Abstract
The development of combined antiretroviral therapy (cART) has increased the lifespan of persons living with HIV (PLWH), with most PLWH having a normal life expectancy. While significant progress has occurred, PLWH continue to have multiple health complications, including HIV associated neurocognitive disorders (HAND). While the exact cause of HAND is not known, persistent neuroinflammation is hypothesized to be an important potential contributor. Molecular imaging using positron emission tomography (PET) can non-invasively evaluate neuroinflammation. PET radiotracers specific for increased expression of the translocator protein18kDa (TSPO) on activated microglia can detect the presence of neuroinflammation in PLWH. However, results from these studies have been inconsistent and inconclusive. Future studies are needed to address key limitations that continue to persist with these techniques before accurate conclusions can be drawn regarding the role of persistent neuroinflammation in PLWH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of combined antiretroviral therapy (cART) has increased the lifespan of persons living with HIV (PLWH), with most PLWH expected to have a normal life expectancy. In 2017, the United Nations Program on HIV/AIDS reported 70% of PLWH know their status, 77% have access treatment, and 82% are virologically suppressed (Joint United Nations Programme on HIV/AIDS (UNAIDS) 2017). Current guidelines aim to reach 90–90-90 by 2020. While significant progress has occurred, PLWH continue to have multiple health complications, including HIV associated neurocognitive disorders (HAND) (McArthur et al. 2010). Approximately 30–50% of PLWH develop HAND even in the cART era (Heaton et al. 2010). The most common cognitive impairments are seen within learning, memory, and executive function (Clifford and Ances 2013). While the exact cause of HAND is not known, persistent neuroinflammation has been hypothesized to be an important contributor.
Unfortunately, cART is only able to suppress the virus, not eradicate it, and remaining low levels of HIV can persist in reservoirs. One of these reservoirs is believed to be the brain (Valcour et al. 2011). HIV enters the brain soon after seroconversion, and because the brain is a protected organ due to the blood brain barrier (BBB), the virus remains despite cART. Microglia, the primary immune cell of the central nervous system (CNS), are a common target of viral infection and, due to low turnover rate, may continue to harbor the virus (González-Scarano and Martín-García 2005). These infected cells, along with the presence of residual virus, can stimulate the immune system. While a normal immune response for an infection is appropriate when properly implemented, persistent neuroinflammation can cause synaptic dysfunction and neuronal death that could translate to neurological impairment commonly seen as HAND (Spudich and González-Scarano 2012).
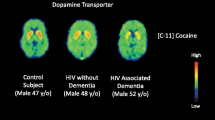
Unlike the blood, the brain is difficult to repeatedly evaluate. As a result, neuroinflammation is frequently assessed by measuring levels of CSF and blood-plasma neuroinflammatory biomarkers, such as pro-inflammatory cytokines and chemokines. These biomarkers, however, are peripheral measures of inflammation and do not provide a topography of inflammatory changes within the brain. Alternatively, neuroimaging techniques provide a non-invasive and direct method of assessing changes in the brain, and molecular imaging using positron emission tomography (PET) has been used in research to appraise neuroinflammation (Masters and Ances 2014). During a PET scan, individuals are injected with a radiotracer that has a high affinity for a cellular marker of interest. After a specific period of time, an image is acquired to determine the location of potential increases in radioligand uptake. To study neuroinflammation, PET imaging studies commonly use radiotracers specific for 18 kDa translocator protein (TSPO) (Fig. 1).
Originally known as peripheral benzodiazepine receptor (PBR), TSPO is primarily located in the mitochondrial matrix and was initially studied due to its role in binding benzodiazepine. Further studies observed correlations between TSPO PET tracer binding and histochemical localization of activated microglia (Banati et al. 2000) (Kuhlmann and Guilarte 2000). Subsequent studies have supported these findings by using both TSPO PET imaging and histochemistry in human and animal models (Gulyás et al. 2009) (Cicchetti et al. 2002). TSPO-targeted PET tracer binding has since become the primary molecular imaging method for researching neuroinflammation and has been used in a wide range of neurological disorders (e.g. Alzheimer disease (Kreisl et al. 2016) and multiple sclerosis (Rissanen et al. 2014)). Since 2005, an increasing number of molecular imaging studies have used TSPO PET radioligands to investigate the potential role of neuroinflammation in the development of HAND in PLWH despite receiving cART. This review will provide an overview of the methods and results from these studies and discuss future directions to better our understanding of the neurological consequences of HIV.
TSPO PET Imaging Methods
The first TSPO ligand used to measure activated microglia was 11C-(R)-PK11195, and this first-generation tracer is still widely used in neuroimaging studies of neurodegeneration. However, PK11195 has high levels of nonspecific binding and may have difficulties penetrating the blood brain barrier (BBB) (Chauveau et al. 2009). PK11195 therefore has a low signal to noise (SNR), and multiple second-generation radiotracers have been developed. These tracers, including 11C-PBR28 and 11C-DPA713, have a greater affinity for the receptor, better ability to cross the BBB, and have an overall higher SNR (Knezevic and Mizrahi 2018). The binding affinities of these newer molecular imaging compounds, however, are highly dependent on the single nucleotide polymorphism (SNP) rs6971, an alanine-threonine substitution in the TSPO gene (Owen et al. 2014). Individuals who are homozygous for this polymorphism show no significant ligand uptake, while heterozygous individuals show a substantially reduced level of uptake. As a result, a genotype analysis is performed before scanning to determine the genotype of participants (high-affinity binders (HAB), mixed-affinity binders (MAB), or low-affinity binders (LAB)). LAB individuals are typically excluded from studies. For studies including both MAB and HAB participants, genotype is commonly defined as a fixed factor in statistical analyses (Kreisl et al. 2013a).
While correcting for genotype accounts for the majority of binding affinity differences, variations can still occur amongst individuals. One method to account for this variability is to use a metabolite-corrected plasma input function in order to normalize the concentration of ligand in the brain tissue to the concentration in blood plasma. Though considered the optimum for quantifying ligand uptake, this method requires the placement of an arterial line and repeated blood sampling (Kreisl et al. 2013b). Furthermore, plasma analysis can be time intensive and expensive. An alternative method is to use a reference region for normalizing binding values. A reference tissue is defined as a region that has no specific binding, but this may be difficult as TSPO is expressed throughout the brain. Instead, many studies use a “pseudo-reference region”, a region expected to be unaffected by disease-pathology (Kreisl et al. 2017). While it depends on the disease being studied, common pseudo-reference regions include the cerebellum, total white matter, and total grey matter.
TSPO PET Imaging Studies in PLWH
Methodology
To date, six studies have used PET imaging of TSPO to look at neuroinflammation in PLWH, but significant differences exist amongst the studies with regard to methodology (Table 1). Some studies (e.g. Wiley et al., Hammoud et al., and Coughlin et al.) compared PLWH with and without cognitive impairment to HIV-uninfected controls (HIV- controls), but others only included unimpaired PLWH. Not only have these studies used different participant populations, they also used different criteria for defining degree of cognitive impairment. Some studies determined cognitive status based on the older Memorial Sloan-Kettering (MSK) dementia scale while others employed a computerized assessment tool (CogState; CogState Ltd., Melbourne, Australia). Only two studies (Wiley et al. and Rubin et al.), classified HAND using standards similar to the currently recommended Frascati criteria (Antinori et al. 2007).
Each group also used a different approach to process PET data. Regional binding in PET studies can be analyzed using a voxel-based cluster approach across the entire brain or by using predefined or manually drawn regions-of-interest (ROIs). Wiley et al. employed a voxel-based cluster method, while Hammoud et al., Coughlin et al., Vera et al., and Rubin et al. used an ROI method. In one study (Garvey et al.), both methods were used. Another important difference amongst the various studies was the choice of pseudo-reference region used for comparison. Hammoud et al. used manually drawn white matter ROIs as pseudo-reference regions. In contrast, Wiley et al. chose the pseudo-reference region to be the cluster with normal ligand kinetics, determined using three HIV- controls. However, Garvey et al., Coughlin et al., Vera et al., and Rubin et al. used cortical grey matter as a pseudo-reference region.
Results
Each of the molecular imaging studies that used TSPO concentrated on potential differences between PLWH compared to HIV- controls. Varying results were observed when comparing unimpaired participants to HIV- controls. Both Hammoud et al. and Wiley et al. did not observe significant differences between asymptomatic PLWH (cognitively normal) and HIV- control participants. Garvey et al. also observed no differences between these two groups when a ROI based analysis was used. However when using a whole brain voxel-based analysis, Garvey and colleagues observed that asymptomatic PLWH had increased inflammation within the corpus callosum, anterior and posterior cingulate, temporal cortex, and frontal cortex compared to HIV- controls. Coughlin et al. observed increased inflammation in the white matter, cingulum, occipital cortex, and temporal cortex when a more lenient false discovery rate q-value of 10% was applied. However when the false discovery rate threshold was reduced to 5%, these relationships were no longer seen. Vera et al. showed increased tracer uptake in the parietal lobe, occipital lobe, and globus pallidus of asymptomatic PLWH compared to HIV- controls.
Three studies performed further analyses comparing impaired and unimpaired PLWH, but results varied greatly amongst studies. Both Hammoud et al. and Wiley et al. saw no significant differences, while Coughlin et al. saw greater inflammation within the frontal cortex of impaired PLWH. Hammoud et al. and Wiley et al. also compared impaired PLWH to cognitively normal HIV- controls. The results from Hammoud et al. showed increased binding in the thalamus, putamen, frontal cortex, temporal cortex, and occipital cortex, but Wiley et al. observed no significant differences between impaired PLWH and HIV- controls.
Instead of comparing PLWH and controls, Rubin et al. reanalyzed an existing PLWH cohort (Coughlin et al.). Rubin and colleagues studied the correlation between tracer binding and different cognitive domains. Similar analyses were performed by Garvey et al. and Vera et al., but results from these studies vary. Garvey et al. noted correlations between executive function and increased uptake in the anterior cingulate, posterior cingulate, and corpus callosum. Rubin et al. observed significant correlations between executive function and the frontal cortex and hippocampus. However, Vera et al. saw no correlations between binding and executive function. Conversely, Vera et al. observed significant correlations between global cognition and increased binding in multiple regions, while Rubin et al. did not detect a correlation between global function and binding potential. Overall, these studies found correlations between certain cognitive domains and tracer uptake in various brain regions, but the findings were not consistent. For instance, Rubin et al. found increased tracer binding in the occipital region correlated with working memory and processing speed. However, Vera et al., identified increased uptake within the occipital lobe correlating with composite accuracy and visual learning performance.
Limitations of TSPO Studies in PLWH
Although all six PET neuroimaging studies of inflammation have similar aims, the results and conclusions have varied. Differences exist in criteria for defining cognitive impairment. Not only did this result in varying participant selection, it prevents comparisons to be made amongst studies. Only Garvey et al. and Vera et al. showed statistically significant increases in inflammation when comparing HIV- controls to asymptomatic PLWH. Other studies showed potential trends towards significance when more liberal thresholds were used. Overall, these results suggest that there may be mild increases in neuroinflammation within asymptomatic PLWH, but further studies are needed. However, no overlapping regions of increased binding were observed, which may reflect the presence of more global inflammation. The lack of significant results may also be due to small sample sizes. Most studies included less than ten unimpaired HIV+ participants, and when demographic data was provided, it showed the participants were predominately male.
Another important limitation is the variety of TSPO ligands used. Only three studies used PK11195 ligand while the other studies used different second-generation ligands. Choice of ligand can significantly affect the results. Two key disadvantages of PK1195 are the ligand’s high levels of nonspecific binding and its low permeability across the BBB. Each of these factors contribute to the generally low SNR for PK11195. While second generation tracers such as PBR28 and DPA-713 have greater SNR, they are also affected by genotype. Potential differences observed amongst the various studies may reflect inherent differences in these tracers. Additional studies in PLWH using a consistent tracer will allow for more confident conclusions to be drawn.
As previously discussed, a more consistent image processing approach will be important for future studies. Differences in the choice of pseudo-reference region can greatly affect results. Although grey matter was the defined pseudo-reference region for three molecular imaging studies, a number of studies have identified increased uptake within this same region. If grey matter is in fact affected, using it as a pseudo-reference region could skew the results and cause other affected regions to not be identified. A potentially more accurate pseudo-reference region could be the cerebellar grey matter, which is often not affected by HIV. This same region is also a commonly used pseudo-reference region for molecular imaging studies in other neurodegenerative (including Alzheimer disease (Lyoo et al. 2015)). Additional analyses using multiple pseudo-reference regions should be explored.
Finally, recent studies have challenged the reliability of TSPO as a marker of neuroinflammation. While TSPO has been linked with a variety of cellular processes, such as steroidogenesis and inflammation, the exact function of TSPO is not known, and extensive research has produced conflicting results (Notter et al. 2018). Initial studies hypothesized that TSPO was necessary for transport of cholesterol across the outer mitochondrial membrane and, therefore, was vital for steroidogenesis (Krueger and Papadopoulos 1990). More recent studies, have challenged this claim and have shown no changes in steroid synthesis in TSPO knock-out mice (Tu et al. 2014). TSPO has also been linked to apoptosis via mitochondrial permeability transition, but this too has been challenged by recent TSPO knock-out mice studies (Veenman and Gavish 2012). Most importantly for this review, the function of TSPO in the inflammatory process and the proteins that are activated by microglia have not been well characterized. TSPO has been shown to correlate with both pro-inflammatory and anti-inflammatory processes (Bae et al. 2014). A recent study noted a decrease in TSPO expression in pro-inflammatory activated microglia (Owen et al. 2017). The uncertainty regarding the definitive function and expression of TSPO has caused some to question the reliability of using TSPO tracer binding as a measure of microglial activity and, therefore, a secondary measure of inflammation.
Future Directions
While cART has improved the quality of life for PLWH, this population still faces considerable cognitive challenges. Many studies have used PET imaging of TSPO to investigate the potential role of neuroinflammation with regard to HAND, but the current findings are inconsistent. Additional studies with a larger sample size that uses the Frascati HAND criteria are necessary. A significant obstacle that also needs to be addressed is the lack of a common and decisive methodology for this research, and a test-retest analysis would help resolve this issue. Using the various analysis methods, a test-retest study would compare each methods’ results and evaluate their repeatability. This is important to determine a reliable standard methodology, ensuring the results arise from disease pathology and not due to differences in analyses.
So far neuroinflammation in PLWH has only been studied cross-sectionally, but a longitudinal study could be an insightful next step. Previous studies have shown that most asymptomatic PLWH remain cognitively stable over time, but some individuals experience neurocognitive deterioration despite viral suppression with cART (Sanford et al. 2018). A longitudinal study using TSPO PET imaging could evaluate if observed changes in cognitive status correlate to changes in ligand binding. In addition to cognitive status, future studies could analyze the correlation between TSPO PET imaging measurements and HIV biomarkers, as Vera et al. briefly tested. Cerebrospinal fluid (CSF) and plasma measurements including CD4/CD8 ratio, CSF HIV RNA, sCD14, sCD163, and plasma 16 s rRNA can provide important biomarkers for evaluating the effects of HIV throughout the body. Comparing these measures to molecular imaging markers of neuroinflammation would provide valuable insight into the relationship between neuroinflammation and HIV.
Future TSPO PET studies in PLWH should address concerns regarding the reliability of TSPO as a marker of neuroinflammation. Due to these concerns and complications caused by genetic effects on TSPO tracer binding, there has been an increasing interest in investigating alternative, more reliable PET radiotracers that measure microglial activation. One such alternative PET tracer target is the P2X7 receptor (P2X7R), a ligand-gated cation receptor that plays a vital role in pro-inflammatory processes in microglia (Beaino et al. 2017). Multiple PET radiotracers targeting P2X7R have been developed, and a few, such as [3H]A-740003 and [11C]SMW139, are undergoing clinical evaluation (Janssen et al. 2018).
Overall, the six studies using TSPO PET imaging show some could allow us to understand the role of neuroinflammation in PLWH, but to date the results are not able to provide a clear conclusion. Additional PET studies of neuroinflammation in PLWH are needed in order to better understand and improve treatment.
References
Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB et al (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69(18):1789–1799. https://doi.org/10.1212/01.WNL.0000287431.88658.8b
Bae K-R, Shim H-J, Balu D, Kim SR, Yu S-W (2014) Translocator protein 18 KDa negatively regulates inflammation in microglia. J NeuroImmune Pharmacol 9(3):424–437. https://doi.org/10.1007/s11481-014-9540-6
Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G et al (2000) The peripheral benzodiazepine binding site in the brain in multiple sclerosis. Quantitative in vivo imaging of microglia as a measure of disease activity. Brain 123(11):2321–2337. https://doi.org/10.1093/brain/123.11.2321
Beaino W, Janssen B, Kooij G, van der Pol SMA, van Het Hof B, van Horssen J, Windhorst AD, de Vries HE (2017) Purinergic receptors P2Y12R and P2X7R: potential targets for PET imaging of microglia phenotypes in multiple sclerosis. Journal of Neuroinflammation 14(1):1–16. https://doi.org/10.1186/s12974-017-1034-z
Chauveau F, Van Camp N, Dolle F, Kuhnast B, Hinnen F, Damont A, Boutin H, James M, Kassiou M, Tavitian B (2009) Comparative evaluation of the translocator protein Radioligands 11C-DPA-713, 18F-DPA-714, and 11C-PK11195 in a rat model of acute Neuroinflammation. J Nucl Med 50(3):468–476. https://doi.org/10.2967/jnumed.108.058669
Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, Isacson O (2002) Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur J Neurosci 15(6):991–998. https://doi.org/10.1046/j.1460-9568.2002.01938.x
Clifford DB, Ances BM (2013) HIV-associated neurocognitive disorder. Lancet Infect Dis 13(11):976–986. https://doi.org/10.1016/S1473-3099(13)70269-X
Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV, Roosa HV et al (2014) Regional brain distribution of translocator protein using [ 11C]DPA-713 PET in individuals infected with HIV. Journal of NeuroVirology 20(3):219–232. https://doi.org/10.1007/s13365-014-0239-5
Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, Winston A (2014) Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. Aids 28(1):67–72. https://doi.org/10.1097/01.aids.0000432467.54003.f7
González-Scarano F, Martín-García J (2005) The Neuropathogenesis of AIDS. Nat Rev Immunol 5(1):69–81. https://doi.org/10.1038/nri1527
Gulyás B, Makkai B, Kása P, Gulya K, Bakota L, Várszegi S, Beliczai Z et al (2009) A comparative autoradiography study in post mortem whole hemisphere human brain slices taken from Alzheimer patients and age-matched controls using two Radiolabelled DAA1106 analogues with high affinity to the peripheral benzodiazepine receptor (PBR) Syst. Neurochem Int 54(1):28–36. https://doi.org/10.1016/j.neuint.2008.10.001
Hammoud DA, Endres CJ, Chander AR, Guilarte TR, Wong DF, Sacktor NC, McArthur JC, Pomper MG (2005) Imaging glial cell activation with [ 11 C]- R -PK11195 in patients with AIDS. J Neurovirol 11(4):346–355. https://doi.org/10.1080/13550280500187351
Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ et al (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: charter study. Neurology 75(23):2087–2096. https://doi.org/10.1212/WNL.0b013e318200d727
Janssen B, Vugts DJ, Windhorst AD, Mach RH (2018) PET imaging of microglial activation - beyond targeting TSPO. Molecules 23(3):1–14. https://doi.org/10.3390/molecules23030607
Joint United Nations Programme on HIV/AIDS (UNAIDS) (2017) Ending Aids Progress Towards the 90–90-90 Targets. Global Aids Update. https://doi.org/UNAIDS/JC2900E. Accessed 12 July 2018
Knezevic D, Mizrahi R (2018) Molecular imaging of Neuroinflammation in Alzheimer’s disease and mild cognitive impairment. Prog Neuro-Psychopharmacol Biol Psychiatry 80(January 2017). Elsevier):123–131. https://doi.org/10.1016/j.pnpbp.2017.05.007
Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, Zoghbi SS et al (2013a) A genetic polymorphism for translocator protein 18 kda affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. Journal of Cerebral Blood Flow and Metabolism 33(1). Nature Publishing Group):53–58. https://doi.org/10.1038/jcbfm.2012.131
Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, Corona W et al (2013b) In vivo Radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 136(7):2228–2238. https://doi.org/10.1093/brain/awt145
Kreisl, William C., Chul Hyoung Lyoo, Jeih San Liow, Monica Wei, Joseph Snow, Emily Page, Kimberly J. Jenko, et al. 2016. “11C-PBR28 binding to translocator protein increases with progression of Alzheimer’s Disease.” Neurobiol Aging 44. Elsevier Inc: 53–61. https://doi.org/10.1016/j.neurobiolaging.2016.04.011
Kreisl WC, Henter ID, Innis RB (2017) Imaging Translocator Protein as a Biomarker of Neuroinflammation in Dementia. Advances in Pharmacology. 1st ed. Vol. 82. Elsevier Inc. https://doi.org/10.1016/bs.apha.2017.08.004
Krueger KE, Papadopoulos V (1990) Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J Biol Chem 265(25):15015–15022 http://www.jbc.org/content/265/25/15015. Accessed 5 Oct 2018
Kuhlmann AC, Guilarte TR (2000) Cellular and subcellular localization of peripheral benzodiazepine receptors after Trimethyltin neurotoxicity. J Neurochem 74(4):1694–1704. https://doi.org/10.1046/j.1471-4159.2000.0741694.x
Lyoo CH, Ikawa M, Liow J-S, Zoghbi SS, Morse CL, Pike VW, Fujita M, Innis RB, Kreisl WC (2015) Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect Neuroinflammation measured with PET Radioligand binding to translocator protein. J Nucl Med 56(5):701–706. https://doi.org/10.2967/jnumed.114.146027
Masters M, Ances B (2014) Role of neuroimaging in HIV-associated neurocognitive disorders. Semin Neurol 34(01):089–102. https://doi.org/10.1055/s-0034-1372346
McArthur JC, Steiner J, Sacktor N, Nath A (2010) Human immunodeficiency virus-associated neurocognitive disorders mind the gap. Ann Neurol 67(6):699–714. https://doi.org/10.1002/ana.22053
Notter T, Coughlin JM, Sawa A, Meyer U (2018) Reconceptualization of translocator protein as a biomarker of Neuroinflammation in psychiatry. Mol Psychiatry 23(1). Nature Publishing Group):36–47. https://doi.org/10.1038/mp.2017.232
Owen DR, Qi Guo NJ, Kalk AC, Kalogiannopoulou D, Dimber R, Lewis YL et al (2014) Determination of [11C]PBR28 binding potential in vivo: A first human TSPO blocking study. J Cereb Blood Flow Metab 34(6). Nature Publishing Group):989–994. https://doi.org/10.1038/jcbfm.2014.46
Owen DR, Narayan N, Wells L, Healy L, Smyth E, Rabiner EA, Galloway D et al (2017) Pro-inflammatory activation of primary microglia and macrophages increases 18 KDa translocator protein expression in rodents but not humans. J Cereb Blood Flow Metab 37(8):2679–2690. https://doi.org/10.1177/0271678X17710182
Rissanen E, Tuisku J, Rokka J, Paavilainen T, Parkkola R, Rinne JO, Airas L (2014) In vivo detection of diffuse inflammation in secondary progressive multiple sclerosis using PET imaging and the Radioligand 11C-PK11195. J Nucl Med 55(6):939–944. https://doi.org/10.2967/jnumed.113.131698
Rubin LH, Sacktor N, Creighton J, Du Y, Endres CJ, Pomper MG, Coughlin JM (2018) Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS 32(12):1661–1667. https://doi.org/10.1097/QAD.0000000000001858
Sanford R, Ances BM, Meyerhoff DJ, Price RW, Fuchs D, Zetterberg H, Spudich S, Louis Collins D (2018) Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary human immunodeficiency virus infection. Clin Infect Dis, no. August 67:1–8. https://doi.org/10.1093/cid/ciy362
Spudich S, González-Scarano F (2012) HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb Perspect Med 2(6):1–17. https://doi.org/10.1101/cshperspect.a007120
Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM, Selvaraj V (2014) Peripheral benzodiazepine receptor/translocator protein global Knock-out mice are viable with no effects on steroid hormone biosynthesis. J Biol Chem 289(40):27444–27454. https://doi.org/10.1074/jbc.M114.578286
Valcour V, Sithinamsuwan P, Letendre S, Ances B (2011) Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep 8(1):54–61. https://doi.org/10.1007/s11904-010-0070-4
Veenman L, Gavish M (2012) The role of 18 KDa mitochondrial translocator protein (TSPO) in programmed cell death, and effects of steroids on TSPO expression. Curr Mol Med 12:398–412. https://doi.org/10.2174/156652412800163343
Vera JH, Qi G, Cole JH, Boasso A, Greathead L, Kelleher P, Rabiner EA et al (2016) Neuroinflammation in treated HIV-positive individuals. Neurology 86(15):1425–1432. https://doi.org/10.1212/WNL.0000000000002485
Wiley CA, Lopresti BJ, Becker JT, Boada F, Lopez OL, Mellors J, Meltzer CC, Wisniewski SR, Mathis CA (2006) Positron emission tomography imaging of peripheral benzodiazepine receptor binding in human immunodeficiency virus-infected subjects with and without cognitive impairment. J Neurovirol 12(4):262–271. https://doi.org/10.1080/13550280600873868
Funding
The study was supported by grants from the National Institute for Nursing Research (R01NR014449 and R01NR015738), the National Institute of Mental Health (R01MH118031), the Paula O and Rodger Riney Research Fund, and the Daniel J Brennan MD Research Fund.
Author information
Authors and Affiliations
Contributions
A.B and B.M.A conceived the study; A.B. analyzed the data; and A.B and B.M.A wrote the paper.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boerwinkle, A., Ances, B.M. Molecular Imaging of Neuroinflammation in HIV. J Neuroimmune Pharmacol 14, 9–15 (2019). https://doi.org/10.1007/s11481-018-9823-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-018-9823-4