pharmacological drugs. Dopamine receptors are broadly expressed on different immune cells with multifunctional effects depending on the dopamine concentration available and the pattern of expression of five dopamine receptors displaying different affinities for dopamine. Thus, impaired dopaminergic signalling through different dopamine receptors may result in altered behaviour of immunity, contributing to the development and progression of autoimmune pathologies. In this review we discuss the current evidence involving the dopaminergic system in inflammatory bowel disease, multiple sclerosis and Parkinson’s disease. In addition, we summarise and analyse the therapeutic approaches designed to attenuate disease development and progression by targeting the dopaminergic system.

Targetting the dopaminergic system in autoimmunity.
Effector T-cells (Teff) orchestrate inflamamtion involved in autoimmunity, whilst regulatory T-cells (Tregs) suppress Teff activity promoting tolerance to self-constituents. Dopamine has emerged as a key regulator of Teff and Tregs function, thereby dopamine receptors have becoming important therapeutic targets in autoimmune disorders, especially in those affecting the brain and the gut, where dopamine levels strongly change with inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autoimmunity is a complex condition where the immune system is unable to distinguish between self and non-self-antigens due to the loss of immune tolerance. To date, 81 autoimmune diseases have been identified with an overall prevalence of 5%, constituting a serious health issue worldwide (Hayter and Cook 2012). Extensive research has shown dopaminergic pathways as key regulators of autoimmunity (Pacheco 2017; Pinoli et al. 2017), especially in inflammatory bowel disease (IBD) (Pacheco et al. 2014; Contreras et al. 2016), multiple sclerosis (MS) (Prado et al. 2012; Prado et al. 2013; Cosentino et al. 2016; Osorio-Barrios et al. 2018; Prado et al. 2018), and Parkinson’s disease (PD) (Gonzalez et al. 2013; Christiansen et al. 2016; Elgueta et al. 2017; Kustrimovic et al. 2018).
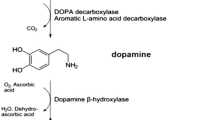
Dopamine is a catecholaminergic neurotransmitter present in central and peripheral tissues involved in sodium balance, blood pressure, renal function, glucose homeostasis, voluntary movement, cognition, reward, sleep, memory, sympathetic regulation and retinal processes (Beaulieu and Gainetdinov 2011; Pinoli et al. 2017). It is mainly synthesized in the brain from the precursor L-dihydroxyphenylalanine (L-DOPA). Other sources of active dopamine are some immune cells, such as dendritic cells and T cells (Pacheco et al. 2014; Levite 2016; Papa et al. 2017) and some gut commensals, such as Clostridium species (Asano et al. 2012). There are five distinct receptors to which dopamine binds with different affinities. The D1-like subtype comprises D1 dopamine receptor (DRD1) and DRD5, whilst the D2-like subtype comprises DRD2, DRD3 and DRD4 (Vallone et al. 2000; Beaulieu and Gainetdinov 2011; Pinoli et al. 2017). The D1-like subtype is often coupled to the Gαs/olf family of G proteins that stimulate cyclic adenosine monophosphate (cAMP) production, whereas the D2-like subtype classically activates the Gαi/o family of G proteins to inhibit cAMP production (Beaulieu and Gainetdinov 2011). Stimulation of one or more of these receptors has been implicated either in promoting or dampening the development or progression of autoimmune diseases. The present review integrates the current literature about dopaminergic pathways involved in IBD, MS and PD.
Inflammatory Bowel Disease
Epidemiology of Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is an umbrella term used to describe a group of chronic inflammatory conditions triggered by the break of immune tolerance in the gastrointestinal tract. The most common pathologies falling under this term are Crohn’s disease (CD) and ulcerative colitis (UC) (Mayer 2010; Pacheco et al. 2014). Results from gene-expression studies highlight IBD as one the most complex autoimmune diseases affecting at least 163 loci along the human genome, some of them shared by both CD and UC (Jostins et al. 2012). Inflammatory lesions in CD patients are generally transmural, multifocal and contain granulomas. They can affect the entire gastrointestinal tract from the mouth to the anus. In the case of UC patients, inflammation is superficial and limited to the colon (Brown and Mayer 2007; Pacheco et al. 2014). IBD is associated with abdominal pain, weight loss, diarrhoea, passage of blood or mucus or both (Baumgart and Sandborn 2007), as well as alternating phases of relapse and remission, increasing the risk of developing intestinal cancer (Geremia et al. 2014). IBD prevalence fluctuates between 605 and 827 cases per 100.000 individuals in Europe and North America with an estimated health-care cost of €4.6–5.6 billion annually (Burisch et al. 2013; Ng et al. 2018).
Physiopathology of Inflammatory Bowel Disease
Development and progression of IBD involves several signalling pathways interacting simultaneously, such as impairment of barrier function and loss of permeability (i.e. defective tight junctions in the intestinal epithelial cell layer), altered immune response (T lymphocyte signalling) (Izcue et al. 2009; Martini et al. 2017), environmental factors (i.e. smoking and intestinal microbiota) (Bernstein et al. 2006; Mayer 2010) and genetic predisposition (i.e. IL-23 receptor and ATG16L mutations) (Rioux et al. 2007; Mayer 2010). Current understanding of IBD pathobiology comes from the use of different experimental animal models, which resemble one or two major characteristics of the disease (Kiesler et al. 2015).
One of the first events occurring during IBD is the disruption of the intestinal epithelial barrier permeability, which allows bacterial breakthrough and development of intestinal inflammation (Olson et al. 2006). Studies with both mouse strains, IL-10 deficient and senescence-accelerated mouse (SAMP), which develop spontaneous colitis and ileitis while aging respectively, have shown that dysregulated epithelial barrier response precedes intestinal inflammation in vivo (Madsen et al. 1999; Olson et al. 2006). Moreover, different subsets of mesenchymal cells present in the gut lamina propria undergo remodeling processes during chronic gut inflammation, which may contribute to either epithelial barrier breakdown or repair and regeneration (Kinchen et al. 2018). Defective T cell signalling is another well characterized event leading to the development of IBD (Powrie and Mason 1990). In the T cell transfer model of colitis, regulatory T cells (Tregs) maintain intestinal homeostasis preventing disease development, whereas effector T cells (Teff) promote gut inflammation in the presence of triggering intestinal microbiota (Powrie and Mason 1990; Izcue et al. 2009). Moreover, a subtype of Teff, namely Th17 cells, have shown substantial plasticity with both protective and pathogenic functions following IBD (Feng et al. 2011; Gagliani et al. 2015). For example, in the presence of TGF-β1 the aryl hydrocarbon receptor promotes transdifferentiation of Th17 into Tregs, allowing resolution of inflammation following IBD (Gagliani et al. 2015). Whereas IL-17, IL-12, IL-23 and IFN-γ promote transdifferentiation of Th17 into Th1 cells leading to the development of a severe colitis (Feng et al. 2011; Nizzoli et al. 2018). Of note, the central role of Teff in promoting gut inflammation in IBD is carried out by recruiting and stimulating the function of cells from the innate immune system, such as neutrophils and monocytes/macrophages. On the other hand, the key anti-inflammatory activity of Tregs is exerted by dampening Teff function. Furthermore, several other immune pathways involving myeloid antigen-presenting cells of the intestine have been described to contribute to the development and progression of IBD. In experimental models of acute colitis, ablation of dendritic cells has a time-dependent effect, leading to either amelioration or exacerbation of disease progression (Berndt et al. 2007). Recently, IL-33 has been shown to ameliorate colitis development by stimulating differentiation of goblet cells and macrophage switching from an inflammatory to anti-inflammatory phenotype (Seo et al. 2017).
Dopaminergic Mechanisms Involved in Inflammatory Bowel Disease
Dopamine has been detected in the gastrointestinal tract of humans and mice, where is involved in gastrointestinal motility and gut homeostasis (Zizzo et al. 2016). It is produced by the mesenteric tissue (Eisenhofer et al. 1997), gut microbiota (Asano et al. 2012) and enteric nerves (Pacheco et al. 2014). Under inflammatory conditions, such as those found in IBD, the levels of this catecholamine are dysregulated both in humans and animal models (Table 1). In biopsy specimens of inflamed gut mucosa from IBD patients, dopamine levels are reduced compared to healthy controls (Magro et al. 2002). A similar situation has been observed in experimental rodent models of IBD (Magro et al. 2004; Tolstanova et al. 2015). This decrease in dopamine levels has been attributed to the IFN-γ-mediated inhibition on L-DOPA uptake by epithelial cells (Magro et al. 2004), decreased expression levels of tyrosine hydroxylase (TH) - the enzyme that synthesizes dopamine -, and reduced expression of the dopamine transporter (DAT) in the colon (Tolstanova et al. 2015).
Another hallmark of IBD, where the dopaminergic system plays a relevant role, is the altered T cell signalling. This process might be mediated by the release of dopamine stored in endogenous compartments of Tregs, which subsequently acts in an autocrine/paracrine manner stimulating DRD5, attenuating their suppressive activity and thereby, leading to upregulation of Teff function (Cosentino et al. 2007). Dopamine receptors are present in the gut mucosa and adjacent tissues. For instance, DRD1 and DRD2 have been found mainly located in some subsets of sub-mucosal and myenteric neurons. On the other hand, DRD3 and DRD5 are present in both the nerve ending layer of the intestine wall and the gut mucosa, whereas DRD4 is located in the mucosal layer (Li et al. 2006; Zizzo et al. 2016). Signalling through the DRD3 has been shown to act as a negative regulator of Th2 differentiation, promoting a Th1 phenotype in a chronic model of IBD induced by the transfer of naive CD4+ T cells into T lymphopenic recipient mice (Contreras et al. 2016). The negative regulation of Th2 differentiation exerted by DRD3 in CD4+ T cells involves the upregulation of SOCS5, (Contreras et al. 2016) and the reduction of cAMP levels and attenuation of late extracellular signal-regulated kinase (ERK) phosphorylation (Franz et al. 2015). In contrast, signalling mediated by DRD5 in CD4+ T cells leads to an early increase of ERK phosphorylation which potentiates T cell activation (Franz et al. 2015).
According to the involvement of the dopaminergic system in maintaining gut homeostasis, the development of IBD in some cases has been associated with genetic predisposition involving polymorphisms and mutations of different components of the dopaminergic system. For instance, D1-like receptor gene polymorphisms have been associated with CD4+ T cell counts and proper Tregs function in the blood of healthy patients (Cosentino et al. 2015; Cosentino et al. 2018). Moreover a genetic polymorphism in the DRD2 has been associated with susceptibility to develop refractory CD in patients (Magro et al. 2006).
Current and Future Treatments for Inflammatory Bowel Disease
The main goal of IBD treatment is to relieve symptoms, diminish the inflammatory response, allow lesions to heal and prevent relapse. Current therapeutic approaches include the use of pharmacological drugs to inhibit inflammation, stimulate anti-inflammatory pathways, disturbance of T cell function, inhibition of leukocyte recruitment, among others. In severe cases, surgical management is recommended (Brown and Mayer 2007). However, no treatment strategy is free of side effects. Importantly, some studies performed in animal models have highlighted the importance of targeting dopamine receptors to attenuate IBD progression (Table 2). For example, in adult mice dopamine acts as an endogenous inhibitor of intestinal motility, promoting relaxation, mainly through D2-like receptors (Zizzo et al. 2016). This observation, has led to the use of pharmacological drugs targeting D2-like receptors for the management of gastrointestinal motor disorders (Tonini et al. 2004). Of note, is important to mention that chronic blockade of central DRD2 has been associated with the development of extrapyramidal reactions and hyperprolactinaemia (Tonini et al. 2004).
Pharmacological modulation of the dopaminergic system in animal models of IBD highlights DRD2 as a promising target for the development of future treatments (Table 2). In this regard, it has been shown that DRD2 agonists, such as quinpirole and cabergoline, attenuate IBD severity by reducing nitric oxide production, recruitment of neutrophils in the intestine (Oehlers et al. 2017), and vascular permeability (Tolstanova et al. 2015). It is noteworthy that quinpirole is also a DRD3 agonist, although its affinity for this receptor is considerable lower than the affinity for DRD2 (Ki ≈ 4,8 and 24 nM for DRD2 and DRD3 respectively) (Seeman and Van Tol 1994). Interestingly, another study also found attenuation of IBD using beberine, a non-selective antagonist of both D1- and 2 like receptors (Kawano et al. 2015). Beberine treatment decreased the secretion of IFN-γ and IL-17 from lymphocytes of the mesenteric lymph nodes of DSS-treated mice, leading to reduced intestinal inflammation (Kawano et al. 2015), thus suggesting a relevant role not only for DRD2, but also for other dopamine receptors in the control of gut inflammation.
Importantly, case reports of successful repurposing of pre-existing drugs to indirectly target the dopaminergic system have shown promising therapeutic results in CD and UC patients (Kast and Altschuler 2001; Furlan et al. 2006; Check et al. 2010, 2011a). The use of dextroamphetamine sulphate and clonidine, commonly used in the treatment of narcolepsy and hypertension, respectively, ameliorates some symptoms and colonic histopathological abnormalities in IBD patients (Furlan et al. 2006; Check et al. 2010, 2011a), by either reducing the hyperfunction (Check et al. 2011a) or attenuating the hypofunction of both the sympathetic and parasympathetic nervous systems (Check et al. 2011b). In this regard, CD has been associated with parasympathetic hyperactivity and sympathetic neuropathy (Check et al. 2011b), whilst the opposite alterations have been reported for UC, including sympathetic hyperactivity with parasympathetic dysfunction (Check et al. 2011a). Thereby, these facts might partially explain why these two drugs were reported to exert positive outcomes even though they have opposite functions. In the same line, the anti-depressant bupropion, has shown therapeutic effects in IBD patients that do not respond to conventional treatments (Kast and Altschuler 2001) (Table 2). The authors suggested that these drugs might exert their main protective effects indirectly targeting central dopamine receptors, the β-adrenergic receptor or reduction of TNF-α levels (Lechin et al. 1985; Furlan et al. 2006). However, properly designed double-blinded randomized trials are needed to assess the effectiveness and safety of repurposing these drugs in IBD. The involvement of the dopaminergic system in the control of gut homeostasis, alterations of the dopaminergic system associated to IBD and the therapeutic approaches targeting dopaminergic system as treatment for IBD are integrated in the illustration shown in Fig. 1.
Altered dopaminergic signalling and its pharmacological targets in inflammatory bowel disease. Schematic representation of dopaminergic components in the gut mucosa under homeostasis, IBD, and IBD exposed to therapeutic approaches targeting dopaminergic system. a In healthy conditions, dopamine, DAT and TH are present at optimal levels in the intestine (i). The extent of neutrophils infiltration in the gut mucosa is low (ii) and the ratio of Tregs-to-Teff is high in steady-state conditions (iii). b However, during IBD, the expression of TH and DAT is down-regulated and L-DOPA uptake is significantly decreased in the epithelium of the intestine, thus promoting a strong reduction in the levels of dopamine in the gut mucosa (i). Neutrophils infiltration and recruitment into the gut mucosa is increased (ii). Low dopamine levels favour the selective stimulation of DRD3 in Teff, promoting their expansion (reducing the Tregs-to-Teff ratio) and a strong production of IFN-γ (iii). c Pharmacological stimulation of DRD2 reduces neutrophil infiltration (i), whereas inhibition of DRD3 on CD4+ T cells attenuates IFN-γ production, promoting a Th2 phenotype on these cells (ii). In both cases there is a significant reduction in mucosal inflammation, ameliorating colitis symptoms and thus, restoring the gain of proper body weight. Abbreviations: DA: dopamine, DAT: dopamine transporter, TH: tyrosine hydroxylase, Treg: regulatory T cells, Teff: effector T cells
Multiple Sclerosis
Epidemiology of Multiple Sclerosis
Multiple sclerosis (MS) is a chronic demyelinating disease induced by autoimmune response against the axonal myelin sheath in different areas of the brain and spinal cord, leading to a progressive neurological disability. It mainly affects young adults and involves more than 100 loci accounting for approximately 30% of the overall disease risk (International Multiple Sclerosis Genetics et al. 2013; Dendrou et al. 2015). There are also environmental risk factors (i.e. smoking and infectious agents) likely acting together with MS risk-conferring genes contributing to the development and progression of the diseases (Dendrou et al. 2015). The disease course and symptomatology are heterogeneous, including impairments of visual, sensory, motor, neurocognitive, and autonomic functions. The most common form affecting MS patients is relapsing-remitting MS, which is characterized by two alternating phases. In the first one there are neurological impairment and inflammation, followed by a remission period of clinical recovery (Dendrou et al. 2015). It has an overall prevalence of 58.3 per 100.000 individuals (Hayter and Cook 2012) with an estimated health-care cost of €42–53 million annually (Ernstsson et al. 2016; Buijs et al. 2018) depending on disease severity.
Physiopathology of Multiple Sclerosis
Experimental autoimmune encephalitis (EAE), the animal model of MS, has provided compelling evidence of the multicellular complexity of this autoimmune pathology that involves myelin-reactive CD4+ T cells, imbalanced CD8+ T cells, inflammatory B cells, and NK function, production of autoantibodies, and a genetic predisposition and non-genetic factors (Dendrou et al. 2015; Li et al. 2015; Laroni et al. 2016; Jelcic et al. 2018).
Th1 and Th17 cells producing IFN-γ and IL-17A respectively, are the main CD4+ T cells subsets promoting inflammation in MS. Patients carrying the HLA-DR15 haplotype, the strongest genetic-risk factor for MS, have elevated self-reactive CD4+ T cells compared to MS patients devoid of this haplotype (Jelcic et al. 2018). Myelin protein-derived antigens such as basic protein (MBP), proteolipid protein and myelin oligodendrocyte glycoprotein (MOG) have been suggested as the main targets recognized by autoreactive CD4+ T cells in MS patients (Dendrou et al. 2015). Furthermore, CD8+ T cells have been found in the white and grey matter of cortical demyelinating lesions of MS patients (Frischer et al. 2009). Interestingly, a pathogenic CD8+ T cell subset expressing intermediate levels of CD161, which displays increased migratory properties, has recently been detected in the central nervous system (CNS) of MS patients (Nicol et al. 2018).
Other well-known players of MS are NK and B cells. Immune-modulating therapies targeting NK cells in MS, have suggested a relevant immune-regulatory role of these cells on CD4+ T cell proliferation and activity, which is dysregulated during disease progression (Gross et al. 2016; Laroni et al. 2016). Addressing the relevance of B cells in MS, it has been shown that a single administration of rituximab (an anti-CD20 monoclonal antibody that mediates the depletion of B cells) reduced brain lesions and clinical relapses for 48 weeks (Hauser et al. 2008). In this regard, B cell subsets can play antibody-dependent or –independent pathogenic functions in MS. For example, the GM-CSF-producing B cell subset that activates inflammatory myeloid cells, is increased in MS patients compared to age and sex-matched healthy controls (Li et al. 2015). On the other hand, the memory B cell subset (CD19+CD27+HLA-DR++ RASGRP2+) contributes to T cell proliferation and homeostasis (Jelcic et al. 2018). These memory B cells can activate and propagate pathogenic T cells, which induce inflammation in the brain.
Dopaminergic Dysregulation in Multiple Sclerosis
Dopamine has been detected in the midbrain, hindbrain and spinal cord of mice (Sharples et al. 2014), where it has been involved in stabilization of ongoing locomotor activity, without affecting coordination on its own (Sharples et al. 2015), and controlling of micturition (Hou et al. 2016). Peripheral blood mononuclear cells (PBMC) of MS patients display altered expression of dopaminergic receptors (Giorelli et al. 2005; Prado et al. 2018), reduced expression of TH and production of dopamine (Zaffaroni et al. 2008), compared to healthy controls (Table 1). Furthermore, PBMCs from MS patients display higher IL-17 production compared to those obtained from healthy controls. This effect was mediated by in vitro activation of D2-like receptors and reverted by the stimulation of D1-like receptors (Melnikov et al. 2016). Interestingly, it has been shown that after 12 months of IFN-β treatment, alterations in the dopaminergic system of MS patients were reverted. In this regard, PBMC from IFN-β-treated MS patients display an increased production of catecholamines (norepinephrine, epinephrine and dopamine), higher mRNA levels of TH and DRD5, and a reduction of DRD2 mRNA levels, compared to their baseline levels before starting the treatment (Zaffaroni et al. 2008). Furthermore, in relapsing-remitting MS patients, IFN-β treatment reduced DRD5 and TH mRNA expression in Tregs compared to their baseline expression, thus, contributing to the recovery of normal suppressive function by abolishing dopamine-mediated inhibition of regulatory T cells function (Cosentino et al. 2012). Of note, although DRD3 mRNA levels were also reduced in Tregs following IFN-β treatment, it is unlikely that signalling through this receptor could be involved in proper regulatory T cell function given that functional impairment of Tregs has been associated to D1-like receptors activation (Cosentino et al. 2007).
In EAE, the animal model of MS, there are also several studies highlighting the role of D1-like dopamine receptors, specially DRD5, in the development of the disease (Nakano et al. 2008; Prado et al. 2012; Osorio-Barrios et al. 2018; Prado et al. 2018). In this regard, the complete DRD5-deficiency, or either confined to dendritic cells or bone marrow, has led to a delayed onset and less severe EAE compared to the control group (Prado et al. 2012; Osorio-Barrios et al. 2018; Prado et al. 2018). It has been suggested that DRD5 activation targets a broad spectrum of different immune cells with opposite functional outcomes depending on the disease stage. For example, it has been shown that stimulation of DRD5 present on dendritic cells increases the production of IL-12 and IL-23, thus promoting the differentiation of naïve CD4+ T cells toward the Th1 and Th17 phenotypes early after the onset of the disease (Prado et al. 2012; Prado et al. 2018). This inflammatory effect was due to the release of dopamine contained in dendritic cells and the subsequent stimulation of DRD5-dependent inhibition of STAT3 phosphorylation in dendritic cells (Prado et al. 2018). Nevertheless, it has recently been shown that DRD5-signalling in Tregs favours a stronger suppressive activity in late stages of EAE (Osorio-Barrios et al. 2018). Furthermore, in apparent controversy, experiments performed in PBMCs obtained from MS patients and from healthy subjects carrying specific dopamine receptors polymorphisms have shown that stimulation of D1-like receptors in Tregs attenuates their suppressive activity (Cosentino et al. 2007; Cosentino et al. 2018). These discrepancies observed between studies performed in EAE and MS could be due to one or more of the following explanations: i) maybe the effects observed in human PBMCs were mediated by DRD1-stimulation rather than DRD5-activation; ii) DRD5-signalling could be coupled to different effects in human Tregs and mouse Tregs; iii) DRD5-mediated effects in Tregs might be different in those Tregs circulating in peripheral blood in comparison with those infiltrating the CNS. Anyway, irrespective of which of these hypotheses are correct, the evidence obtained from animal models and MS patients indicates an important regulatory role of D1-like dopamine receptors in the suppressive activity of Tregs.
Current and Future Treatments for Multiple Sclerosis
Long-standing treatments used for MS reduce relapses, but do not substantially halt disease progression and neuroaxonal damage. A recent study showed that increased DRD3 and DRD5 mRNA expression in regulatory T cells may be associated with the risk of developing MS in patients with clinically isolated syndrome (Cosentino et al. 2016) (Table 1). IFN-β treatment, which is associated with improvement of clinical MS manifestation, has been shown to involve increased production of catecholamines (norepinephrine, epinephrine and dopamine) and mRNA levels of DRD5, and a reduction of DRD2 mRNA levels in PBMC obtained from MS patients, suggesting that these alterations in the dopaminergic system might be involved in ameliorating the clinical course of the disease (Zaffaroni et al. 2008). In this regard, several studies have shown the therapeutic potential of targeting dopamine receptor using human PBMCs in vitro or the EAE animal model.
In monocyte-derived dendritic cells isolated from healthy volunteers, inhibition of D2-like or D1-like receptors can lead to a higher or lower IL-17 production, respectively, compared to non-treated cells (Nakano et al. 2008). Furthermore, EAE mice treated with a D2-like receptor antagonist developed an accelerated progression of EAE. In contrast, EAE mice treated with a D1-like receptor antagonist showed an improved clinical score compared to control group. The authors suggested that this therapeutic effect was mediated by a reduction of IL-17 production and increased IFN-γ secretion by T cells (Nakano et al. 2008).
According to the beneficial effect observed for D2-like dopamine receptors mediated signalling in EAE, the use of pharmacological agonists targeting DRD2, such as bromocriptine, arylpiperazine and pramipexole in MS or EAE have reported promising therapeutic results (Dijkstra et al. 1994; Popovic et al. 2015; Lieberknecht et al. 2017). Bromocriptine, a DRD2 agonist normally used to treat PD, has been shown to decrease EAE severity and duration compared to the control group (Dijkstra et al. 1994). Regarding the mechanism underlying, it is likely that bromocriptine treatment reduces the production of inflammatory cytokines, such as TNF-α, similarly to what has been observed under inflammatory stress induced by LPS inoculation (Mastronardi et al. 2001). Furthermore, mice treated with a high dose of pramipexole, a DRD2/3 agonist, did not develop neurological signs upon EAE induction. These animals presented a significant reduction in astrogliosis, demyelination, and production of reactive oxygen species in the CNS as well as an attenuated production of inflammatory cytokines in the draining lymph nodes (Lieberknecht et al. 2017). Although, the specific cell type responsible for the pramipexole-mediated therapeutic effect was not addressed in this study, it is likely that this drug affected multiple cell types concertedly, as D2-like receptors are expressed in several immune cells, including, lymphocytes, dendritic cells and astrocytes among others. Importantly, therapeutic effects targeting the dopaminergic system have been reported not only in EAE but also in human MS patients. In this regard, a case report of two MS patients showed that bromocriptine treatment alleviated paroxysmal symptoms (Khan and Olek 1995), potentially by reducing IL-17 production by CD4+ T cells (Melnikov et al. 2016). Moreover, there are two ongoing phase II clinical trials using a DRD2/3 antagonist, domperidone, in patients with secondary progressive MS (NCT02308137) and in relapsing-remitting MS (NCT02493049) in Canada (see Table 2).
Interestingly, a recent study performed in the EAE animal model described an important DRD5-mediated dopaminergic autocrine loop in dendritic cells which promotes an inflammatory response and, consequently, a stronger severity in EAE development. In this regard, the depletion of dopamine contained in dendritic cells or the abolition of DRD5-signalling in dendritic cells attenuated significantly the development of EAE (Prado et al. 2018).
Interestingly, the use of psychostimulant drugs can modulate dopamine levels and CD4+ T cell response in MS patients affected by fatigue and cognitive deficits. For instance, increased absolute number and percentage of peripheral blood CD4+ T cells, the main players in MS pathology, have also been reported in patients with depression (Foley et al. 1992). In addition, the prevalence of depression in MS patients is much higher than general population, reaching a frequency of about 20% (Siegert and Abernethy 2005). Moreover, the treatment of patients with anti-depressive therapy has been associated with decreased T cell responsiveness to MOG. Thus, these results together suggest that depression could promote the pathogenic CD4+ T cell response in MS (Mohr et al. 2001). Furthermore, 80–97% of MS patients experience fatigue, which arises due to dopamine imbalance in the CNS. In this regard, the use of amantadine or IFN-β, have shown not only to ameliorate fatigue, but also to increase extracellular dopamine levels (Melanson et al. 2010; Dobryakova et al. 2015). Thus, the evidence suggests the involvement of dopamine mediating a connection between depression and the pathogenic CD4+ T cell response associated to MS.
There is increasing evidence that B cells are involved in the pathogenesis of MS. In this regard, a single administration of rituximab, which deplete B cells, can lead to a significant reduction of brain lesions associated to clinical relapses for 48 weeks (Hauser et al. 2008), and decreased frequency of autoreactive T cells present in peripheral blood of MS patients (Jelcic et al. 2018). Although, the role of dopaminergic signalling on B cells has not been yet studied in MS or EAE, it is likely that DRD2, DRD3 or DRD5, which have been found to be expressed on B cells (McKenna et al. 2002), might play a relevant role in the regulation of the autoimmune response involved in this disorder. Interestingly, DRD2 signalling in B cells negatively correlated with disease progression in rheumatoid arthritis, another autoimmune disorder dependent of autoreactive T and B cells (Wei et al. 2015). Future studies should address the contribution of the dopaminergic system on B cell signalling and its involvement in autoimmunity. The alterations of dopaminergic system involved in MS/EAE and the therapeutic approaches targeting the dopaminergic system as treatment for MS are integrated in the illustration shown in Fig. 2.
Targeting the dopaminergic system in MS/EAE attenuates the pathogenic immune response, ameliorating disease manifestation. Scheme illustrating some immune and dopaminergic components of the CNS and PBMC under steady-state conditions, during MS, and in MS exposed to therapeutic approaches targeting dopaminergic system. a In homeostatic conditions some dendritic cells, which express DRD5, infiltrate the CNS and homeostatic astrocytes express DRD2 (i). PBMC do not infiltrate the CNS, express TH, synthesize dopamine and express the DRD5. It is noteworthy that stimulation of D1-like receptors attenuates the suppressive activity of Treg (ii). b Stimulation of DRD5 signalling on dendritic cells promotes a strong production of IL-12 and IL-23, inducing the differentiation of CD4+ T cells into Th1 and Th17 phenotypes respectively in the CNS. Of note, pathogenic Teff not only produce IFN-γ and IL-17, but also the key pro-inflammatory cytokine GM-CSF (i). Reduction in TH expression and dopamine synthesis in PBMC, favour the stimulation of high affinity dopamine receptors. In this regard, the stimulation of D1-like receptors (presumably DRD5) on PBMCs also increases IL-17 production (ii). c Furthermore, the systemic administration of a D1-like antagonists (SCH23390) inhibits disease development by reducing the frequency of Teffs producing IL-17, IFN-γ and GM-CSF in the CNS (i). Stimulation of DRD2 also attenuates disease progression by reducing immune cell infiltration, astrogliosis, demyelination in the CNS (ii). (iii) The systemic administration of the DRD5 antagonist (SCH23390) also exerts an anti-inflammatory effect at the level of PBMC, by reducing IL-17 production. Abbreviations: CNS: Central nervous system, PBMCs: peripheral mononuclear cells, TH: tyrosine hydroxylase, Treg: regulatory T cells, Teff: effector T cells
Parkinson’s Disease
Epidemiology of Parkinson’s Disease
PD is the second most common chronic neurodegenerative disorder of the aging brain in the United States, affecting about 0.01–1.2% of the population between 45 and 65 years, respectively. With increasing life expectancy and the aging of the population, it has been estimated that approximately 1.34 million new cases will be diagnosed with PD by 2050 in the US. The annual costs are $22,800 per person in the US (Kowal et al. 2013). It is considered a multifactorial disease, including genetic (approximately 10–15%) (Thacker and Ascherio 2008; Ascherio and Schwarzschild 2016; Lunati et al. 2018) and environmental ones (i.e. exposure to solvents, pesticides) (Elbaz et al. 2016; Abbas et al. 2018). To date 15 causal genes and over 25 genetic risk factors have been identified (Verstraeten et al. 2015). Patients with PD can present a variety of clinical symptoms, including slowness of voluntary movements and rigidity, tremor, postural instability, cognitive impairment and dementia, originated from a loss of striatal dopaminergic innervations in the brain. Symptoms are often unilateral becoming more pronounced meanwhile the disease and age progresses (Verstraeten et al. 2015). Non-motor symptoms include olfactory, cognitive and autonomic impairments (De Virgilio et al. 2016). Of note, PD risk is lower among smokers, tea and coffee drinkers, people who perform physical activities, non-steroidal anti-inflammatory drug consumers, among others (Ascherio and Schwarzschild 2016). Parkinson’s disease diagnosis is based on the presence of motor impairment, poor levodopa response, early dementia, ataxia, among others (Verstraeten et al. 2015).
Physiopathological Features of Parkinson’s Disease
The main hallmarks of PD are the reduction in striatal dopamine levels, loss of dopaminergic neurons in the substantia nigra projecting to the striatum (nigrostriatal pathway), generation of protein inclusions formed by the aggregation of misfolded α-synuclein (Lewy bodies) and impairment in the control of voluntary movements (Elbaz et al. 2016; Spillantini and Goedert 2018). Of note, not all PD patients present evident detection of Lewy bodies (Verstraeten et al. 2015), which adds more complexity to the understanding of the disease. The loss of dopaminergic neurons causes most of the motor symptoms observed in PD (Hirsch and Hunot 2009). Furthermore, non-dopaminergic neurons (e.g. norepinephrinergic, cholinergic and serotoninergic) are also affected in a lower degree in PD (Hirsch and Hunot 2009). There are several animal models that have allowed a better understanding of the disease development and progression, including those induced by dugs affecting mitochondrial respiration, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone, annonacin, 6-hydroxydopamine (6-OHDA) (Hirsch and Hunot 2009), and those genetic animal models including the α-synuclein overexpression (Hallett et al. 2012) or those transgenic animals expressing mutant versions of proteins involved in mitochondrial and lysosomal homeostasis such as parkin, pink1, Dj-1, LRRK2, ATP13A2 among others (Winklhofer and Haass 2010).
Several mouse models of PD have shown a pivotal contribution of neuroinflammation, which is required to promote the loss of dopaminergic neurons in the substantia nigra. Importantly, it has been consistently shown that the infiltration and accumulation of peripheral T cells (CD4 and CD8) play a fundamental role favouring neuroinflammation in PD (Brochard et al. 2009). Neuroinflammation involves the activation and recruitment of microglial cells (Kurkowska-Jastrzebska et al. 1999) and astrogliosis (Brochard et al. 2009). It is noteworthy that a similar scenario has been observed in postmortem substantia nigra obtained from PD patients, including a strong T cell infiltration, microglial activation, astrogliosis and also the production of inflammatory factors (e.g. TNF-α, IL-1β) and autoreactive antibodies (McGeer et al. 1988; Damier et al. 1993; Chen et al. 1998; Brochard et al. 2009; Hirsch and Hunot 2009; Selvaraj et al. 2012; Mercado et al. 2018). According to the fundamental role of T cells in chronic neuroinflammation and to the pivotal role of neuroinflammation in the loss of dopaminergic neurons, it has been shown that loss of dopaminergic neurons of the nigrostriatal pathway and the progression of PD are dependent on CD4+ but not CD8+ T cell infiltration in the substantia nigra (Brochard et al. 2009; Gonzalez et al. 2013). In this regard, several lines of evidences have shown that PD involves an autoimmune response mediated by CD4+ T cells specific to neo-antigens contained in Lewy bodies, including nitrated and phosphorylated forms of α-synuclein (Gonzalez et al. 2014, 2015). The generation of these neoantigens and their relevance triggering antigen-specific CD4+ T cell mediated responses have been observed in both, animal models and human PD patients (Gonzalez and Pacheco 2014; Sulzer et al. 2017).
Role of Dopaminergic System in Parkinson’s Disease
There are four major dopaminergic pathways in the mammalian brain, the nigrostriatal, mesolimbic, mesocortical and tuberoinfundibular systems (Beaulieu and Gainetdinov 2011). Among them, the nigrostriatal is substantially affected during the development and progression of PD. The progressive degeneration of dopaminergic neurons of the nigostriatal pathway is accompanied by the reduction of striatal dopamine (Ehringer and Hornykiewicz 1998) and decreased levels of TH and DAT in both striatum and substantia nigra of PD patients compared to healthy controls. Interestingly, it has been determined that maximal reduction of dopaminergic neurons (quantidied as TH and DAT expression) was reached 4 years after the disease is diagnosed (Kordower et al. 2013). Furthermore, it has been shown that dopaminergic neurons of PD patients undergo a downregulation of different genes involved in mitochondrial and synaptic function, protein degradation, cell survival, and programmed cell death, oxidative stress-induced cell response and ubiquitin-proteasome system compared to dopaminergic neurons of the substantia nigra from control subjects (Table 1) (Simunovic et al. 2009).
Maturation and function of neurons in the nigrostriatal pathway are controlled by microRNA (miRNA)-133b, which is specifically expressed in midbrain dopaminergic neurons. It was undetectable in the midbrain of PD patients and in two different mouse models of PD (Kim et al. 2007). However, miRNA-133b deficient-mice do not develop PD, and present a normal number of midbrain dopaminergic neurons and no alterations in striatal dopamine levels and in motor performance (Heyer et al. 2012). Thus, these results suggest that reduction of miRNA-133b levels in dopaminergic neurons of the nigrostriatal pathway is induced as a consequence of the pathogenic process associated to PD, but it is not the cause of neurodegeneration. On the other hand, the expression of dopamine receptors has been shown to be differentially affected during the development of PD. In this regard, DRD3 expression has been consistently decreased in the striatum of MPTP intoxicated macaques (Bezard et al. 2003; Guigoni et al. 2005). Conversely, DRD2 mRNA levels are increased in the caudate nucleus and putamen of a macaque model of PD, without affecting the protein levels (Guigoni et al. 2005).
Dopaminergic signalling is not only affected during disease progression, but also after the treatment with levodopa (Table 2). For instance, it has been described that levodopa treatment increases DRD3 and DRD1 expression in striatal neurons of non-dyskinetic and dyskinetic groups (Bezard et al. 2003; Guigoni et al. 2005), whilst DRD2 mRNA recovers normal levels in dyskinetic macaques intoxicated with MPTP (Guigoni et al. 2005). Thus, whereas DRD3 signalling has been co-related with both development and attenuation of levodopa-induced dyskinesia (LID) (Bezard et al. 2003), DRD1 signalling has been only directly associated with LID (Guigoni et al. 2005). Interestingly, it has been suggested the existence of a crosstalk between DRD1 and DRD3 during the development of dyskinesia. In this regard, a recent study performed in a mouse model of PD showed that levodopa treatment resulted in the overexpression of DRD3 in both DRD1 and DRD2 positive neurons. Furthermore, experiments performed with the DRD3 antagonist PG01037 and DRD1-deficient mice showed that inhibition of DRD1- and DRD3-signalling act synergistically attenuating the incidence of dyskinesia (Solis et al. 2017).
DRD3-signalling has also been shown to promote the development of PD by favouring neuroinflammation (Elgueta et al. 2017) and the pathogenic CD4+ T cell response associated to PD (Gonzalez et al. 2013). In this regard, it has been described that the selective DRD3-stimulation in CD4+ T-cells promotes a strong production of IFN-γ and TNF-α in the substantia nigra inducing the inflammatory M1 phenotype on the microglial cells and the consequent degeneration of the dopaminergic neurons of the nigrostriatal pathway in a mouse model of PD induced by MPTP (Gonzalez et al. 2013). Accordingly, it has been recently demonstrated that the systemic DRD3-antagonism results in a significant attenuation of neurodegeneration and in a significant reduction of motor impairment in two different mouse models of the disease (Elgueta et al. 2017). Furthermore, a recent study performed in the mouse model of PD induced by MPTP has shown that DRD1-signalling in microglial cells and astrocytes promoted a potent anti-inflammatory effect attenuating neurodegeneration of the nigrostriatal pathway. This potent effect was mediated by an increase in cAMP levels, which subsequently induced the ubiquitination and degradation of the NLRP3 inflammasome in glial cells (Yan et al. 2015). Another study addressing the role of dopaminergic signalling in glial cells in the regulation of neuroinflammation found that DRD2-signalling in astrocytes promotes a relevant anti-inflammatory effect mediated by αB-crystallin. In this regard, it was shown that mice bearing DRD2-deficiency confined to astrocytes presented an exacerbated susceptibility to MPTP-induced neurodegeneration and that wild type (WT) mice treated with a DRD2-agonist displayed attenuated neurodegeneration of the nigrostriatal pathway (Shao et al. 2013). Structural changes have been observed in the striatal microcircuit of a 6-OHDA mouse model of PD. Medium spiny neurons (MSN) expressing either DRD1 or DRD2 display a reduced number of spines, leading to an enhancement of excitability. Although, levodopa treatment recovered the number of spines in MSN expressing DRD2, these new spines are weak and insufficient to recover the loss of synaptic transmission (Suarez et al. 2014, 2016). Taken together these studies suggest that high dopamine levels, such as those found in the nigrostriatal pathway under homeostatic conditions, would promote the stimulation of low-affinity dopamine receptors, including DRD1 and DRD2, thus inducing anti-inflammatory effects and favouring homeostasis. Conversely, pathologic conditions involving decreased dopamine levels, such as those found in the nigrostriatal pathway of PD patients and animal models, would induce a selective stimulation of high-affinity dopamine receptors, specially DRD3 (displaying the highest affinity for dopamine), which exert inflammatory effects, favouring neuroinflammation and consequent neurodegeneration.
Treatment Options for Parkinson’s Disease
To date, currently available pharmacological treatments for PD are geared to restore dopaminergic transmission, helping to alleviate symptoms. Nevertheless, these drugs do not affect the neurodegenerative process, thereby they do not cure or slow down disease progression. Some D2-like agonists, such as ropinirole or rotigotine have been used to increase the dopaminergic signalling in the striatum as a palliative therapy for PD, which improves motor symptoms. Some inhibitors of the enzymes that catabolize dopamine, including monoamine oxidase and catechol-O-methyltransferase, have also used to increase the availability of endogenous dopamine as a therapy to dampen motor impairment (Stoker et al. 2018). The drug most commonly used to treat PD is levodopa, the precursor of dopamine, which is administered together with dopa-decarboxylase inhibitors to increase the half-life of the drug and to limit the development of some side-effects (Stoker et al. 2018). This treatment is effective reducing motor symptoms, specially at early stages of the disease progression (Poewe et al. 2010). However, long-term levodopa treatment has been shown to involve the development of complications related to fluctuations in motor response and appearance of involuntary movements (dyskinesia) (Poewe 2010; De Virgilio et al. 2016), which have been associated with signalling through D1- and D2-like receptors (Bezard et al. 2003; Guigoni et al. 2005; Huot et al. 2012).
As discussed above, DRD1 and DRD2 signalling in glial cells has been associated with potent anti-inflammatory effects in animal models. Accordingly, it has been shown that the systemic administration of the DRD1-agonist A-68930 strongly attenuated the loss of dopaminergic neurons of the nigrostraiatal pathway in mice intoxicated with MPTP, an effect that was abrogated in DRD1-deficient mice (Yan et al. 2015). Similarly, according to the anti-inflammatory effect of DRD2-signalling in astrocytes, the systemic administration of the DRD2/DRD3-agonist quinpirole exerted a significant therapeutic effect reducing the extent of neuroinflammation and neurodegeneration of dopaminergic neurons of the substantia nigra in a mouse model of PD induced by MPTP (Shao et al. 2013).
Several studies in animal models of PD, have pointed to the DRD3 receptor as a potential target for the development of future therapies to either stop of slow-down the progression of the disease or to attenuate the emergence of side-effects associated with current treatments (Bezard et al. 2003; Elgueta et al. 2017) (Table 2). In this regard, chronic pharmacological antagonism of DRD3 using PG01037 has been shown to significantly attenuate the loss of dopaminergic neurons of the nigrostriatal pathway, to abolish the development of motor impairment, and to reduce microglia activation in a mouse model of PD induced by the chronic administration of MPTP. Furthermore, the therapeutic effect of the systemic administration of PG01037 at the level of neurodegeneration was also reproduced in a mouse model of PD induced by the stereotaxic delivery of 6-OHDA (Elgueta et al. 2017). Interestingly, the therapeutic effect of PG01037 was accompanied by an increased astrogliosis (Elgueta et al. 2017), thus suggesting that DRD3-antagonism induces an exacerbated activation of astrocytes with anti-inflammatory properties. In this regards, neuronal death has been inversely correlated with the number of activated astrocytes in necropsies of PD patients (Damier et al. 1993). Moreover, an increased astrogliosis has been shown to constitute a critical process necessary to achieve tissue healing and functional recovery in models of spinal cord injury (Herrmann et al. 2008; Anderson et al. 2016). Since astrocytes are heterogeneous, future studies should point to dissect which astrocyte sub-populations are involved in controlling the beneficial effects of astrogliosis in PD, and to decipher the key factors involved in their turning on/off protective effects, as well as the relative contribution of DRD1, DRD2 and DRD3 signalling.
Addressing the therapeutic potential of DRD3 in avoiding LID in PD, a recent study showed that the administration of the DRD3-antagonist PG01037 reduced LID, an effect that was due to a synergistic crosstalk between DRD3 and DRD1 signalling (Solis et al. 2017). On the other hand, another study showed that the treatment of parkinsonian mice with the selective DRD3-agonist, SK609, led to a reduction in the development of motor impairment induced by 6-OHDA (Simms et al. 2016). Moreover, it has been shown that the administration of BP897, a partial DRD3 agonist and DRD2 antagonist, attenuated LID without affecting the therapeutic effects of levodopa in a macaque PD model. However, although the interaction of the drug with DRD2-mediated effects was limited, it could not be completely excluded from the effect observed. In addition, the use of two DRD3 antagonists, nafadotride or ST198 also reduced dyskinesia, but PD symptoms reappeared (akinesia, rigidity) (Bezard et al. 2003). A similar effect was observed using SK609 in combination with levodopa treatment in a rat model of PD, induced by L-DOPA (Simms et al. 2016). Moreover, the use of pramipexole in combination with levodopa treatment in PD patients has shown promising results alleviating LID (Utsumi et al. 2013). Taken together these results indicate that DRD3 signalling has a dual role, participating in the regulation of dyskinesia and also in the therapeutic action of levodopa.
According to the relevance of dopaminergic drugs in attenuating LID in animal models of PD, some clinical trials are currently evaluating the therapeutic potential of these drugs in humans. In this regard, two clinical trials are evaluating the efficacy of buspiron, an antagonist of DRD3 and DRD4, alone or in combination with amantadine in reducing LID in the US (NCT02589340) and France (NCT02617017). However, buspirone is also a 5-HT1A agonist, and probably its efficacy attenuating LID also depends on neurons expressing this receptor (Politis et al. 2014) and in the consequent effect on the firing rate of neurons from the subthalamic nucleus (Sagarduy et al. 2016).
The use of cell-based therapies to restore loss of dopaminergic neurons has also shown promising results (Ma et al. 2010; Kikuchi et al. 2017). In a non-human primate model of PD induced by MPTP, transplantation of dopaminergic neurons derived from induced pluripotent stem cell (iPSC) led to an improvement of symptoms with mild inflammation and recovery of midbrain dopaminergic neurons (Kikuchi et al. 2017). Prompted by these results, in October 2018 the first in-human trial of dopaminergic precursor cells implantation into the brain of a PD patient has begun in Japan. The surgeries aim to restore dopaminergic neuron deficit and motor abilities in six patients (doi: https://doi.org/10.1038/d41586-018-07407-9). Another line of research is the use of spinal cord stimulation to attenuate parkinsonian symptoms. Although it is not clear the exact mechanism involved in this process, it has been suggested that spinal cord stimulation may trigger structural changes in the nigrostriatal circuit leading to locomotor improvement (Santana et al. 2014; Yadav et al. 2014). The involvement of the dopaminergic system in homeostasis of the nigrostriatal pathway, alterations of dopaminergic system associated to PD and the therapeutic approaches targeting dopaminergic system as treatment for PD are integrated in the illustration shown in Fig. 3.
Targeting the dopaminergic system as therapy for Parkinson’s disease. Schematic representation of dopaminergic components in the nigrostriatal pathway under homeostasis, PD, and PD exposed to therapeutic approaches targeting dopaminergic system. a In healthy conditions, dopaminergic neurons located in the substantia nigra release dopamine in the striatum. Striatal GABAergic neurons, which regulate the control of voluntary movements, express DRD3 and DRD2 and respond to dopamine produced by neurons of the nigrostriatal pathway. Homeostatic astrocytes express DRD1 and DRD2, which exert anti-inflammatory effects (i). b In PD, there is an oxidative stress which leads to the generation of Lewy bodies and the progressive loss of dopaminergic neurons of the nigrostriatal pathway, and a consequent reduction of dopamine levels in the striatum (i). Decreased dopamine levels promote a shift from the stimulation of low-affinity dopamine receptors (DRD1 and DRD2) to a selective stimulation of high-affinity dopamine receptors (DRD3). Thus, GABAergic neurons receive attenuated input for DRD2 stimulation, triggering the motor impairment. In addition, microglial cells and astrocytes loss their anti-inflammatory stimulation mediated by DRD1 and DRD2, thus favouring neuroinflammation (ii). Lewy bodies stimulate Toll-like receptors in microglial cells, leading to further microglial activation and neuroinflammation. Moreover, Lewy bodies are captured by dendritic cells and presented to T cells in the cervical lymph nodes, triggering a CD4+ T cell response specific to neo-antigens contained in Lewy bodies. Activated CD4+ T cell acquire Teff phenotypes and infiltrate the brain. The selective stimulation of DRD3 expressed in Teff induces a strong IFN-γ production, which further contributes to induce a pro-inflammatory phenotype in microglial cells, increasing the extent of activated microglia and astrogliosis (iii). c The antagonism of DRD3-signalling attenuates IFN-γ-mediated pro-inflammatory effects of Teff, promotes activated astrocytes with anti-inflammatory properties, reduced microglial activation and thus dampening neurodegeneration (i). A combination of levodopa and pramiprexole, a DRD2/3 agonist, increases DRD2 expression and stimulation in GABAergic neurons, attenuating motor manifestations (ii). Moreover, the systemic administration of quinpirole, a DRD2/DRD3-agonist, promotes the DRD2-stimulation in astrocytes, inducing anti-inflammatory effects, dampening microglial activation and consequently attenuating neurodegeneration (iii). Similarly, the systemic administration of the DRD1-agonist A-68930, stimulates DRD1-signalling in microglial cells and astrocytes, triggering anti-inflammatory effects that attenuates neuroinflammation and the consequent neurodegeneration (iv). Levodopa, the current treatment to treat PD patients’ symptoms, increases DRD3 and DRD1 expression in the brain (v), and when used in combination with pramiprexole increases DRD2 signalling (ii). Abbreviations Teff: effector T cells
Conclusions and Future Perspectives
Alterations of the neuroimmune communications mediated by the dopaminergic system have been involved in the physiopathology of IBD, MS and PD. Taking into consideration the dopamine levels available in particular tissues in homeostasis or in inflammation, the differential expression of dopamine receptors in different immune cells, and the mechanisms by which dopaminergic signalling regulates the function of immune cells, the dopaminergic system becomes a very attractive therapeutic target to manipulate the pathogenic immune response involved in autoimmune disorders. A thorough understanding of the role of this neurotransmitter and signalling pathways associated, will in the future contribute not only to the repurposing of already available pharmacological drugs for the usage in autoimmune pathologies, but also to the development of new therapeutic strategies geared to target specific dopamine receptors in particular cell types, increasing the therapeutic efficacy and reducing adverse effects associated with current off-target drugs. This kind of therapy complemented with genome sequencing analysis usage for diagnosis, will reduce off-target effects among different patients affected by these autoimmune pathologies.
References
Abbas MM, Xu Z, Tan LCS (2018) Epidemiology of Parkinson's disease-east versus west. Mov Disord Clin Pract 5:14–28
Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200
Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N (2012) Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol 303:G1288–G1295
Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol 15:1257–1272
Baumgart DC, Sandborn WJ (2007) Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369:1641–1657
Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217
Berndt BE, Zhang M, Chen GH, Huffnagle GB, Kao JY (2007) The role of dendritic cells in the development of acute dextran sulfate sodium colitis. J Immunol 179:6255–6262
Bernstein CN, Rawsthorne P, Cheang M, Blanchard JF (2006) A population-based case control study of potential risk factors for IBD. Am J Gastroenterol 101:993–1002
Bezard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P (2003) Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat Med 9:762–767
Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S (2009) Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 119:182–192
Brown SJ, Mayer L (2007) The immune response in inflammatory bowel disease. Am J Gastroenterol 102:2058–2069
Buijs S, Krol M, de Voer G (2018) Healthcare utilization and costs of multiple sclerosis patients in the Netherlands: a healthcare claims database study. J Comp Eff Res 7:453–462
Burisch J, Jess T, Martinato M, Lakatos PL, EpiCom E (2013) The burden of inflammatory bowel disease in Europe. J Crohns Colitis 7:322–337
Check JH, Katsoff B, Cohen R (2010) Novel highly effective medical treatment of severe treatment refractory Crohn's disease using sympathomimetic amines: case report. Inflamm Bowel Dis 16:1999–2000
Check JH, Katsoff B, Cohen R (2011a) Case report showing that a woman with ulcerative colitis refractory to standard therapy responded well to the sympathomimetic amine dextroamphetamine sulfate. Inflamm Bowel Dis 17:870–871
Check JH, Cohen R, Katsoff B, Check D (2011b) Hypofunction of the sympathetic nervous system is an etiologic factor for a wide variety of chronic treatment-refractory pathologic disorders which all respond to therapy with sympathomimetic amines. Med Hypotheses 77:717–725
Chen S, Le WD, Xie WJ, Alexianu ME, Engelhardt JI, Siklos L, Appel SH (1998) Experimental destruction of substantia nigra initiated by Parkinson disease immunoglobulins. Arch Neurol 55:1075–1080
Christiansen JR, Olesen MN, Otzen DE, Romero-Ramos M, Sanchez-Guajardo V (2016) Alpha-Synuclein vaccination modulates regulatory T cell activation and microglia in the absence of brain pathology. J Neuroinflammation 13:74
Contreras F, Prado C, Gonzalez H, Franz D, Osorio-Barrios F, Osorio F, Ugalde V, Lopez E, Elgueta D, Figueroa A, Lladser A, Pacheco R (2016) Dopamine receptor D3 signaling on CD4+ T cells favors Th1- and Th17-mediated immunity. J Immunol 196:4143–4149
Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, Saporiti F, Meloni F, Marino F, Lecchini S (2007) Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood 109:632–642
Cosentino M, Zaffaroni M, Trojano M, Giorelli M, Pica C, Rasini E, Bombelli R, Ferrari M, Ghezzi A, Comi G, Livrea P, Lecchini S, Marino F (2012) Dopaminergic modulation of CD4+CD25(high) regulatory T lymphocytes in multiple sclerosis patients during interferon-beta therapy. Neuroimmunomodulation 19:283–292
Cosentino M, Ferrari M, Kustrimovic N, Rasini E, Marino F (2015) Influence of dopamine receptor gene polymorphisms on circulating T lymphocytes: a pilot study in healthy subjects. Hum Immunol 76:747–752
Cosentino M, Zaffaroni M, Legnaro M, Bombelli R, Schembri L, Baroncini D, Bianchi A, Clerici R, Guidotti M, Banfi P, Bono G, Marino F (2016) Dopaminergic receptors and adrenoceptors in circulating lymphocytes as putative biomarkers for the early onset and progression of multiple sclerosis. J Neuroimmunol 298:82–89
Cosentino M, Kustrimovic N, Ferrari M, Rasini E, Marino F (2018) cAMP levels in lymphocytes and CD4(+) regulatory T-cell functions are affected by dopamine receptor gene polymorphisms. Immunology 153:337–341
Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F (1993) Glutathione peroxidase, glial cells and Parkinson's disease. Neuroscience 52:1–6
De Virgilio A, Greco A, Fabbrini G, Inghilleri M, Rizzo MI, Gallo A, Conte M, Rosato C, Ciniglio Appiani M, de Vincentiis M (2016) Parkinson's disease: autoimmunity and neuroinflammation. Autoimmun Rev 15:1005–1011
Dendrou CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol 15:545–558
Dijkstra CD, van der Voort ER, De Groot CJ, Huitinga I, Uitdehaag BM, Polman CH, Berkenbosch F (1994) Therapeutic effect of the D2-dopamine agonist bromocriptine on acute and relapsing experimental allergic encephalomyelitis. Psychoneuroendocrinology 19:135–142
Dobryakova E, Genova HM, DeLuca J, Wylie GR (2015) The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol 6:52
Ehringer H, Hornykiewicz O (1998) Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Parkinsonism Relat Disord 4:53–57
Eisenhofer G, Aneman A, Friberg P, Hooper D, Fandriks L, Lonroth H, Hunyady B, Mezey E (1997) Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab 82:3864–3871
Elbaz A, Carcaillon L, Kab S, Moisan F (2016) Epidemiology of Parkinson's disease. Rev Neurol 172:14–26
Elgueta D, Aymerich MS, Contreras F, Montoya A, Celorrio M, Rojo-Bustamante E, Riquelme E, Gonzalez H, Vasquez M, Franco R, Pacheco R (2017) Pharmacologic antagonism of dopamine receptor D3 attenuates neurodegeneration and motor impairment in a mouse model of Parkinson's disease. Neuropharmacology 113:110–123
Ernstsson O, Gyllensten H, Alexanderson K, Tinghog P, Friberg E, Norlund A (2016) Cost of illness of multiple sclerosis - a systematic review. PLoS One 11:e0159129
Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y (2011) Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J Immunol 186:6313–6318
Foley FW, Traugott U, LaRocca NG, Smith CR, Perlman KR, Caruso LS, Scheinberg LC (1992) A prospective study of depression and immune dysregulation in multiple sclerosis. Arch Neurol 49:238–244
Franz D, Contreras F, Gonzalez H, Prado C, Elgueta D, Figueroa C, Pacheco R (2015) Dopamine receptors D3 and D5 regulate CD4(+)T-cell activation and differentiation by modulating ERK activation and cAMP production. J Neuroimmunol 284:18–29
Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H (2009) The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132:1175–1189
Furlan R, Ardizzone S, Palazzolo L, Rimoldi A, Perego F, Barbic F, Bevilacqua M, Vago L, Bianchi Porro G, Malliani A (2006) Sympathetic overactivity in active ulcerative colitis: effects of clonidine. Am J Physiol Regul Integr Comp Physiol 290:R224–R232
Gagliani N, Vesely MCA, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS, Ching T, Weaver C, Zi X, Pan X, Fan R, Garmire LX, Cotton MJ, Drier Y, Bernstein B, Geginat J, Stockinger B, Esplugues E, Huber S, Flavell RA (2015) Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523:221–225
Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A (2014) Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev 13:3–10
Giorelli M, Livrea P, Trojano M (2005) Dopamine fails to regulate activation of peripheral blood lymphocytes from multiple sclerosis patients: effects of IFN-beta. J Interf Cytokine Res 25:395–406
Gonzalez H, Pacheco R (2014) T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J Neuroinflammation 11:201
Gonzalez H, Contreras F, Prado C, Elgueta D, Franz D, Bernales S, Pacheco R (2013) Dopamine receptor D3 expressed on CD4+ T cells favors neurodegeneration of dopaminergic neurons during Parkinson's disease. J Immunol 190:5048–5056
Gonzalez H, Elgueta D, Montoya A, Pacheco R (2014) Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J Neuroimmunol 274:1–13
Gonzalez H, Contreras F, Pacheco R (2015) Regulation of the neurodegenerative process associated to Parkinson's disease by CD4+ T-cells. J NeuroImmune Pharmacol 10:561–575
Gross CC, Schulte-Mecklenbeck A, Runzi A, Kuhlmann T, Posevitz-Fejfar A, Schwab N, Schneider-Hohendorf T, Herich S, Held K, Konjevic M, Hartwig M, Dornmair K, Hohlfeld R, Ziemssen T, Klotz L, Meuth SG, Wiendl H (2016) Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc Natl Acad Sci U S A 113:E2973–E2982
Guigoni C, Aubert I, Li Q, Gurevich VV, Benovic JL, Ferry S, Mach U, Stark H, Leriche L, Hakansson K, Bioulac BH, Gross CE, Sokoloff P, Fisone G, Gurevich EV, Bloch B, Bezard E (2005) Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Parkinsonism Relat Disord 11(Suppl 1):S25–S29
Hallett PJ, McLean JR, Kartunen A, Langston JW, Isacson O (2012) Alpha-Synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol Dis 47:258–267
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH, Group HT (2008) B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 358:676–688
Hayter SM, Cook MC (2012) Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev 11:754–765
Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28:7231–7243
Heyer MP, Pani AK, Smeyne RJ, Kenny PJ, Feng G (2012) Normal midbrain dopaminergic neuron development and function in miR-133b mutant mice. J Neurosci 32:10887–10894
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol 8:382–397
Hou S, Carson DM, Wu D, Klaw MC, Houle JD, Tom VJ (2016) Dopamine is produced in the rat spinal cord and regulates micturition reflex after spinal cord injury. Exp Neurol 285:136–146
Huot P, Johnston TH, Koprich JB, Aman A, Fox SH, Brotchie JM (2012) L-745,870 reduces L-DOPA-induced dyskinesia in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease. J Pharmacol Exp Ther 342:576–585
International Multiple Sclerosis Genetics C et al (2013) Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet 45:1353–1360
Izcue A, Coombes JL, Powrie F (2009) Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol 27:313–338
Jelcic I et al (2018) Memory B cells activate brain-homing, autoreactive CD4(+) T cells in multiple sclerosis. Cell 175(85–100):e123
Jostins L et al (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491:119–124
Kast RE, Altschuler EL (2001) Remission of Crohn's disease on bupropion. Gastroenterology 121:1260–1261
Kawano M, Takagi R, Kaneko A, Matsushita S (2015) Berberine is a dopamine D1- and D2-like receptor antagonist and ameliorates experimentally induced colitis by suppressing innate and adaptive immune responses. J Neuroimmunol 289:43–55
Khan OA, Olek MJ (1995) Treatment of paroxysmal symptoms in multiple sclerosis with bromocriptine. J Neurol Neurosurg Psychiatry 58:253
Kiesler P, Fuss IJ, Strober W (2015) Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol 1:154–170
Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, Mizuma H, Takara S, Takahashi R, Inoue H, Morita S, Yamamoto M, Okita K, Nakagawa M, Parmar M, Takahashi J (2017) Human iPS cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature 548:592–596
Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A (2007) A MicroRNA feedback circuit in midbrain dopamine neurons. Science 317:1220–1224
Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, Fawkner-Corbett D, Ashley N, Cubitt L, Mellado-Gomez E, Attar M, Sharma E, Wills Q, Bowden R, Richter FC, Ahern D, Puri KD, Henault J, Gervais F, Koohy H, Simmons A (2018) Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell 175(372–386):e317
Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT (2013) Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain 136:2419–2431
Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A (2013) The current and projected economic burden of Parkinson's disease in the United States. Mov Disord 28:311–318
Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A (1999) The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp Neurol 156:50–61
Kustrimovic N, Comi C, Magistrelli L, Rasini E, Legnaro M, Bombelli R, Aleksic I, Blandini F, Minafra B, Riboldazzi G, Sturchio A, Mauri M, Bono G, Marino F, Cosentino M (2018) Parkinson's disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J Neuroinflammation 15:205
Laroni A, Armentani E, Kerlero de Rosbo N, Ivaldi F, Marcenaro E, Sivori S, Gandhi R, Weiner HL, Moretta A, Mancardi GL, Uccelli A (2016) Dysregulation of regulatory CD56(bright) NK cells/T cells interactions in multiple sclerosis. J Autoimmun 72:8–18
Lechin F, van der Dijs B, Insausti CL, Gomez F, Villa S, Lechin AE, Arocha L, Oramas O (1985) Treatment of ulcerative colitis with clonidine. J Clin Pharmacol 25:219–226
Levite M (2016) Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol (Oxf) 216:42–89
Li ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD (2006) Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J Neurosci 26:2798–2807
Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, Moore CS, Michel L, Althekair F, Rajasekharan S, Gommerman JL, Prat A, Fillatreau S, Bar-Or A, Canadian BciMST (2015) Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med 7:310ra166
Lieberknecht V, Junqueira SC, Cunha MP, Barbosa TA, de Souza LF, Coelho IS, Santos AR, Rodrigues AL, Dafre AL, Dutra RC (2017) Pramipexole, a dopamine D2/D3 receptor-preferring agonist, prevents experimental autoimmune encephalomyelitis development in mice. Mol Neurobiol 54:1033–1045
Lunati A, Lesage S, Brice A (2018) The genetic landscape of Parkinson's disease. Rev Neurol (Paris) 174:628–643
Ma Y, Tang C, Chaly T, Greene P, Breeze R, Fahn S, Freed C, Dhawan V, Eidelberg D (2010) Dopamine cell implantation in Parkinson's disease: long-term clinical and (18)F-FDOPA PET outcomes. J Nucl Med 51:7–15
Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN (1999) Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis 5:262–270
Magro F, Vieira-Coelho MA, Fraga S, Serrao MP, Veloso FT, Ribeiro T, Soares-da-Silva P (2002) Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci 47:216–224
Magro F, Fraga S, Ribeiro T, Soares-da-Silva P (2004) Decreased availability of intestinal dopamine in transmural colitis may relate to inhibitory effects of interferon-gamma upon L-DOPA uptake. Acta Physiol Scand 180:379–386
Magro F, Cunha E, Araujo F, Meireles E, Pereira P, Dinis-Ribeiro M, Veloso FT, Medeiros R, Soares-da-Silva P (2006) Dopamine D2 receptor polymorphisms in inflammatory bowel disease and the refractory response to treatment. Dig Dis Sci 51:2039–2044
Martini E, Krug SM, Siegmund B, Neurath MF, Becker C (2017) Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 4:33–46
Mastronardi CA, Yu WH, McCann S (2001) Lipopolysaccharide-induced tumor necrosis factor-alpha release is controlled by the central nervous system. Neuroimmunomodulation 9:148–156
Mayer L (2010) Evolving paradigms in the pathogenesis of IBD. J Gastroenterol 45:9–16
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38:1285–1291
McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ (2002) Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol 132:34–40
Melanson M, Grossberndt A, Klowak M, Leong C, Frost EE, Prout M, Le Dorze JA, Gramlich C, Doupe M, Wong L, Esfahani F, Gomori A, Namaka M (2010) Fatigue and cognition in patients with relapsing multiple sclerosis treated with interferon beta. Int J Neurosci 120:631–640
Melnikov M, Belousova O, Murugin V, Pashenkov capital Em C, Boysmall ka CoCA (2016) The role of dopamine in modulation of Th-17 immune response in multiple sclerosis. J Neuroimmunol 292:97–101
Mercado G, Castillo V, Soto P, Lopez N, Axten JM, Sardi SP, Hoozemans JJM, Hetz C (2018) Targeting PERK signaling with the small molecule GSK2606414 prevents neurodegeneration in a model of Parkinson's disease. Neurobiol Dis 112:136–148
Mohr DC, Goodkin DE, Islar J, Hauser SL, Genain CP (2001) Treatment of depression is associated with suppression of nonspecific and antigen-specific T(H)1 responses in multiple sclerosis. Arch Neurol 58:1081–1086
Nakano K, Higashi T, Hashimoto K, Takagi R, Tanaka Y, Matsushita S (2008) Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun 373:286–291
Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG (2018) Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390:2769–2778
Nicol B, Salou M, Vogel I, Garcia A, Dugast E, Morille J, Kilens S, Charpentier E, Donnart A, Nedellec S, Jacq-Foucher M, le Frère F, Wiertlewski S, Bourreille A, Brouard S, Michel L, David L, Gourraud PA, Degauque N, Nicot AB, Berthelot L, Laplaud DA (2018) An intermediate level of CD161 expression defines a novel activated, inflammatory, and pathogenic subset of CD8(+) T cells involved in multiple sclerosis. J Autoimmun 88:61–74
Nizzoli G, Burrello C, Cribiu FM, Lovati G, Ercoli G, Botti F, Trombetta E, Porretti L, Todoerti K, Neri A, Giuffre MR, Geginat J, Vecchi M, Rescigno M, Paroni M, Caprioli F, Facciotti F (2018) Pathogenicity of in vivo generated intestinal Th17 lymphocytes is IFNgamma dependent. J Crohns Colitis 12:981–992
Oehlers SH, Flores MV, Hall CJ, Wang L, Ko DC, Crosier KE, Crosier PS (2017) A whole animal chemical screen approach to identify modifiers of intestinal neutrophilic inflammation. FEBS J 284:402–413
Olson TS, Reuter BK, Scott KG, Morris MA, Wang XM, Hancock LN, Burcin TL, Cohn SM, Ernst PB, Cominelli F, Meddings JB, Ley K, Pizarro TT (2006) The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med 203:541–552
Osorio-Barrios F, Prado C, Contreras F, Pacheco R (2018) Dopamine receptor D5 signaling plays a dual role in experimental autoimmune encephalomyelitis potentiating Th17-mediated immunity and favoring suppressive activity of regulatory T-cells. Front Cell Neurosci 12:192
Pacheco R (2017) Targeting dopamine receptor D3 signalling in inflammation. Oncotarget 8:7224–7225
Pacheco R, Contreras F, Zouali M (2014) The dopaminergic system in autoimmune diseases. Front Immunol 5:117
Papa I, Saliba D, Ponzoni M, Bustamante S, Canete PF, Gonzalez-Figueroa P, McNamara HA, Valvo S, Grimbaldeston M, Sweet RA, Vohra H, Cockburn IA, Meyer-Hermann M, Dustin ML, Doglioni C, Vinuesa CG (2017) TFH-derived dopamine accelerates productive synapses in germinal centres. Nature 547:318–323
Pinoli M, Marino F, Cosentino M (2017) Dopaminergic regulation of innate immunity: a review. J NeuroImmune Pharmacol 12:602–623
Poewe W (2010) Parkinson disease: treatment of the nonmotor symptoms of Parkinson disease. Nat Rev Neurol 6:417–418
Poewe W, Antonini A, Zijlmans JC, Burkhard PR, Vingerhoets F (2010) Levodopa in the treatment of Parkinson's disease: an old drug still going strong. Clin Interv Aging 5:229–238
Politis M, Wu K, Loane C, Brooks DJ, Kiferle L, Turkheimer FE, Bain P, Molloy S, Piccini P (2014) Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson's disease patients. J Clin Invest 124:1340–1349
Popovic M, Stanojevic Z, Tosic J, Isakovic A, Paunovic V, Petricevic S, Martinovic T, Ciric D, Kravic-Stevovic T, Soskic V, Kostic-Rajacic S, Shakib K, Bumbasirevic V, Trajkovic V (2015) Neuroprotective arylpiperazine dopaminergic/serotonergic ligands suppress experimental autoimmune encephalomyelitis in rats. J Neurochem 135:125–138
Powrie F, Mason D (1990) OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med 172:1701–1708
Prado C, Contreras F, Gonzalez H, Diaz P, Elgueta D, Barrientos M, Herrada AA, Lladser A, Bernales S, Pacheco R (2012) Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity. J Immunol 188:3062–3070
Prado C, Bernales S, Pacheco R (2013) Modulation of T-cell mediated immunity by dopamine receptor d5. Endocr Metab Immune Disord Drug Targets 13:184–194
Prado C, Gaiazzi M, Gonzalez H, Ugalde V, Figueroa A, Osorio-Barrios FJ, Lopez E, Lladser A, Rasini E, Marino F, Zaffaroni M, Cosentino M, Pacheco R (2018) Dopaminergic stimulation of myeloid antigen-presenting cells attenuates signal transducer and activator of transcription 3-activation Favouring the development of experimental autoimmune encephalomyelitis. Front Immunol 9:571
Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, Barmada MM, Datta LW, Shugart YY, Griffiths AM, Targan SR, Ippoliti AF, Bernard EJ, Mei L, Nicolae DL, Regueiro M, Schumm LP, Steinhart AH, Rotter JI, Duerr RH, Cho JH, Daly MJ, Brant SR (2007) Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet 39:596–604
Sagarduy A, Llorente J, Miguelez C, Morera-Herreras T, Ruiz-Ortega JA, Ugedo L (2016) Buspirone requires the intact nigrostriatal pathway to reduce the activity of the subthalamic nucleus via 5-HT1A receptors. Exp Neurol 277:35–45
Santana MB, Halje P, Simplicio H, Richter U, Freire MAM, Petersson P, Fuentes R, Nicolelis MAL (2014) Spinal cord stimulation alleviates motor deficits in a primate model of Parkinson disease. Neuron 84:716–722
Seeman P, Van Tol HH (1994) Dopamine receptor pharmacology. Trends Pharmacol Sci 15:264–270
Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, Singh BB (2012) Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J Clin Invest 122:1354–1367
Seo DH, Che X, Kwak MS, Kim S, Kim JH, Ma HW, Kim DH, Kim TI, Kim WH, Kim SW, Cheon JH (2017) Interleukin-33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci Rep 7:851
Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek E, Chen T, Li SB, Xu M, Zhou JN, Hu G, Zhou JW (2013) Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature 494:90–94
Sharples SA, Koblinger K, Humphreys JM, Whelan PJ (2014) Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front Neural Circuits 8:55
Sharples SA, Humphreys JM, Jensen AM, Dhoopar S, Delaloye N, Clemens S, Whelan PJ (2015) Dopaminergic modulation of locomotor network activity in the neonatal mouse spinal cord. J Neurophysiol 113:2500–2510
Siegert RJ, Abernethy DA (2005) Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry 76:469–475
Simms SL, Huettner DP, Kortagere S (2016) In vivo characterization of a novel dopamine D3 receptor agonist to treat motor symptoms of Parkinson's disease. Neuropharmacology 100:106–115
Simunovic F, Yi M, Wang Y, Macey L, Brown LT, Krichevsky AM, Andersen SL, Stephens RM, Benes FM, Sonntag KC (2009) Gene expression profiling of substantia nigra dopamine neurons: further insights into Parkinson's disease pathology. Brain 132:1795–1809
Solis O, Garcia-Montes JR, Gonzalez-Granillo A, Xu M, Moratalla R (2017) Dopamine D3 receptor modulates l-DOPA-induced dyskinesia by targeting D1 receptor-mediated striatal signaling. Cereb Cortex 27:435–446
Spillantini MG, Goedert M (2018) Neurodegeneration and the ordered assembly of alpha-synuclein. Cell Tissue Res 373:137–148
Stoker TB, Torsney KM, Barker RA (2018) Emerging treatment approaches for Parkinson's disease. Front Neurosci 12:693
Suarez LM, Solis O, Carames JM, Taravini IR, Solis JM, Murer MG, Moratalla R (2014) L-DOPA treatment selectively restores spine density in dopamine receptor D2-expressing projection neurons in dyskinetic mice. Biol Psychiatry 75:711–722
Suarez LM, Solis O, Aguado C, Lujan R, Moratalla R (2016) L-DOPA oppositely regulates synaptic strength and spine morphology in D1 and D2 striatal projection neurons in dyskinesia. Cereb Cortex 26:4253–4264
Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, Peters B, Frazier A, Lindestam Arlehamn CS, Sette A (2017) T cells from patients with Parkinson's disease recognize alpha-synuclein peptides. Nature 546:656–661
Thacker EL, Ascherio A (2008) Familial aggregation of Parkinson's disease: a meta-analysis. Mov Disord 23:1174–1183
Tolstanova G, Deng X, Ahluwalia A, Paunovic B, Prysiazhniuk A, Ostapchenko L, Tarnawski A, Sandor Z, Szabo S (2015) Role of dopamine and D2 dopamine receptor in the pathogenesis of inflammatory bowel disease. Dig Dis Sci 60:2963–2975
Tonini M, Cipollina L, Poluzzi E, Crema F, Corazza GR, De Ponti F (2004) Review article: clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment Pharmacol Ther 19:379–390
Utsumi H, Okuma Y, Kano O, Suzuki Y, Iijima M, Tomimitsu H, Hashida H, Kubo S, Suzuki M, Nanri K, Matsumura M, Murakami H, Hattori N, Tokyo Parkinson's Disease Study G (2013) Evaluation of the efficacy of pramipexole for treating levodopa-induced dyskinesia in patients with Parkinson's disease. Intern Med 52:325–332
Vallone D, Picetti R, Borrelli E (2000) Structure and function of dopamine receptors. Neurosci Biobehav Rev 24:125–132
Verstraeten A, Theuns J, Van Broeckhoven C (2015) Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet 31:140–149
Wei L, Zhang C, Chen HY, Zhang ZJ, Ji ZF, Yue T, Dai XM, Zhu Q, Ma LL, He DY, Jiang LD (2015) Dopamine receptor DR2 expression in B cells is negatively correlated with disease activity in rheumatoid arthritis patients. Immunobiology 220:323–330
Winklhofer KF, Haass C (2010) Mitochondrial dysfunction in Parkinson's disease. Biochim Biophys Acta 1802:29–44
Yadav AP, Fuentes R, Zhang H, Vinholo T, Wang CH, Freire MA, Nicolelis MA (2014) Chronic spinal cord electrical stimulation protects against 6-hydroxydopamine lesions. Sci Rep 4:3839
Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R (2015) Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160:62–73
Zaffaroni M, Marino F, Bombelli R, Rasini E, Monti M, Ferrari M, Ghezzi A, Comi G, Lecchini S, Cosentino M (2008) Therapy with interferon-beta modulates endogenous catecholamines in lymphocytes of patients with multiple sclerosis. Exp Neurol 214:315–321
Zizzo MG, Cavallaro G, Auteri M, Caldara G, Amodeo I, Mastropaolo M, Nuzzo D, Di Carlo M, Fumagalli M, Mosca F, Mule F, Serio R (2016) Postnatal development of the dopaminergic signaling involved in the modulation of intestinal motility in mice. Pediatr Res 80:440–447
Acknowledgements
This work was supported by Programa de Apoyo a Centros con Financiamiento Basal AFB-170004 (to Fundación Ciencia & Vida) from “Comisión Nacional de Investigación Científica y Tecnológica de Chile (CONICYT)” and by grants FONDECYT-1170093 (to R.P.) from “Fondo Nacional de Desarrollo Científico y Tecnológico de Chile”, and MJFF-10332.01 (to R.P.) and MJFF-15076 (to R.P.) from Michael J. Fox Foundation for Parkinson Research. PV is supported by “Progama PostDoc Hinge” from Fundación Ciencia & Vida.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vidal, P.M., Pacheco, R. Targeting the Dopaminergic System in Autoimmunity. J Neuroimmune Pharmacol 15, 57–73 (2020). https://doi.org/10.1007/s11481-019-09834-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-019-09834-5