Abstract
Purpose
Animal studies suggested that halogenated hydrocarbons such as 1,1-difluoroethane (DFE) sensitized myocardial tissues to catecholamines and might cause fatal arrhythmia. In this paper, we report a case of a fatality that was associated with DFE abuse, and quantified DFE concentrations in postmortem specimens using gas chromatography–mass spectrometry (GC–MS).
Methods
Femoral vein blood, cardiac blood, and urine samples were taken from the autopsy for toxicological analysis. We have established a detailed procedure for quantification of DFE in human blood and urine by GC–MS and have presented its validation data.
Results
The concentrations of DFE in this case were 481, 591 and 201 µg/mL in femoral vein blood, cardiac blood and urine samples, respectively, which were much higher than those in previous cases measured by gas chromatography–flame ionization detection. Thus, in the absence of other remarkable autopsy findings, the cause of death was determined to be DFE intoxication.
Conclusions
To the best of our knowledge, this is the first case report of quantification of DFE in human blood and urine specimens by GC–MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
1,1-Difluoroethane (DFE) is a halogenated hydrocarbon, known as HFC-152a that is commonly used as a propellant in aerosolized products, such as keyboard cleaners [1]. However, it may be a substance of abuse, because of its inhalation-induced euphoria [2, 3]. It is known that intentional inhalation of DFE can cause symptoms, such as confusion, tremors, pulmonary irritation, and coma [4]. In addition, animal studies suggested that halogenated hydrocarbons such as DFE sensitized myocardial tissues to catecholamines and might cause fatal arrhythmia [3, 5]. However, there are a limited number of forensic studies regarding postmortem fatal tissue concentrations of DFE [2, 3, 6, 7]. The analysis of inhaled substances of abuse poses a challenge for forensic investigation, because of their high volatility, short half-life, and rapid elimination [1]. In this case study, we report a fatality that was associated with DFE abuse and determined concentrations of DFE in postmortem femoral vein blood, cardiac blood, and urine samples using gas chromatography–mass spectrometry (GC–MS). This is the first case report to quantify DFE concentrations in human blood and urine specimens using GC–MS; in the previous reports [3, 6, 7], gas chromatography–flame ionization detection (GC–FID) was exclusively used for quantification.
Case history and autopsy findings
A homosexual man in his forties was found dead lying with his back on a sofa in a hotel room. He was holding a spray cleaner containing compressed DFE (Fig. 1) and had a history of DFE inhalant abuse. An eye mask was found on his forehead; his neck, chest, and limbs were fitted with a belt-like object, and blood was found in his anal area. The door of the room could not be locked automatically, allowing free entry or exit from the room. Accidental death, suicide, and homicide were considered. Although the present decedent had a medical history of immunodeficiency, the details of his physical condition were not available.
The postmortem interval was estimated to be 2–3 days. His height and weight were 170 cm and 72.5 kg, respectively. External examinations demonstrated conjunctival petechiae and anal fissures with small amounts of blood. Internal examinations showed congestion in organs, including the lungs (left weight, 574 g; right weight, 691 g), and a foreign object in the rectum. Otherwise, by macroscopic observation, there were no notable findings externally and internally.
Laboratory test results were positive for hepatitis B, HIV, and syphilis infections, although no sperm was detected in the anal canal. Femoral vein blood, cardiac blood, and urine samples were taken for toxicological screening and analysis.
Materials and methods
Reagents and materials
Standard DFE (0.2 mg/mL in methanol) and internal standard (IS) chloroethane solutions (2 mg/mL in methanol) were purchased from AccuStandard® (New Haven, CT, USA); methanol (LC/MS grade) from Wako Pure Chemical (Osaka, Japan); Alumi-Seal vials (5 mL), polytetrafluoroethylene (PTFE) silicon septums (20 mm), and TS-S type alumi-seals from GL Sciences (Tokyo, Japan); gas-tight syringes (100 μL) from Trajan Scientific and Medical (Ringwood, Australia). A Reacti-Therm™ Heating Module (Pierce, Rockford, IL, USA) was used to incubate samples. Blank blood and urine samples were collected from volunteers with no history of drug abuse after obtaining informed consent.
Basic toxicological analyses
The alcohol concentrations in femoral vein blood and urine samples were analyzed using headspace GC–FID (GC–14B; Shimadzu, Kyoto, Japan). A Triage® drugs of abuse (DOA) panel was purchased from Sysmex (Kobe, Japan) and used to screen for amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, opiates, phencyclidine, and tricyclic antidepressants in urine samples. Drug screening of cardiac blood and urine samples was performed using liquid chromatography–QTrap tandem mass spectrometry (LC–MS/MS) (type 3200) and liquid chromatography–quadrupole time-of-flight mass spectrometry (LC–QTOF-MS) (all AB Sciex, Framingham, MA, USA).
Analytical procedure for DFE
Samples were prepared using the headspace method. A 50 μL sample of blood or urine, 20 μL of IS, and 200 μL of methanol were placed and immediately sealed in the 5 mL glass vials with each PTFE silicon septum and aluminum sealing. Vials were incubated at 50 °C for 10 min, and 80 µL of headspace was manually injected into the GC–MS.
GC–MS conditions
GC–MS was performed using a 7890A gas chromatograph connected to a 5975C mass-selective detector (Agilent Technologies, Santa Clara, CA, USA). Chromatographic separation was performed using an Agilent DB-5MS UI capillary column of 30 m in length, 0.25 mm in internal diameter, and 0.25 µm in film thickness (Agilent Technologies) using high-purity helium carrier gas at a flow rate of 1.0 mL/min. The injection temperature was 200 °C, and the column temperature was maintained at 40 °C for 2 min, increased at 50 °C/min to 300 °C, and then held at 300 °C for 2 min. The total run time was 9.2 min. The split ratio was set at 25:1. Electron ionization was achieved at 70 eV. The temperatures of transfer line, ion source, and quadrupole were 280, 230, and 150 °C, respectively. MS analyses were performed in the selected ion monitoring (SIM) and scan modes. Ions at m/z 47.1, 49.0, 51.0, 64.0, 65.0, and 66.0 were monitored in the SIM mode, and target ions of DFE and IS were m/z 51.0 and 64.0, respectively. The scan range was set at m/z 45.0–200.0.
Method validation
DFE calibration curves were prepared at concentrations of 40, 80, 160, 320, 540, and 800 µg/mL for both blood and urine. Volumes of DFE methanol solution and pure methanol were adjusted to 200 µL. Intraday accuracy and precision were determined in five replicate analyses of quality control (QC) samples at low (60 µg/mL), medium (300 µg/mL), and high (720 µg/mL) concentrations. Interday accuracy and precision (n = 15) were assessed in three repetitions of intraday assays. Accuracy (%bias) and precision (%CV: coefficient of variation) were calculated according to previous studies [8, 9]. Analytical criteria were adopted from the Food and Drug Administration (FDA) [10].
Results and discussion
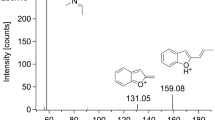
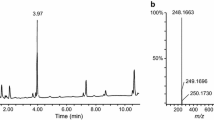
In the present case, the alcohol concentration in femoral vein blood and urine was < 0.1 mg/mL. Triage® DOA screening of urine samples was negative. No drugs that could be associated with death were found in the screening of cardiac blood or urine samples using LC–MS/MS and LC–QTOF-MS. Quantitative analyses revealed DFE concentrations of 481, 591 and 201 µg/mL in femoral vein blood, cardiac blood, and urine samples, respectively (Table 1). In these chromatographic analyses, retention times of DFE and IS were 1.51 and 1.64 min, respectively (Fig. 2). The major mass spectra peaks of DFE were observed at m/z 47.1, 51.0, and 65.0 (Fig. 3). Retention times and mass spectra of femoral vein blood, cardiac blood, and urine samples were all consistent with those of the DFE reference standard solution.
DFE concentrations in the present case of DFE inhalation together with the reported cases are summarized in Table 1. The concentrations in the present femoral vein and cardiac blood samples were higher than those in the previous cases. Moreover, the findings of conjunctival petechiae and lung congestion were consistent with other cases [3, 6], indicating that DFE intoxication was the cause of death in the present case.
In the previous cases, DFE quantifications were determined using headspace GC–FID [3, 6, 7]. It is well known that GC–MS is one of the most reliable analytical techniques and provides excellent chromatographic resolution, precise retention times and quantification with high MS sensitivity [9, 11, 12]. The regression equation for blood was y = 1.63x − 0.0147, and had a coefficient of determination of 0.9995. The limit of detection (LOD: signal/noise ≥ 3) and the limit of quantification (LOQ: signal/noise ≥ 10) for blood were 8 and 40 µg/mL, respectively. The equation for urine was y = 1.54x − 0.0159, and had a coefficient of determination of 0.9916. The LOD and LOQ were also 8 and 40 µg/mL, respectively. Tables 2 and 3 show the accuracy and precision data of the present method for blood and urine, respectively. According to the FDA guidance criteria, both accuracy and precision should be less than 15% [10]. Thus, this method using GC–MS may be suitable for accurate DFE quantification.
Avella et al. [13] previously showed that uncontrolled losses of volatile gas should be considered during assessments of DFE concentrations in biological samples. In agreement, Vance et al. [14] quantified DFE concentrations in iliac vein blood samples and reported that samples from previously opened tubes always had lower concentrations of DFE than those from sealed tubes. Thus, we took care to maintain satisfactory sample conditions.
Previous studies suggest that lack of oxygen contributes to death in the cases of inhalant abuse [5, 15]. However, for anoxia to occur, subjects need to fit a bag tightly over the head [6, 15]. In the present case, the decedent did not have a plastic bag or a gas mask on his face. Hence, the cause of death may not have been associated with lack of oxygen.
Avella et al. [3] reported that volatile gases such as DFE may be rapidly taken up by the blood, and distribution to other tissues may be dependent on concentration, compound solubility, and blood perfusion to each tissue. Previous studies revealed that adipose tissues retain volatile compounds longer than other tissues, and therefore, may be useful to distinguish between acute and chronic intoxication [3, 16]. However, in the present case, a sample of adipose tissues was not collected.
As shown in Table 1, the DFE concentration in urine sample was higher in the present case than in previously reported cases. However, the excretion time of inhaled DFE was unknown in this case. Studies suggest that without knowledge of the final time of inhalation, accurate pharmacokinetic and postmortem distribution models are difficult to construct [1, 17].
Vance et al. [14] reported cases in which the evidence of DFE abuse from aerosol cans was not always available. Thus, routine screening tests may be necessary for volatile compounds such as halogenated hydrocarbons. In addition, in cases of unexplained death, it may be necessary to collect sealed samples from which DFE cannot escape due to its low boiling point (− 25 °C).
Although police investigations revealed the presence of sexual items and used condoms in the hotel room of the present decedent, no suicide note was found, and thus, the manner of death was determined to be accidental. Although several cases of suicidal and accidental deaths due to DFE inhalation have been reported [2, 3, 6, 7, 18], spray cleaners that contain DFE are widely available in shops and on the Internet for use with electronics and computers. Therefore, further studies are required to determine the incidence of deaths from DFE inhalation and to increase awareness among people regarding the dangers of DFE. In addition, it may be necessary to regulate information leading to suicides using DFE.
Conclusions
In this case report, we encountered a case of fatal DFE poisoning. Thus, we have established a detailed procedure for quantification of DFE in human blood and urine by GC–MS, thereby presenting its validation data. The concentrations of DFE in this case were 481, 591 and 201 µg/mL in femoral vein blood, cardiac blood and urine samples, respectively, which were much higher than those previously measured by GC–FID. We, therefore, diagnosed that this victim had died of acute DFE poisoning. To our knowledge, this is the first demonstration of quantification of DFE in human blood and urine by GC–MS.
References
Hahn T, Avella J, Lehrer M (2006) A motor vehicle accident fatality involving the inhalation of 1,1-difluoroethane. J Anal Toxicol 30:638–642
Xiong Z, Avella J, Wetli CV (2004) Sudden death caused by 1,1-difluoroethane inhalation. J Forensic Sci 49:627–629
Avella J, Wilson JC, Lehrer M (2006) Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE). Am J Forensic Med Pathol 27:58–60
Jufer RA, Callery RT (2003) A case of fatal difluoroethane intoxication (abstract no. K31). Proceedings of the American Academy of Forensic Sciences, Chicago IL Annual Meeting IX: 318–319. https://www.aafs.org/up-content/uploads/ProceedingsChicago2003.pdf. Accessed Mar 2018
Shepherd RT (1989) Mechanism of sudden death associated with volatile substance abuse. Hum Toxicol 8:287–297
Sakai K, Maruyama-Maebashi K, Takatsu A, Fukui K, Nagai T, Aoyagi M, Ochiai E, Iwadate K (2011) Sudden death involving inhalation of 1,1-difluoroethane (HFC-152a) with spray cleaner: three case reports. Forensic Sci Int 206:e58–e61
Sasaki C, Shinozuka T, Irie W, Nakamae T, Murakami C, Nakamaru N, Furukawa M, Nakamura S, Kurihara K (2009) A fatality due to inhalation of 1,1-difluoroethane (HFC-152a) with a peculiar device. Forensic Toxicol 27:45–48
Causon R (1997) Validation of chromatographic methods in biomedical analysis. Viewpoint and discussion. J Chromatogr B 689:175–180
Saka K, Uemura K, Shintani-Ishida K, Yoshida K (2008) Determination of amobarbital and phenobarbital in serum by gas chromatography-mass spectrometry with addition of formic acid to the solvent. J Chromatogr B 869:9–15
US Department of Health and Human Services, Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Center for Vaterinary Medicine (CVM) (2018) Bioanalytical method validation: guidance for industry. https://www.fda.gov/downloads/drugs/guidances/ucm070107.Pdf. Accessed May 2018
Villas-Bôas SG, Mas S, Akesson M, Smedsgaard J, Nielsen J (2005) Mass spectrometry in metabolome analysis. Mass Spectrom Rev 24:613–646
Demeestere K, Dewulf J, Roo KD, Wispelaere PD, Langenhove HV (2008) Quality control in quantification of volatile organic compounds analysed by thermal desorption-gas chromatographymass spectrometry. J Chromatogr A 1186:348–357
Avella J, Lehrer M, Zito SW (2008) A validated method for the quantitation of 1,1-difluoroethane using a gas in equilibrium method of calibration. J Anal Toxicol 32:680–687
Vance C, Swalwell C, McIntyre IM (2012) Deaths involving 1,1-difluoroethane at the San Diego County Medical Examiner’s Office. J Anal Toxicol 36:626–633
Harris D (2006) Volatile substance abuse. Arch Dis Child Educ Pract Ed 91:ep93–ep100
Woollen BH, Marsh JR, Mahler JD, Auton TR, Makepeace D, Cocker I, Blain PG (1992) Human inhalation pharmacokinetics of chlorodifluoromethane (HCFC22). Int Arch Occup Environ Health 64:383–387
Broussard LA, Brustowicz T, Pittman T, Atkins KD, Presley L (1997) Two traffic related fatalities related to the use of difluoroethane. J Forensic Sci 42:1186–1187
Iseki K, Tomonaga A, Hayashida A, Seino K, Shinozaki K, Haneda T, Yamazaki K (2012) Fatal suffocation following inhalation of 1,1-difluoroethane (DFE) (in Japanese with English abstract). J Jpn Assoc Acute Med 23:775–780
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no financial or other relations that could lead to conflicts of interest.
Ethical approval
The analyses of toxic substances were requested by judicial authorities with documentation. The blank blood and urine (about 10 mL, respectively) were collected from a healthy volunteer with informed consent.
Rights and permissions
About this article
Cite this article
Torimitsu, S., Fujii, Y., Saka, K. et al. Fatal intoxication with 1,1-difluoroethane (DFE) due to inhalation of a spray cleaner: analysis by GC–MS. Forensic Toxicol 37, 245–249 (2019). https://doi.org/10.1007/s11419-018-0435-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-018-0435-8