Abstract
Rare earth (RE)–doped glasses with high quantum efficiency and strong photoluminescence (PL) characteristics are essential for next generation photonic and optoelectronic devices. To attain strong PL emission and related attributes of Er3+-doped in glass system, the silver nanoparticles (NPs) were embedded. UV-Vis absorption and microscopic measurements divulged the existence of metallic silver NPs. The PL emission of Er3+-doped in titled glasses was improved in visible and infrared spectral ranges as AgNO3 concentration escalated to higher level. In addition, the quantum efficiency also enhanced as the AgNO3 doping level escalated to a higher level. The enhancements in PL emission intensity and quantum efficiency were ascribed to the local field induced by surface plasmons of Ag NPs. The outcomes suggest that the high concentration of Ag NPs routed in Er3+-doped glass system is useful in fabricating the optical amplifiers and solid-state lasers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal nanoparticles (NPs) routed in different optically transparent hosts such as polymers [1], silica films [2], colloidal solutions [3], and glasses [2] are of enormous curiosity to the scientific community owing to their potential functionalities in bio-sensing [4], bio-imaging [5], molecular species sensing by surface-enhanced scattering spectroscopy [6], plasmonics [7], luminescence [8], and nonlinear optical [9]. Among all other hosts for routing the metal NPs, the glass hosts are exceptionally attractive because of high metal solubility along with uniform distribution, which allows for the tuning of optical and other properties of hosts based on the NPs concentration [2]. In particular, the metal NPs embedment in rare earth (RE) containing glass matrices is significantly attracted the research community for tuning the photoluminescence (PL) and quantum efficiency magnitudes of RE3+ ions loaded in glass specimens [10]. Usually when the RE concentration is increased in the composition to tune the PL and related parameters, at higher concentration of RE ions, the properties are deteriorated due to concentration quenching effect which limits their practical use in the devices. By embedding NPs to RE-activated glasses provides an alternative way to tune the PL and related attributes significantly without immediate quenching in the properties which are desired for specific applications. The co-doping of a small quantity of metal NPs to the optimized glass composition significantly improves the PL and other optical features by the local electric field modified [11,12,13] and/or transfer of energy (from NPs to RE ions) phenomena [11, 14]. This luminescence enhancement of RE ions due to the inclusion of NPs in the composition was first observed by Malta et al. [15] in 1985 for Eu3+ ions–doped materials with co-doping of silver (Ag) NPs. Then onwards, this method has been widely followed by many glass researchers for developing the glassy materials for photonic applications [16,17,18,19,20] by achieving significant enhancement in PL properties of RE-doped glasses, and different attributions were proposed for the observed enhancement. Few examples are as follows: Silva et al. [16] have achieved the luminous up conversion enhancement in Er3+-loaded PbO–GeO2 glass specimens by co-doping with Ag NPs and highlighted that the enhancement was due to local field effect. Dousti et al. [17] have observed the improved emission curves of Er3+ activated heavy metal oxide vitreous systems caused by the surface plasmon resonance (SPR) of silver NPs. Jiani et al. [18] have shown the significant fluorescence amplification of trivalent erbium ions in silver NPs co-doped B2O3–Bi2O3–TiO2 glass system and highlighted due to both the energy transfer and local field effects. The contribution of Ag NPs on the 1.5 μm emission amplification of trivalent erbium ions activated in complex silicate based glass has recently been examined by Rajaramakrishna et al. [20] and underlined the local electric field evinced and energy transfer routes are the acceptable claims for emission gained by the Er3+ ions.
Notably, among numerous glass families of interest, the borate vitreous compositions have attracted the scientific community for photonic applications since the borate matrix can dissolve high amounts of RE ions and NPs [21, 22]. In addition, the borate glasses possess wide transparency from visible to infrared regions, strong thermo–chemico–mechanical stability, and low glass transition temperatures [22]. Furthermore, the inclusion of high molar mass oxides in a borate glass system greatly decreases the energy of phonons present in the host and improves the PL intensity of RE ions by reducing non-radiative emissions [23]. The Sb2O3 is one of the high molar mass oxides, and it also serves as the thermal reducing agent to obtain Ag0 NPs from its ionic state [24]. Therefore, the selection of Sb2O3 in the glass composition provides a straightforward, low-cost strategy to fabricate metal NPs containing glasses without following additional heat treatments at different temperatures or for different durations. Among all RE ions, the Er3+ ion is one of the most promising RE ions in the lanthanide group since it possesses a favorable energy level scheme to be exploited in a wide variety of applications which includes solid-state lasers, microchip lasers, eye safe lasers, and many other advanced photonic systems [25, 26].
Thus, Er3+ loaded in glass specimens has received significant attention as an active host for lasers, upconverters, amplifiers, and so forth. It was identified that only a few investigations are found in the open literature with improvement in 1.53 μm band PL emission by the inclusion of metal NPs in the glass hosts [25, 27,28,29,30]. The current investigation aims to prepare Er3+-activated borate-based glasses embedded with silver NPs to enhance PL emission at the 1.53 μm emission band and in the visible region. The efficacy of silver NPs concentration on spectroscopic and PL attributes of Er3+ ions has been analyzed, and the mechanism trustworthy for enhancement of PL and related attributes has been discussed in detail.
Materials and Methods
The glass specimens bearing the compositional formula of (mol %) 10Sb2O3–20Na2O–69B2O3–1Er2O3–xAgNO3 (x = 0 – 0.8 mol %) were fabricated through the melting and quenching process. Before proceeding for characterization, the fabricated glass specimens were designated as Er1Ag–x; in the codes, the “x” indicates the AgNO3 content added in the composition to obtain the Ag NPs. The glass free from Er2O3 and Ag NPs was coded as Er0Ag–0. The glass compositional mixtures of 30 g were kept in a high-temperature furnace by loading into the porcelain crucibles. The mixtures were melted at 950 °C and rapidly quenched on pre-annealed brass plates. After the quenching, immediatley the glasses were moved to annealing furnace that was already programmed with 350 °C for 4 h. This process was used to remove internal stress in the glass samples that had developed during quick quenching from high temperatures. X-ray diffraction (XRD) profiles with 2θ range from 10 to 90° of step size of 0.05° were recorded using Rigaku Ultima IV X-ray diffractometer. The optical absorbance spectra were collected utilizing Carry 5000 UV–Vis–NIR spectrophotometer from 200 to 1700 nm. The presence and morphology of Ag NPs in the Er1Ag–0.2 glass specimen were divulged by transmission electron microscopy (TEM) micrograph captured with the help of FEI Technai G2 20 TEM. The photonic measurements of all the glasses were recorded using an Edinburg Spectro Fluorimeter (FLS 980) provided with flash lamp (xenon) as the source of excitation.
Results and Discussion
The XRD profiles Er1Ag–0, Er1Ag–0.2, and Er1Ag–0.8 glass samples are presented in Fig. 1. The observed broad diffused hump with no sharp crystallization peak suggests the missing of long-range atomic arrangement in the studied glasses. These signatures also affirm the non-crystalline characteristic of the glass specimens examined in the current work. The TEM micrograph of the Er1Ag–0.2 glass is displayed in Fig. 2a. The size distribution of silver NPs and the average nanoparticle diameter were analyzed utilizing ImageJ computer software. The size of the Ag NPs computed utilizing ImageJ software has been plotted as a histogram and presented in Fig. 2b. The histogram profile reveal the size of metallic silver NPs lies in the range of 2.50 - 12.46 nm, with 7.78 nm median size. The TEM image displayed in Fig. 2a also unveils that the silver NPs embedded in Er1Ag–0.2 glass are spherical in form and are distributed uniformly in the sample. Additionally, the TEM image of the Er1Ag–0.2 glass host shows some Ag NPs with non-spherical shapes.
The selective formation of Ag0 NPs from their counter ionic species has been occurred in the current system based on the following thermochemical equations [31],
The reaction, i.e., Sb3+ + 2Ag+ → Sb5+ + 2Ag0, has the E0 = 1.02 V and the ΔG = –197 kJ. These magnitude of E0 and ΔG divulge the reaction is plausible to induce in the system. However, the reaction 3Sb3+ + 2Er3+ → 3Sb5+ + 2Er0 owes the E0 = –5.76 V and the ΔG = 3334 kJ, the negative sign on E0 and positive magnitude of ΔG clearly suggest that the later reaction is not plausible to induce in the currently investigated glass system. These two reactions and their inferences clearly unveil the antimony selectively reducing Ag+ to Ag0 (first reduction reaction) and not reducing Er3+ to Er0 (second reduction reaction) in the glass matrix considered in the current investigation.
Optical absorption spectra of Er1Ag–0 (doped with only 1 mol % of Er2O3 and free from Ag NPs), Er1Ag–0.2, and Er1Ag–0.8 glass specimens in the range of 200–1700 nm are displayed in Fig. 3. The inset of Fig. 3 displays the absorption spectra of Er0Ag–0 (free from Er2O3 and Ag NPs) and Er1Ag–0 glasses with designation to the absorption peaks observed in Er1Ag–0 glass. The noticed Er3+ characteristic absorption peaks were assigned be referring the articles [25, 31]. The spectrum of Er0Ag–0 glass does not demonstrate any absorption peaks while the Er1Ag–0 glass demonstrates the characteristic absorption bands of Er3+ ions. The comparison of absorption spectrum of Er1Ag–0 glass specimen with that of Er0Ag–0 glass clearly unveils the existence of Er3+ ions in Er1Ag–0 glass. Furthermore, from Fig. 3, it is evident that the characteristic absorption peaks of Er3+ ions are also present in Ag NPs embedded glass specimens, which illustrates the presence of Er3+ ions in the Ag NPs embedded glass specimens too. Figure 3 displays that the spectra of AgNO3-containing glasses exhibited a broad and robust peak at ~ 430 nm, which corresponds to the SPR of silver nanoparticles. It can also be identified that the SPR amplitude increases with the rise of AgNO3 content due to the rise in the concentration of Ag NPs formed upon loading the more and more concentration of AgNO3 in the composition [32]. Furthermore, from Fig. 3, it can be identified that the center of SPR peak has been slightly shifted towards longer wavelength with increase of Ag NPs concentration, which is manifested to growth of silver NPs routed in the glass hosts [22]. In addition, it is also manifested to increase of concentration of formation of Ag NPs occurred due to loading of more and more AgNO3 in the glass composition [25, 33].
Usually, the amplitude of Er3+ absorption characteristic peaks is corroborated to asymmetry and covalency around the Er3+ ions and the rigidity of the glass is based on the hypothesis provided by Judd and Ofelt, famously referred to as Judd-Ofelt (JO) hypothesis [34, 35]. The JO parameters Ωt (t = 2, 4, 6) computed from UV-Vis linear absorption results [25, 36] and quality factor (χ) are furnished in Table 1 for Er1Ag–0, Er1Ag0.2, and Er1Ag–0.8 glasses. From Table 1, it can be observed that the Ω2 enhances with the increase of AgNO3 content. This enhance effect in Ω2 with the inclusion of more and more Ag NPs in the composition suggests that the trivalent erbium cations are occupied in strongly non-symmetric sites in the high amount of Ag NPs bearing glasses, which also instantiate that ligand field in erbium ions sorrounding is highly covalent in Ag NPs routed glasses [25]. Table 1 also shows the Ω4 and Ω6 increase with the rise of AgNO3 quantity, which divulges that long-range structure related parameters (density and rigidity) of the glasses increases with the formation of Ag NPs in the glass system. This enhance behaviour of Ω4 and Ω6 also divulge that large Er3+ cations are dissolved in the glass matrix (solubility of Er3+ increases); thereby, the interionic separation with erbium ions decreases. This ultimately enhances the force of repulsion between surrounding similar Er3+ cations, thereby enhances the Ω6 [25]. Furthermore, the χ of the studied glass specimens enhanced with the increase of AgNO3 content, which indicates that a high concentration of Ag NPs routed glass matrix is beneficial for having high stimulated emission cross-section [18, 25, 27, 37, 38].
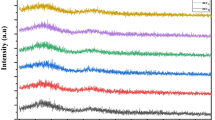
The PL emission spectral outcomes of Er1Ag–x glass samples are exhibited in Fig. 4 recorded for irradiation of 376 nm. The three PL emission lines evidenced at 538, 552, and 626 nm are related to 2H11/2 → 4I15/2, 4S3/2 → 4I15/2, and 4F9/2 → 4I15/2 transitions occurring within trivalent erbium ions, respectively. In Fig. 4, a weak PL signal has appeared at around 505 nm in addition to the PL emission peaks related to Er3+ ions. This weak PL signal and its intensity variation are not related to Er3+ ions, which might be related to the glass host or Ag NPs. In-depth structural and spectroscopic investigations are essential to address this, which will be a part of a future study. The 4S3/2 → 4I15/2 transition of Er3+ occurred at 552 nm is highly intense compared to other three PL emission lines. From Fig. 4 it can be noticed that emission intensity enhances with escalation of AgNO3 content in the glass composition. In similar lines, the PL emission spectra in infrared region for all the glass specimens collected under the excitation of 980 nm are depicted in Fig. 5. All the glass specimens display a strong PL emission peak poisoned at 1535 nm owing to 4I13/2 → 4I15/2 inherent transition of Er3+. The spectral results displayed in Fig. 5 divulge that intensity of PL emission lines improves with AgNO3 content in the glass matrix. The luminescence improvement factor (LIF), defined as the ratio of PL emission intensity magnitude of Er3+ ions in NPs bearing glass to the NPs free glass. The LIF factors for green light transition are 1.15, 1.96, 2.57, and 2.79 for Er1Ag–0.2, Er1Ag–0.4, Er1Ag–0.6, and Er1Ag–0.8 glass samples, respectively. Similarly, for 1535 nm transition the magnitudes were found to be 1.54, 1.93, 2.42, and 2.83 for Er1Ag–0.2, Er1Ag–0.4, Er1Ag–0.6, and Er1Ag–0.8 glass specimens, respectively. The increment of PL emission of erbum ions occurred in metallic Ag NPs routed glasses is because of the transfer of energy from metallic silver NPs to the corresponding energy levels of trivalent erbium ions and / or evinced local electric field produced by localized surface plasmons of Ag NPs [25].
The attenuations, such as an increase in the broadness and shift (towards red region) of maxima of the SPR band as seen in UV-Vis absorption results, expose the reduction in interparticle distances (dAg–Ag) of silver NP and their subsequent growth with the increase of silver NPs quantity in the glass matrices. It has been documented that the diameter of the NPs and the separation between them are oppositely corroborated in the local field boost formula [22]. These variations clearly unveil the boosting of local electric field with increase of metallic silver NPs content in the glass host. Therefore, the improved attenuation noticed in PL properties with Ag0 NPs is due to the boosted local field provoked metallic Ag NPs around the trivalent erbium ions [31]. Since, the boosted local field effectively enhances the rate of pumping of trivalent erbium ions to higher energy levels, which results in the high PL emission in different spectral regions depending the energy difference between the ground state and excited energy states of trivalent erbium ions [31].
In addition, when the metallic silver NPs routed glass host irradiated with electromagnetic radiation, the NPs routed in the glass host causes a weak emission which enhances the pumping rates of tripositive erbium ions to their excited energy levels. This boosted the photons content in upper energy levels of erbium ions [39]. Therefore, the energy transfer from metallic silver NPs to higher energy levels of erbium cations is contributory factor for the strong PL emission noticed for erbium ions PL peaks in Ag NPs routed glasses. Since the position of SPR peak of metallic silver lies very close to that of irradiated light utilized for recording the PL emission profiles in the visible region. Hence, it is believed that the transfer energy from metallic silver NPs to excited erbium ions energy levels is a highly acceptable origin contrast to the local field evinced origin for improved emission observed for visible spectra range.
The stimulated emission cross-section (σemi) [40] magnitudes were estimated for Er1Ag–0, Er1Ag–0.2, and Er1Ag–0.8 glass samples using corresoponding radiative properties and PL emission outcomes, and which are 4.79 × 10−21, 5.92 × 10−21, and 9.26 × 10−21 cm2, respectively. The full width at half maximum (FWHM) factors computed from the PL emission spectral results, the results are 82.31, 95.62, and 121.27 nm respectively for Er1Ag–0, Er1Ag–0.2, and Er1Ag0.8 glass samples. The attenuation in these results clearly divulge that the formation of metallic Ag NPs in erbium ions activated glass hosts greatly boosts the emission cross-sections and bandwidths of PL emission lines of erbium ions in infrared spectral range. Furthermore, the gain bandwidth (FWHM × σemi) factors are found to be 3.94, 5.66, and 11.23 × 10−26 cm3 respectively for Er1Ag–0, Er1Ag–0.2, and Er1Ag–0.8 glass specimens. The greatness of gain bandwidth and related results noticed in the present study contrast to those achieved in other works [25, 27, 41] illustrate that the Er1Ag–0.8 glass host is largely suitable for fiber optic, photonic amplifier, and infrared laser functionalities.
The PL lifetime results for Er1Ag–0, Er1Ag–0.2, and Er1Ag–0.8 glass hosts recorded by monitoring 4I13/2 → 4I15/2 infrared emission transition with pumping of 980 nm are provided in Fig. 6 and all the outcomes were matched well with single exponential decay mechanism [27]. The decay lifetime (τexp) magnitudes were computed to be 2.54, 2.83, and 3.61 ms, respectively for Er1Ag–0, Er1Ag–0.2, and Er1Ag–0.0.8 glass samples. The τexp magnitudes boosted with silver NPs content in accordance with the PL emission intensity trend that noticed in infrared spectra region. This is corresponds to evinced local field stimulated by localized surface plasmons of metallic Ag NPs and energy transfer from Ag0 NPs to the upper energy levels of tripositive erbium ions [25]. The quantum efficiency (η) factors were computed by following the procedure elaborated in the reference [25]. Table 2 furnishes the τexp, τrad, and η magnitudes for Er1Ag–0, Er1Ag–0.2, and Er1Ag–0.8 glass hosts and it unveil the increase behaviour with respect to Ag0 NPs content in the host. The high quantum efficiency result achieved for Er1Ag–0.8 glass system divulge that this glass hosts is benefical for photonic and optoelectronic functionalities to execute their function in infrared spectral range [42, 43].
Summary
The Er3+ activated in borate glass specimens embedded with various concentrations of Ag NPs was synthesized by the standard melt–quench process. The XRD outcomes affirm the glassy nature of the glass samples examined in the current investigation. The TEM micrograph divulged the presence of spherical-shaped Ag NPs with uniform distribution in the tested glass with a median size of 7.78 nm. The absorption outcomes further confirmed the existence of silver NPs by exhibiting the SPR peak in the spectra of the tested Ag NPs embedded glasses. The absorption results also unveiled the growth of silver NPs with an elevation of AgNO3 content in the system. The PL emission intensity of both visible and infrared transitions is increased as the function of Ag NPs due to evinced local field provoked by localized surface plasmons of metallic Ag NPs and transfer of energy from metallic Ag NPs to corresponding energy levels of trivalent erbium ions. The maximum values of phenomenal parameters in the device fabrication mentioned in the Table 1 and η achieved for Er1Ag–0.8 glass divulge that a high concentration of silver NPs embedded glass specimens are beneficial for optical and optoelectronic functionalities.
Research Data Policy and Data Availability
The authors declare that the data supporting the findings of this study are available within the article. The raw data that support the findings are available on request from the corresponding author.
References
Gushiken NK, Paganoto GT, Temperini MLA, Teixeira FS, Salvadori MC (2020) ACS Omega 5:10366–10373
Sendova M, Jiménez JA (2012) J Phys Chem C 116:17764–17772
Richter K, Campbell PS, Baecker T, Schimitzek A, Yaprak D, Mudring AV (2013) Phys Status Solidi 250:1152–1164
Malekzad H, Sahandi Zangabad P, Mirshekari H, Karimi M, Hamblin MR (2017) Nanotechnol Rev 6:301–329
Han X, Xu K, Taratula O, Farsad K (2019) Nanoscale 11:799–819
Camelio S, Babonneau D, Vandenhecke E, Louarn G, Humbert B (2021) Nanoscale Adv 3:6719–6727
Kumar P, Mathpal MC, Prakash J, Jagannath G, Roos WD, Swart HC (2020) Mater Res Bull 125:110799
Da Silva DS, De Assumpção TAA, Kassab LRP, De Araújo CB (2014) J Alloys Compd 586:S516–S519
Jagannath G, Eraiah B, Jayanthi K, Keshri SR, Som S, Vinitha G, Pramod AG, Krishnakanth KN, Devarajulu G, Balaji S, Rao SV, Annapurna K, Das S, Allu AR (2020) Phys Chem Chem Phys 22:2019–2032
Som T, Karmakar B (2010) Plasmonics 5:149–159
De Araújo CB, Kassab LRP (2016) in: Glas. Nanocomposites Synth Prop Appl pp 131–144
Wu Y, Shen X, Dai S, Xu Y, Chen F, Lin C, Xu T (2011) J Phys Chem C 115:25040–25045
Liu J, Wang Q, Sang X, Hu H, Li S, Zhang D, Liu C, Wang Q, Zhang B, Wang W, Song F (2021) Nanomaterials 11:1037
Li J, Wei R, Liu X, Guo H (2012) Opt Express 20:10122–10127
Malta OL, Santa-Cruz PA, Desa GF, Auzel F (1985) J Lumin 33:261–272
Mariano D, Reyes L, Kassab P, Lüthi SR, De Araújo CB, Anderson SL, José M, Bell V (2007) Appl Phys Lett 90:081913
Dousti MR, Sahar MR, Ghoshal SK, Amjad RJ, Arifin R (2013) J Mol Struct 1033:79–83
Qi J, Xu T, Wu Y, Shen X, Dai S, Xu Y (2013) Opt Mater 35:2502–2506
Soltani I, Hraiech S, Horchani-Naifer K, Massera J, Petit L, Férid M (2016) J Non Cryst Solids 438:67–73
Rajaramakrishna R, Ruangtaweep Y, Sangwaranatee N, Kaewkhao J (2019) J Non Cryst Solids 521:119522
Gao G, Wei J, Shen Y, Peng M, Wondraczek L (2014) J Mater Chem C 2:8678–8682
Fatima N, Pramod AG, Jagannath G, Rajaramakrishna R, Keshavamurthy K, Ramesh P, Sathish KN, Alhuthali AMS, Sayyed MI, Hegde V, Rao SV, Nadaf YF (2021) Ceram Int 47:16801–16808
Ramesh P, Gangareddy J, Sathish KN, Pramod AG, Hegde V, Pasha UM, Khan S, Annapurna K, Sayyed MI, Alhuthali AMS, Agarkov DA, Kokila MK (2021) Opt Mater 114:110933
Sasai J, Hirao K (2001) J Appl Phys 89:4548
Fares H, Elhouichet H, Gelloz B, Férid M (2014) J Appl Phys 116:123504
Gelija D, Kadathala L, Borelli DPR (2018) Opt Mater 78:172–180
Swetha BN, Devarajulu G, Keshavamurthy K, Jagannath G, Deepa HR (2021) J Alloys Compd 856:158212
Manzani D, Marega E, Nunes LAO, Osorio SPA, Rivera VAG, Ledemi Y, Messaddeq Y (2010) Opt Express 18:25321–25328
Rivera VAG, Ledemi Y, Osorio SPA, Manzani D, Ferri FA, Ribeiro SJL, Nunes LAO, Marega E (2013) J Non Cryst Solids 378:126–134
Qi Y, Zhou Y, Wu L, Yang F, Peng S, Zheng S, Yin D (2014) J Lumin 153:401–407
Som T, Karmakar B (2009) J Appl Phys 105(1–8):013102
Gangareddy J, Bheemaiah E, Gandhiraj V, James JT, Jose JK, Katturi Naga K, Soma VR (2018) Appl Phys B Lasers Opt 124
Jagannath G, Eraiah B, NagaKrishnakanth K, Rao SV (2018) J Non Cryst Solids 482:160–169
Judd BR (1962) Phys Rev 127:750–761
Ofelt GS (1962) J Chem Phys 37:511–520
Serqueira EO, De Morais RF, Dantas NO (2013) J Alloys Compd 560:200–207
Yang J, Dai S, Zhou Y, Wen L, Hu L, Jiang Z (2002) J Appl Phys 93:977
Jlassi I, Elhouichet H, Ferid M, Barthou C (2010) J Lumin 130:2394–2401
Mechergui I, Fares H, Mohamed SA, Nalin M, Elhouichet H (2017) J Lumin 190:518–524
OSA Tech. Dig. (Optica Publ. Group, 1990) (1990)
Fares H, Stambouli W, Elhouichet H, Gelloz B, Férid M (2016) RSC Adv 6:31136–31145
Caetano M, Silva ACA, Filho JCS, De Morais RF, Sales TO, Andrade AA, Dantas NO (2020) J Lumin 228:117599
Aouaini F, Maaoui A, Mohamed NBH, Alanazi MM, El Maati LA (2022) J Alloys Compd 894:162506
Acknowledgements
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R57), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
This research was funded by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R57), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Kempaiah Keshavamurthy: conceptualization, data curation, writing—original draft and editing, Gangareddy Jagannath: methodology, conceptualization, formal analysis, writing—review and editing, Dalal Abdullah Aloraini: data curation, writing—review and editing, Aljawhara H Almuqrin: data curation, M.I. Sayyed: writing—review and editing, K. N. Sathish: writing—review and editing, and P. Ramesh: conceptualization, formal analysis, writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keshavamurthy, K., Jagannath, G., Aloraini, D.A. et al. Silver Nanoparticles Amplified Visible and Infrared Photoluminescence Features of Er3+ Ions Activated in Borate Glasses. Plasmonics 18, 175–182 (2023). https://doi.org/10.1007/s11468-022-01736-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-022-01736-2