Abstract
Nowadays, approximately 3% of the world’s population suffers from psoriasis, an inflammatory dermatosis with high recurrence. Tryptanthrin (TRYP) is a natural alkaloid that possesses anti-inflammatory activities on multiple diseases. The present study aimed to unravel whether TRYP could relieve psoriasis and how it works. Imiquimod (IMQ)-induced psoriatic mouse models were administered saline (model), TRYP (25 and 100 mg/kg), or methotrexate (MTX, 1 mg/kg) and considered as the positive control. TNF-α-induced keratinocytes (HaCaT cells) with TRYP (0, 10, 20 and 50 nM) were used for in vitro verification. Psoriasis area severity index (PASI) and spleen index were evaluated. Th17 cell infiltration in both spleens and lymph nodes was detected by flow cytometry. The expression levels of inflammatory cytokines, glutathione (GSH), malondialdehyde (MDA) and catalase (CAT), as well as superoxide dismutase (SOD), were examined by ELISA, while the NF-κB/MAPK/Nrf2 pathways-related proteins were determined by western blot. TRYP significantly attenuated psoriatic skin lesions, increased GSH, SOD, and CAT levels, reduced spleen index, accumulation of MDA, the abundance of Th17 cells in both the spleen and lymph nodes, and secretion of inflammatory cytokines in IMQ-induced psoriatic mouse models. Mechanically, TRYP suppressed IMQ-activated NF-κB (IκB and p65), MAPK (JNK, ERK1/2, and p38), and activated Nrf2 signaling pathways. Similar alterations for inflammation and oxidative stress parameters and NF-κB/MAPK/Nrf2 pathways were also observed in TNF-α-treated HaCaT cells upon TRYP treatment. Our findings suggested TRYP is effective in protecting against inflammation and oxidative stress in psoriasis-like pathogenesis by modulating the NF-κB/MAPK/Nrf2 pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriasis is a skin disorder due to systemic inflammation, the prevalence rate of which is approximately 3% in the world [1]. Psoriasis is described by scaly erythematous plaques caused by dysregulated keratinocyte proliferation and differentiation, as well as excessive inflammatory response, which has a profound influence on patient quality of life [2, 3]. Until now, psoriasis is incurable but can be controlled with medications. For the treatment of psoriasis, topical therapies with corticosteroid agents are widely used in mild psoriasis, while systemic agents are often used in severe conditions [4]. Some patients with psoriasis will develop resistance to topical therapy, and systemic therapies usually lead to unwanted side effects, such as nausea and gastrointestinal intolerance [5].
Even though the pathogenesis of psoriasis remains unclear, the essential role of inflammatory response and oxidative stress in the occurrence, development, and progression of psoriasis has been gradually uncovered in the past decades [6, 7]. It has been reported that CD4+T cell-mediated autoimmune response plays a central role in the abnormal activation of keratinocytes during psoriasis occurrence [8]. As one of the subtypes of CD4+T cell, the T helper 17 (Th 17) cell has been demonstrated as a key regulator in the pathogenesis of psoriasis in recent years [9, 10]. Cytokines secreted by Th17, especially IL-17A, accelerate the recruitment of inflammatory cells via antimicrobial protein, thereby enhancing the proliferation and suppressing the differentiation of keratinocytes [11]. Notably, anti-IL-17A has been approved by FDA for psoriasis therapy in 2015 [12], proving targeting IL-17A is a credible direction for exploring effective novel agents for psoriasis. Besides, increasing evidence supports that the dysfunction of the antioxidant system accompanied by increased reactive oxidative species (ROS) production in the skin is closely related to the progression of psoriasis. Interestingly, Fumaderm®, a drug displaying potent antioxidant activity, has been proven to be an effective agent for the treatment of psoriasis [13], revealing the potential of oxidative stress as the target for psoriasis therapy.
In recent decades, traditional Chinese herb has aroused immense clinical interest as an alternative medicine for the treatment of psoriasis [14]. Tryptanthrin (TRYP) (Fig. 1A) is a natural alkaloid with anti-inflammatory and anti-breast cancer effects [15, 16], which could be derived from Polygonum tinctorium, Isatis tinctoria, and indigo plants [17]. These three herbs have been demonstrated to effectively alleviate psoriasis [18,19,20]. Some research suggested a potent effect of TRYP on IL-17A inhibition [21]. The potential of TRYP against oxidative stress has also been revealed in recent years [22]. In light of the regulatory role of TRYP in IL-17A and the oxidative stress effect, we hypothesized that TRYP may be a drug candidate for psoriasis therapy. However, there is still no literature supporting this point yet.
The effect of TRYP on scratching behavior and body weight changes in psoriatic mouse models. A The chemical structure of TRYP. B Schematic experimental design of IMQ-induced psoriatic mouse models that received different treatments. C The scratch bouts and D body weight changes of mice from each group on days 0, 3, 5, and 7 (***p < 0.005 significant difference from the control group, ###p < 0.005 significant difference from the model group)
To verify our hypothesis, the present study evaluated the pharmacologic effects of TRYP on imiquimod (IMQ)-induced psoriatic mouse models and TNF-α-induced human keratinocytes. The underlying molecular mechanisms were also preliminarily explored in vivo and in vitro.
Materials and methods
Psoriatic model establishment and treatments
The psoriatic mouse model was established by a topical application with 5% IMQ cream (62.5 mg/day) on a shaved back region for a week, as previously reported [23]. Given that methotrexate (MTX) is a widely used agent for the treatment of psoriasis [24], it was thus selected as the positive drug in animal experiments. TRYP (SML0310; purity 98%) used in this study was purchased from Sigma-Aldrich (MO, USA).
BALB/c male mice (18–25 g, 6–8 weeks) purchased from the Experimental Animal Center of Guangdong Province (Guangzhou, China) were arbitrarily divided into five groups (n = 5/group) after a 7-day acclimation as follows: control, model, model + TRYP-L (models received intragastric administration of a low dose (25 mg/kg) of TRYP), model + TRYP-H (models received intragastric administration of a high dose (100 mg/kg) of TRYP), and model + MTX (models received intragastric administration of 1 mg/kg MTX) groups. The animal experiment design is depicted in Fig. 1B. In brief, the back skin of the mice was shaved 1 day before IMQ treatment (day 0). All treatments were carried out at 12 h intervals after applying IMQ for seven consecutive days. The scratching behavior, body weight, and Psoriasis Area and Severity Index (PASI) of each mouse were recorded on days 0, 3, 5, and 7. The PASI was evaluated according to the extent of erythema, scales, and infiltration on the shaved back skin as follows: 0 means none; 1 means slight; 2 means moderate; 3 means marked; and 4 means severe. On day 7, mice were killed by cervical dislocation after 1 h of treatment to collect spleens, lymph nodes, and skin for further analysis.
To observe histological changes in the skin, the dorsal skin of each mouse was cut and fixed in formalin solution, embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin (H&E).
The experimental protocol regarding animals was approved by Chongqing Traditional Chinese Medicine Hospital Animal Care and Use Committee.
Th17 cells quantification
Spleen samples and inguinal lymph node samples harvested from each group were ground to obtain single-cell suspensions. Cells were subjected to staining with FITC-conjugated anti-mouse CD4 antibody and Alexa Fluor 647-conjugated IL-17A antibody before the flow cytometric analysis. Finally, the percentage of CD4+/IL-17A+ was determined on flow cytometry (BD Biosciences, CA, USA) to evaluate the infiltration of Th17 cells in the spleen and lymph nodes.
Cell culture and viability assessment
Human keratinocyte HaCaT cells purchased from Beyotime (C6282) were cultured in DMEM containing 10% FBS, 100-U/mL penicillin, and 100-μg/mL streptomycin. The cells were maintained in a cell incubator with 5% CO2 at 37 °C.
To obtain a suitable concentration range of TRYP for treatment with HaCaT cells, HaCaT cells were incubated with diverse concentrations of TRYP (0, 10, 20, 50, 100, and 200 nM), followed by subjecting to cell viability analysis using CCK-8 assay. Briefly, after incubation with TRYP for 24 h, the CCK-8 reagent was added to the plates for 2 h additional incubation. Finally, the optical density (OD) at 450 nm was monitored with a microplate reader (Bio-Rad, California, USA).
To mimic psoriatic inflammation in vitro, 20 ng/mL of TNF-α was used to incubate with HaCaT cells for 4 h [25]. The treatment of TRYP on HaCaT cells was subsequently carried out for another 12 h.
Inflammatory cytokines detection
By using commercial ELISA kits, the concentrations of inflammatory cytokines in the protein isolated from skin tissues were determined. The concentrations of IL-1β, IL-6, and IL-17A were also detected in the cell supernatants of diverse groups. The ELISA kits for mouse TNF-α (ab208348), mouse IL-22 (ab223857), IL-1β (mouse: ab197742; human: ab46052), IL-6 (mouse: ab222503; human: ab178013), and IL-17A (mouse: ab199081; human: ab83688) were purchased from Abcam (Cambridge, UK).
RT-PCR
Total RNA was extracted from epidermis layers of skin tissues using TRIzol reagent (Thermo Fisher, Massachusetts, USA). cDNA synthesis was performed using PrimeScript RT reagent kit (Takara, Tokyo, Japan). Using a SYBR Premix EX Taq™ II kit (Takara), qRT-PCR was performed on a Bio-Rad system to detect the expression of studied genes, the primer sequences of which are listed in Supplementary Table 1. Finally, the relative gene expression was calculated with GAPDHs as internal control using the comparative Ct (2−ΔΔCT) method [26].
Oxidative stress parameter examination
The content of superoxide dismutase (SOD), malondialdehyde (MDA), glutathione (GSH), and catalase (CAT) was measured in vitro and in vivo using the corresponding commercial kits (Beyotime, Shanghai, China) following protocols provided by the manufacturers.
Nucleoproteins extraction
Cell nuclei proteins from epidermis layers of skin tissues and HaCaT cells were extracted using a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Beijing, China). Samples were placed in 300 μL of extract buffer A containing 1 μL protease and phosphatase inhibitors. After incubation at 4 °C with agitation for 30 min and centrifugation at 2000×g for 5 min, the supernatant was discarded and the precipitate was washed twice with PBS. Then, 100 μL of cold extract buffer B, containing 0.5 μL protease inhibitor and phosphatase inhibitor, was added. The samples were vigorously mixed at a high speed for 15 s, and the mixture was incubated at 4 °C with agitation for 30 min and subsequently centrifuged at 12,000×g for 10 min. The supernatant contained the nucleoproteins.
Western blot
Total protein isolated from skin tissues or cells by using RIPA buffer (10X) (Cell signaling, Massachusetts, USA) was subjected to quantification with a BCA protein assay kit (Thermo scientific). The total protein or nucleoprotein was separated through SDS‑PAGE gel before being transferred onto PVDF membranes. Afterward, membranes were blocked for 2 h and subsequently incubated overnight with primary antibodies. The information on primary antibodies used in this study is listed in Supplementary Table 2. Thereafter, membranes were rinsed and incubated with HRP-conjugated secondary antibody (ab7090; Abcam) for 2 h. Finally, protein bands were visualized with an ECL Western Blotting Substrate (Thermo Scientific, Massachusetts, USA).
NF-κB DNA-binding activity assay
After the nucleoprotein extraction from HaCaT cells, isolated nuclear protein fractions were then applied to assess NF-κB p65 binding activity by using NF-κB p65 Transcription Factor Assay Kit (ab133112; Abcam) according to the manufacturer's instructions.
Statistical analysis
GraphPad Prism (8.0.1 version; GraphPad Software, CA, USA) was applied in this study. Data were expressed as the mean ± SD from at least three different experiments and analyzed using a one-way analysis of variance, followed by Tukey’s post hoc test. P less than 0.05 represented that the difference was statistically significant.
Results
The effect of TRYP on scratching behavior, body weight changes, and psoriasis-like skin lesions in psoriatic mouse models
To investigate the therapeutic potential of TRYP on psoriasis, IMQ-induced psoriatic mouse models treated with TRYP or MTX (a reference drug that is effective in psoriasis) were utilized. After smearing IMQ cream to the shaved back skin of mice, the mice developed marked scratching behavior on the third day, which could be attenuated by both TRYP and MTX treatments (Fig. 1C). A significant weight loss was observed in psoriatic mouse models without treatment, suggesting systemic inflammation occurred in psoriatic mouse models since the body weight is considered an essential indicator of systemic inflammation (Fig. 1D). Interestingly, TRYP effectively rescued the weight loss of mice induced by IMQ (Fig. 1D).
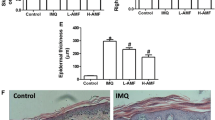
The dorsal skin photographs of mice from each group showed that the model mice displayed typical psoriasis-like skin lesions on the shaved back, including the large areas of deep red plaques and the thickened and widely distributed scaling on the shaved back, compared to control mice (Fig. 2A). Intragastric administration of TRYP, especially of the high dose, considerably improved skin lesions across the treatment period (Fig. 2A). H&E staining was performed to observe the microenvironmental effects in skin lesions after diverse treatments. IMQ caused increased thickening of the epidermis layer and increased immune cell infiltration in the dermis layer. TRYP improved the pathological changes in skin lesions with reduced epidermal thickening, showing an effect similar to that in the MTX group (Fig. 2B). The result of daily PASI scores revealed that the degree of erythema, scale, and thickening in the model group became more and more serious, which was in line with the aforementioned finding (Fig. 2C–F). As expected, a significant decrease in the severity index of skin erythema, scaling, thickness, as well as cumulative scoring was observed in all treatment groups (model + TRYP-L, model + TRYP-H, and model + MTX) as compared to the model group (Fig. 2C–F). These data revealed a comparable therapeutic effect of TRYP to MTX. Collectively, TRYP effectively ameliorated IMQ-induced scratching behavior, body weight loss, as well as psoriatic skin lesion in mice.
TRYP ameliorated psoriasis-like skin lesions in psoriatic mouse models. A Representative images of dorsal skin of mice from different groups on days 0, 3, 5 and 7. B Representative H&E staining of dorsal skin tissues of mice from different groups on day 7. C Erythema, D thickness, and E scales of the back skin was scored on days 0, 3, 5 and 7 on a scale from 0 to 4; F the corresponding cumulative score (0 to 12) was subsequently calculated (*p < 0.05 and ***p < 0.005, significant difference from the control group; #p < 0.05, ##p < 0.01 and ###p < 0.005 significant difference from the model group)
The effect of TRYP on IMQ-induced skin inflammation and oxidative stress in psoriatic mouse models
It has been reported that IMQ is capable of inducing psoriasiform inflammation, accompanied by an increase of splenic Th17 cells in mice [27]. Hence, Th17 (IL-17A+CD4+) cell infiltration in the spleen and lymph nodes of psoriatic mouse models was investigated after the treatment of TRYP. A low percentage of IL-17A+CD4+ T cells in both the spleen and lymph nodes of the control mice was observed (Fig. 3A). Compared with the control group, the IL-17A+CD4+ cell frequency in both the spleen and lymph nodes was markedly increased in the model group (Fig. 3A). TRYP, at either low or high doses, clearly decreased the abundance of IL-17A+CD4+ T cells in lymph nodes, while TRYP at high doses, but not low doses, also significantly reduced the percentage of IL-17A+CD4+ T cells in the spleens (Fig. 3A). Besides, we demonstrated that treatment of TRYP considerably diminished the elevation of spleen index (ratio of spleen weight to body weight) induced by IMQ in mice (Fig. 3B), suggesting the anti-inflammatory activity of TRYP. Psoriasis-related inflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-17A, and IL-22, were subsequently determined in the skin lesions. The outcomes from ELISA revealed that the release of the aforementioned inflammatory cytokines in the model group was more than those in the control group (Fig. 3C). On the other hand, treatments of TRYP, at either low or high doses, or MTX led to a significant reduction in inflammatory cytokines released from the skin of psoriatic mouse models (Fig. 3C). RT-PCR analysis showed that the mRNA expression levels of these inflammatory cytokines in the skin lesions from each group displayed a similar tendency to the result of ELISA (Fig. 3D), further revealing that TRYP effectively reduced the inflammation response in psoriatic skin tissue.
The effect of TRYP on IMQ-induced skin inflammation and oxidative stress in psoriatic mouse models. A The percentage of IL-17A+CD4+ T cells in the total live cells in the spleen (top) and lymph nodes (bottom) isolated from mice of each group. B Representative photographs of the spleen (top) and spleen index (bottom) of mice from each group. C The secretion and D mRNA expression of TNF-α, IL-1β, IL-6, IL-17A, and IL-22 cytokines of skin from each group of mice. The contents of E MDA, F GSH, G SOD, and H CAT in the skin from each group of mice (*p < 0.05 and ***p < 0.005, significant difference from the control group; #p < 0.05, ##p < 0.01, and ###p < 0.005 significant difference from the model group)
Except for inflammation, oxidative stress is considered another critical factor in the development of psoriasis; thus, the changes in MDA, GSH, SOD, and CAT were further examined in the skin lesions. The MDA level in the model group was higher than that in the control group, which could be reduced by TRYP at both high and low doses (Fig. 3E). Topical exposing IMQ induced a significant decrease in the levels of antioxidative related biomarkers (GSH, SOD and CAT) in the skin, and the treatment of TRYP restored the decreased levels (Fig. 3F–H). Moreover, the administration of MTX also displayed a suppression effect on IMQ-induced oxidative stress, as demonstrated by the decrease of MDA content and the increase of GSH, SOD, and CAT levels in the skin of psoriatic mouse models (Fig. 3E–H). Notably, we found that the effect of TRYP at high doses on oxidative stress in psoriasis was slightly greater than that of MTX (Fig. 3E–H). These findings suggested that TRYP was potent in attenuating IMQ-induced oxidative stress in vivo.
TRYP regulated the NF-κB/MAPK/Nrf2 pathways in psoriatic mouse models
Both the NF-κB and MAPK pathways are considered important signals involved in the pathogenesis of psoriasis because of their critical role in inflammation [28]. To understand the mechanism behind the anti-inflammatory effect of TRYP psoriatic mouse models, the expression and phosphorylation of IκB, NF-κB p65, and three MAPKs (JNK, ERK1/2, and p38 MAPK) were analyzed by western blot. The topical administration of IMQ caused a significant increase in the phosphorylation of IκB, NF-κB p65, JNK, ERK1/2, and p38 MAPK, as well as the NF-κB nuclear translocation in the skin (Fig. 4A, B), suggesting that the NF-κB and MAPK pathways were activated in psoriatic skin lesions. After treatment with TRYP, the phosphorylation level of IκB, NF-κB p65, JNK, and ERK1/2, and the NF-κB nuclear translocation in psoriatic skin lesions was reduced, while that of p38 MAPK showed no remarkable change (Fig. 4A, B). Moreover, the regulatory role of TRYP in Nrf2 signaling, a prominent target for oxidative stress, was observed in psoriatic mouse models. In comparison with the control group, nuclear localization of Nrf2 in the model group was significantly increased, which was accompanied by the elevation of HO-1 expression (Fig. 4C). Upon treatment of TRYP, Nrf2 signaling was further activated (Fig. 4C), suggesting TRYP probably ameliorated oxidative stress in psoriatic skin via increasing Nrf2 activation.
TRYP regulated the NF-κB/MAPK/Nrf2 pathways in psoriatic mouse models. A The expression and phosphorylation levels of IκB and NF-κB p65 proteins in the skin of each group of mice. B The expression and phosphorylation levels of JNK, ERK1/2, and p38 MAPK in the skin of each group of mice. C The expression levels of nuclear translocation of Nrf2 and HO-1 in the skin of each group of mice (*p < 0.05 and ***p < 0.005, significant difference from the control group; #p < 0.05 and ###p < 0.005 significant difference from the model group)
TRYP suppressed the inflammation and oxidative stress induced by TNF-α via the NF-κB/MAPK/Nrf2 pathways in HaCaT cells
To further confirm the therapeutic potential of TRYP on psoriasis-like traits, the anti-inflammatory and antioxidative effects were also explored in vitro. To select suitable concentrations of TRYP for in vitro study, HaCaT cells were pretreated with diverse concentrations of TRYP (0, 10, 20, 50, 100, and 200 nM), followed by a CCK-8 assay. As shown in Fig. 5A, at concentrations between 0 and 50 nM, TRYP did not cause cytotoxicity in HaCaT cells; however, up to 100 nM, TRYP began to impair the cell viability of HaCaT cells. Thus, the safe concentrations of TRYP (10, 20, and 50 nM) were chosen for subsequent experiments.
TRYP suppressed the TNF-α-induced activation of inflammation and oxidative stress in HaCaT cells. A The cell viability of HaCaT cells upon different concentrations (0, 10, 20, 50, 100 and 200 nM) of TRYP. HaCaT cells were treated with TNF-α to mimic the psoriasis in vitro and then added 0, 10, 20, and 50 nM TRYP. HaCaT cells without any treatment is considered the control. B The cell viability of HaCaT cells from diverse groups. ELISA determined the levels of C IL-1β, D IL-6, and E IL-17A cytokines in the cell supernatant of HaCaT cells from diverse groups. The contents of F MDA, G GSH, H SOD, and I CAT in the cell supernatant of HaCaT cells from diverse groups (*p < 0.05 and ***p < 0.005, significant difference from the 0 nM or control group; #p < 0.05 and ###p < 0.005 significant difference from the TNF-α group)
Interestingly, TNF-α-treated HaCaT cells exhibited a significant elevation in the levels of secretion of IL-1β, IL-6, and IL-17A, while this increase was blocked by TRYP treatment in a concentration-dependent way (Fig. 5B–D). Besides, the stimulation of TNF-α caused also increased levels of MDA (Fig. 5E) and decreased GSH, SOD, and CAT levels (Fig. 5F–H), suggesting the occurrence of oxidative stress in HaCaT cells. Treatment with TRYP at various concentrations (10, 20 and 50 nM) significantly attenuated the oxidative stress of HaCaT cells induced by TNF-α, as revealed by the reduction of MDA content and the elevation of GSH, SOD, and CAT levels (Fig. 5E–H).
Finally, the regulation of TRYP on the NF-κB/MAPK/Nrf2 pathways was investigated in TNF-α-stimulated HaCaT cells to verify our previous in vivo studies. In HaCaT cells, we found that TNF-α stimulation increased the phosphorylated levels of IκB and NF-κB p65 proteins and the NF-κB nuclear translocation, whereas TRYP treatment concentration-dependently rescued these protein alterations (Fig. 6A). These results suggested that TRYP treatment could suppress the activation of the NF-κB pathway. Consistent with this finding, NF-κB DNA-binding activity assay revealed that the increased DNA-binding activity of p65 induced by TNF-α was reversed by TRYP treatment in a dose-dependent way (Supplementary Figure S1). In addition, the increased level of JNK and ERK1/2 phosphorylation was blocked by TRYP treatment in TNF-α stimulated HaCaT cells (Fig. 6B). There was no obvious change in the phosphorylation of p38 MAPK after exposing HaCaT cells to TNF-α (Fig. 6B). Furthermore, it was obviously observed that the Nrf2 pathways (the expression of Nrf2 and HO-1) were activated by TRYP treatment concentration-dependently in TNF-α-stimulated HaCaT cells (Fig. 6C). Taken together, our results showed that TRYP suppressed oxidative stress and inflammation by regulating the NF-κB/MAPK/Nrf2 pathways.
TRYP regulated the NF-κB/MAPK/Nrf2 pathways in TNF-α-induced HaCaT cells. A The expression and phosphorylation levels of IκB and NF-κB p65 proteins in HaCaT cells of each group. B The expression and phosphorylation levels of JNK, ERK1/2, and p38 MAPK in HaCaT cells of each group. C The expression levels of nuclear translocation of Nrf2 and HO-1 in HaCaT cells of each group (***p < 0.005, significant difference from the control group; #p < 0.05, ##p < 0.01, and ###p < 0.005 significant difference from the TNF-α group)
Discussion
Psoriasis is considered an autoimmune disorder; thus, it is mainly treated with immunosuppressive agents, such as cyclosporin and MTX [24, 28]. Since conventional immunosuppressive drugs have side effects, such as infections and nephrotoxicity, biological treatments with anti-inflammatory antibodies are attracting immense interest. Though several biological treatments show better efficacy, they are also much more expensive than conventional immunosuppressive agents. Therefore, it remains of critical importance to seek a safe and cost-effective drug for the treatment of psoriasis. TRYP is a natural product found in many traditional herbal plants with a potent anti-inflammatory activity [16, 21]. A recent study has shown that TRYP is an active compound for the treatment of psoriasis by its anti-Th17 effect [29]. Moreover, TRYP reportedly suppresses IL-17A production during Th17 polarization [30]. The present study explored for the first time whether TRYP ameliorates psoriatic features in an IMQ-induced psoriatic mouse model and TNF-α-treated keratinocytes in vitro.
Topical administration of IMQ is currently one of the most widely accepted approaches for the establishment of psoriasis animal models in mice, since it is easy to operate and can reproduce histological characteristics and pathological inflammatory phenotype of psoriasis [31]. In this study, TRYP exhibited a similar efficacy to MTX for alleviating psoriatic skin lesion and increased the PASI scores. As noted in previous research, approximately 10% of body weight loss was observed in mice after exposing IMQ for 7 days, which could be restored by TRYP treatment. Th17 cells are considered to contribute to the pathogenesis of psoriasis [10]. TRYP also reduced IMQ-induced Th17 cell infiltration in both spleen and lymph nodes and spleen enlargement, indicating the suppression effect of TRYP on systemic inflammation in psoriatic mouse models.
As reported by previous laboratory and clinical research, various inflammatory cytokines, such as TNF-α, IL-22, and IL-17A, are secreted by immune and inflammatory cells during the progression of psoriasis, which probably and directly act on human keratinocytes, thereby resulting in a variety of pathological symptoms [32]. The level of cytokines, such as IL-17A, IL-22, and TNF-α, secreted by Th17 cells was elevated in the serum of psoriasis patients [33]. Our data showed that TRYP effectively reduced the secretion of TNF-α, IL-1β, IL-6, IL-17A, and IL-22, and also their transcription level in psoriatic skin lesions. As oxidative stress plays a key role in psoriasis pathogenesis [7], our study verified that the effect of TRYP on the psoriatic mouse models was also closely related to its restoration role in the imbalance of reactive oxygen species (ROS) and antioxidants. Consistent with previous reports [34], the up-regulation of MDA and the down-regulation of GSH, SOD, and CAT were observed in the skin lesions of psoriatic mouse models, which confirmed the generation of oxidative stress in the psoriatic skin lesions. The changes in MDA, GSH, SOD, and CAT contents induced by IMQ were partly reversed by the intervention with TRYP. This could explain another mechanism behind TRYP on psoriasis in vivo. Intriguingly, our in vitro experiments based on TNF-α-treated HaCaT cells showed similar findings in vivo, which revealed that TRYP could ameliorate the psoriatic features by suppressing inflammation and oxidative stress in vivo and in vitro.
As a key pro-inflammatory signal, NF-κB has been proven to be involved in inflammation dysregulation during psoriasis [35]. It has been reported that suppressing NF-κB signaling is attributed to relieving symptoms of psoriasis in both psoriatic patients [36] and psoriatic mouse models [34]. Previous studies also have suggested the crucial role of the MAPK pathway in the pathogenesis of psoriasis [37], which was closely related to NF-κB signaling activation in multiple inflammatory diseases [38]. Previous studies have reported increased ERK1/2 activation in cell extracts from lesional psoriatic skin and in the human psoriatic lesion as compared to that in the normal human epidermis [39]. Moreover, it has been demonstrated that JNK is not active in healthy human epidermis, but its activity is increased in psoriasis [40]. To uncover the molecular mechanism underlying the effect of TRYP, our study subsequently investigated whether TRYP attenuates psoriatic skin lesions by suppressing pro-inflammatory activity mediated by the NF-κB/MAPK pathways. Consistent with previous studies, the phosphorylation of JNK and ERK1/2 in IMQ-induced psoriatic skin lesions and TNF-α-treated HaCaT cells both significantly increased compared with those in the control. The data showed that TRYP effectively repressed the NF-κB/MAPK pathways in IMQ-induced psoriatic skin lesions and TNF-α-treated HaCaT cells, including the activation of IκBα and NF-κB, as well as phosphorylation of JNK and ERK1/2.
As one of the key triggers of the antioxidant response, Nrf2 can regulate the transcription of antioxidant genes to protect cells from injury and inflammation induced by oxidative damage, which has recently been considered a promising target in psoriasis [41]. The MAPK signaling system has been implicated in Nrf2 induction by many previous reports [42, 43]. Given that TRYP is capable of enhancing the antioxidant enzyme levels in psoriatic skin lesions, we wonder whether TRYP regulates Nrf2 signaling to attenuate oxidative stress in psoriasis. ERK1/2 and p38 phosphorylation can lead to an activation of Nrf2 in several pathological conditions [44]. This may explain that the activation of Nrf2 was observed in IMQ-induced psoriatic skin lesions. Nrf2 is mainly localized and binds to KEAP1 protein in the cytoplasm under basal conditions, which will quickly translocate into the nucleus to transactivate the antioxidant response once stimulated by oxidative stress or inflammation [45]. In this study, TRYP significantly increased the localization of Nrf2 in the nuclear region in psoriatic models both in vivo and in vitro. Furthermore, previous studies demonstrated that TRYP protected several cells against oxidative stress or inflammation via Nrf2 signaling, implying the effect of TRYP on psoriatic features might be attributed to the activation of the Nrf2 pathway. After the administration of TRYP, the activation of Nrf2 was strengthened but not weakened by the decreased phosphorylation of JNK and ERK1/2. That might be because the phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution to modulating the Nrf2-dependent antioxidant response [46]. These data suggested that the effect of TRYP on Nrf2 activation relied on other mechanisms, but not the MAPK signaling system. It has been widely accepted that Nrf2 regulates antioxidant-responsive element-mediated induction of cytoprotective genes in response to oxidative stress after nuclear localization [47]. As a phase II antioxidant enzyme regulated by Nrf2, HO-1 is an important inducible stress response protein that converts hemoglobin to CO and Fe2+ and reduces the above products to bilirubin, thereby playing antioxidant and anti-inflammatory roles [48]. In line with previous studies [49], our data showed that the expression of HO-1 was significantly increased in psoriasis-like lesions compared to that in normal skin. After the strong activation of Nrf2 by TRYP, HO-1 was further up-regulated, thereby attenuating the inflammation and oxidative stress in both in vivo and in vitro models of psoriasis.
Collectively, TRYP is beneficial for psoriasis-like lesions due to its suppressive effect on immune responses exerted by related cytokines and oxidative stress via regulating NF-κB/MAPK/Nrf2 pathways and IL-17 A-producing Th17 cells (Fig. 7). In the future, we will further elucidate the specific mechanism of TRYP and evaluate its safety in the IMQ-induced psoriatic mouse model.
In conclusion, we found that TRYP treatment could not only ameliorate local symptoms, but also control the systemic immune responses in IMQ-induced psoriatic mice. It is possible that TRYP diminished the infiltration of Th17 cells and the activation of the NF-κB/MAPK pathways, and activated the Nrf2 pathway to attenuate inflammation and oxidative stress in TNF-α-stimulated keratinocytes and psoriatic skin of mice, thereby exerting an anti-psoriatic effect. Our findings provide some scientific information for TRYP as a new drug for psoriasis in the future.
Data availability
All data generated or used during the study appear in the submitted article.
References
Michalek IM, Loring B, John SM (2017) A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol 31:205–212
Langley RG, Krueger GG, Griffiths CE (2005) Psoriasis: epidemiology, clinical features and quality of life. Ann Rheum Dis 64(Suppl 2):ii18–ii23
Koo J, Marangell LB, Nakamura M, Armstrong A, Jeon C, Bhutani T et al (2017) Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol 31:1999–2009
Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. (2009). Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol 61:451-485
Sala M, Elaissari A, Fessi H (2016) Advances in psoriasis physiopathology and treatments: up to date of mechanistic insights and perspectives of novel therapies based on innovative skin drug delivery systems (ISDDS). J Control Release 239:182–202
Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F (2004) The inflammatory response in mild and in severe psoriasis. Br J Dermatol 150:917–928
Pleńkowska J, Gabig-Cimińska M, Mozolewski P (2020) Oxidative stress as an important contributor to the pathogenesis of psoriasis. Int J Mol Sci 21(17):6206
Lowes MA, Suárez-Fariñas M, Krueger JG (2014) Immunology of psoriasis. Annu Rev Immunol 32:227–255
Quaglino P, Bergallo M, Ponti R, Barberio E, Cicchelli S, Buffa E et al (2011) Th1, Th2, Th17 and regulatory T cell pattern in psoriatic patients: modulation of cytokines and gene targets induced by etanercept treatment and correlation with clinical response. Dermatology 223:57–67
Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L et al (2008) Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol 181:4733–4741
Lai Y, Li D, Li C, Muehleisen B, Radek KA, Park HJ et al (2012) The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity 37:74–84
Chen K, Kolls JK (2017) Interluekin-17A (IL17A). Gene 614:8–14
Sheremata W, Brown AD, Rammohan KW (2015) Dimethyl fumarate for treating relapsing multiple sclerosis. Expert Opin Drug Saf 14:161–170
Meng S, Lin Z, Wang Y, Wang Z, Li P, Zheng Y (2018) Psoriasis therapy by Chinese medicine and modern agents. Chin Med 13:16
Yu ST, Chen TM, Tseng SY, Chen YH (2007) Tryptanthrin inhibits MDR1 and reverses doxorubicin resistance in breast cancer cells. Biochem Biophys Res Commun 358:79–84
Recio MC, Cerdá-Nicolás M, Potterat O, Hamburger M, Ríos JL (2006) Anti-inflammatory and antiallergic activity in vivo of lipophilic Isatis tinctoria extracts and tryptanthrin. Planta Med 72:539–546
Honda G, Tosirisuk V, Tabata M (1980) Isolation of an antidermatophytic, tryptanthrin, from indigo plants, Polygonum tinctorium and Isatis tinctoria. Planta Med 38:275–276
Liang CY, Lin TY, Lin YK (2013) Successful treatment of pediatric nail psoriasis with periodic pustular eruption using topical indigo naturalis oil extract. Pediatr Dermatol 30:117–119
Yazdanpanah MJ, Vahabi-Amlashi S, Pishgouy M, Imani M, Banihashemi M, Mohammadpoor AH et al (2021) Comparing the topical preparations of Indigo naturalis from Chinese and Iranian origin in the treatment of plaque-type psoriasis: a preliminary randomized double-blind pilot study. Eur J Integr Med 43:101310
Lin YK, See LC, Huang YH, Chi CC, Hui RC (2018) Comparison of indirubin concentrations in indigo naturalis ointment for psoriasis treatment: a randomized, double-blind, dosage-controlled trial. Br J Dermatol 178:124–131
Kirpotina LN, Schepetkin IA, Hammaker D, Kuhs A, Khlebnikov AI, Quinn MT (2020) Therapeutic effects of tryptanthrin and tryptanthrin-6-oxime in models of rheumatoid arthritis. Front Pharmacol 11:1145
Moon SY, Lee JH, Choi HY, Cho IJ, Kim SC, Kim YW (2014) Tryptanthrin protects hepatocytes against oxidative stress via activation of the extracellular signal-regulated kinase/NF-E2-related factor 2 pathway. Biol Pharm Bull 37:1633–1640
Yue L, Ailin W, Jinwei Z, Leng L, Jianan W, Li L et al (2019) PSORI-CM02 ameliorates psoriasis in vivo and in vitro by inducing autophagy via inhibition of the PI3K/Akt/mTOR pathway. Phytomedicine 64:153054
Yélamos O, Puig L (2015) Systemic methotrexate for the treatment of psoriasis. Expert Rev Clin Immunol 11:553–563
Zhang S, Zhang J, Yu J, Chen X, Zhang F, Wei W et al (2021) Hyperforin ameliorates imiquimod-induced psoriasis-like murine skin inflammation by modulating IL-17A-producing γδ T cells. Front Immunol 12:635076
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta delta C(T)) method. Methods 25:402–408
van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD et al (2009) Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 182:5836–5845
Lowes MA, Bowcock AM, Krueger JG (2007) Pathogenesis and therapy of psoriasis. Nature 445:866–873
Cheng HM, Kuo YZ, Chang CY, Chang CH, Fang WY, Chang CN et al (2020) The anti-TH17 polarization effect of Indigo naturalis and tryptanthrin by differentially inhibiting cytokine expression. J Ethnopharmacol 255:112760
Lee CL, Wang CM, Kuo YH, Yen HR, Song YC, Chou YL et al (2020) IL-17A inhibitions of indole alkaloids from traditional Chinese medicine Qing Dai. J Ethnopharmacol 255:112772
Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT (2007) Mouse models of psoriasis. J Invest Dermatol 127:1292–1308
Ayala-Fontánez N, Soler DC, McCormick TS (2016) Current knowledge on psoriasis and autoimmune diseases. Psoriasis (Auckl) 6:7–32
Tan Y, Qi Q, Lu C, Niu X, Bai Y, Jiang C et al (2017) Cytokine imbalance as a common mechanism in both psoriasis and rheumatoid arthritis. Mediators Inflamm 2017:2405291
Chen H, Lu C, Liu H, Wang M, Zhao H, Yan Y et al (2017) Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-κB pathway. Int Immunopharmacol 48:110–117
Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF (2013) NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci 69:89–94
Lizzul PF, Aphale A, Malaviya R, Sun Y, Masud S, Dombrovskiy V et al (2005) Differential expression of phosphorylated NF-kappaB/RelA in normal and psoriatic epidermis and downregulation of NF-kappaB in response to treatment with etanercept. J Invest Dermatol 124:1275–1283
Haase I, Hobbs RM, Romero MR, Broad S, Watt FM (2001) A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest 108:527–536
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL, Hu G (2012) Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses. PLoS ONE 7:e49701
Johansen C, Kragballe K, Westergaard M, Henningsen J, Kristiansen K, Iversen L (2005) The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Br J Dermatol 152:37–42
Yu XJ, Li CY, Dai HY, Cai DX, Wang KY, Xu YH et al (2007) Expression and localization of the activated mitogen-activated protein kinase in lesional psoriatic skin. Exp Mol Pathol 83:413–418
El Ali Z, Ollivier A, Manin S, Rivard M, Motterlini R, Foresti R (2020) Therapeutic effects of CO-releaser/Nrf2 activator hybrids (HYCOs) in the treatment of skin wound, psoriasis and multiple sclerosis. Redox Biol 34:101521
Lee MS, Lee B, Park KE, Utsuki T, Shin T, Oh CW et al (2015) Dieckol enhances the expression of antioxidant and detoxifying enzymes by the activation of Nrf2-MAPK signalling pathway in HepG2 cells. Food Chem 174:538–546
Jasek-Gajda E, Jurkowska H, Jasińska M, Lis GJ (2020) Targeting the MAPK/ERK and PI3K/AKT signaling pathways affects NRF2, Trx and GSH antioxidant systems in leukemia cells. Antioxidants (Basel) 9(7):633. https://doi.org/10.3390/antiox9070633
Zipper LM, Mulcahy RT (2003) Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol Sci 73:124–134
He F, Ru X, Wen T (2020) NRF2, a transcription factor for stress response and beyond. Int J Mol Sci 21(13):4777
Sun Z, Huang Z, Zhang DD (2009) Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS ONE 4:e6588
Kobayashi M, Yamamoto M (2005) Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 7:385–394
Qiao H, Sai X, Gai L, Huang G, Chen X, Tu X et al (2014) Association between heme oxygenase 1 gene promoter polymorphisms and susceptibility to coronary artery disease: a HuGE review and meta-analysis. Am J Epidemiol 179:1039–1048
Wojas-Pelc A, Marcinkiewicz J (2007) What is a role of haeme oxygenase-1 in psoriasis? Current concepts of pathogenesis. Int J Exp Pathol 88:95–102
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiong, Y., Wang, J., Wang, S. et al. Tryptanthrin ameliorates imiquimod-induced psoriasis in mice by suppressing inflammation and oxidative stress via NF-κB/MAPK/Nrf2 pathways. J Nat Med 77, 188–201 (2023). https://doi.org/10.1007/s11418-022-01664-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-022-01664-9