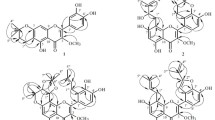
Abstract
Four new geranyl flavonoids 1–4 and four known flavonoids 5–8 were obtained from the leaves of Artocarpus communis collected in Indonesia. The planar structures of flavonoids were elucidated by analyses of MS and NMR spectroscopic data. Absolute configurations of 1 and 2 were determined by ECD spectroscopy. Analyses by HPLC with a chiral-phase column and ECD spectra confirmed that 3 and 4 were stereoisomeric mixtures and 7 and 8 were racemic mixtures. The compounds obtained in this study inhibited the enzymatic activities of ubiquitin-specific protease 7 (USP7) and the chymotrypsin-like activity of the proteasome. Among the geranyl flavonoids tested in this experiment, the USP7 inhibitory activity of 6 (IC50 value, 0.094 μM) was 55 times more potent than the commercially available positive control, P5091 (IC50 value, 5.2 μM).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Medicinal plants have been used as folk medicines in Indonesia, and the bioactive metabolites of these traditional medicines have attracted much interest as new drug candidates [1, 2]. The genus Artocarpus is distributed in the tropical and subtropical areas of Asia and used as traditional medicines to treat inflammation, diabetes, malarial fever, abscesses, and diarrhea [3] as well as a food in South-East Asia [4]. The constituents of Artocarpus species are well-studied and a variety of phenolic compounds including flavonoids, arylbenzofurans, and stilbenoids were reported as the major bioactive components [5]. Flavonoids isolated from Artocarpus species show a wide range of biological activities such as cytotoxicity [6, 7], antibacterial and antifungal [8], antimalarial [9], anti-inflammatory [10, 11], and tyrosinase inhibitory activities [9]. Continuing research on the constituents of the traditional medicinal plants is necessary in order to utilize the traditional ethnopharmacological knowledge for the development of new drugs. In this study, we investigated the biologically active constituents of the leaves of A. communis (family Moraceae) collected in Indonesia and obtained four new geranyl flavonoids along with four known congeners.
Results and Discussion
Separation of eight geranyl flavonoids (1–8)
The leaves of A. communis were extracted with EtOH. The extract was concentrated and partitioned between EtOAc and H2O, and then the organic layer was concentrated and partitioned between n-haxane and 90% MeOH–H2O. The concentrated aqueous MeOH fraction was subjected to silica gel column chromatography followed by HPLC to afford eight compounds. The structures of four new geranyl flavonoids were determined to be (S,E)-2-(3,4-dihydroxyphenyl)-8-(3,7-dimethylocta-2,6-dien-1-yl)-5,7-dihydroxychroman-4-one (1), (S)-5,7-dihydroxy-8-((2E,5E)-7-hydroxy-3,7-dimethylocta-2,5-dien-1-yl)-2-(4-hydroxyphenyl)chroman-4-one (2), (2S)-5,7-dihydroxy-8-((E)-6-hydroxy-3,7-dimethylocta-2,7-dien-1-yl)-2-(4-hydroxyphenyl)chroman-4-one (3), and 1-(2,4-dihydroxyphenyl)-3-(8-hydroxy-2-(3-hydroxy-4-methylpent-4-en-1-yl)-2-methyl-2H-chromen-5-yl)propan-1-one (4) (Fig. 1) by detailed analyses of the spectroscopic data. In addition to 1–4, four known flavonoids sophoraflavanone A [12, 13], 2-geranyl-2′,3,4,4′-tetrahydroxydihydrochalcone [14], 1-(2,4-dihydroxyphenyl)-3-[8-hydroxy-2-methyl-2-(4-methyl-3-pentenyl)-2H-1-benzopyranyl]-1-propanone [14], and 1-(2,4-dihydroxyphenyl)-3-[8-hydroxy-2-methyl-2-(4-hydroxy-4-methyl-2-pentenyl)-2H-1-benzopyran-5-yl]-1-propanone (8) [15] were identified (Fig. 1).
Structure elucidation of four new geranyl flavonoids (1–4)
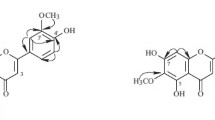
Compound 1 was obtained as pale yellow solid. The molecular formula of C25H28O6 was confirmed by HRESIMS. Interpretation of 1H and 13C NMR spectra together with HSQC spectrum revealed the presence of a carbonyl carbon at δC 196.6 (C-4), five oxygenated aromatic carbons at δC 161.1 (C-5), 164.4 (C-7), 159.7 (C-8a), 145.2 (C-3′), and 145.5 (C-4′), eleven sp2 carbons at δC 101.8 (C, C-4a), 95.2 (CH, C-6), 107.0 (C, C-8), 129.8 (C, C-1′), 114.2 (CH, C-2′), 115.3 (CH, C-5′), 117.6 (CH, C-6′), 122.4 (CH, C-2″), 133.8 (C, C-3″), 124.1 (CH, C-6″), and 130.6 (C, C-7″), four methylenes at δC 42.1 (C-3), 21.2 (C-1″), 39.3 (C-4″), and 26.2 (C-5″), one methine at δC 78.2 (C-2), and three methyls at δC 25.5 (C-8″), 17.5 (C-9″), and 15.8 (C-10″) (Table 1). The COSY correlations indicated the presence of three spin systems, H-2 (δH 5.33)/H-3 (δH 3.10), H-1″ (δH 3.08)/H-2″ (δH 5.09), and H-4″ (δH 1.86)/H-5″ (δH 1.96)/H-6″ (δH 5.01) (Fig. 2a). The HMBC correlations from two methyls at δH 1.58 (Me-8″) and δH 1.51 (Me-9″) to C-7″ and C-6″, and from a methyl proton at δH 1.55 (H-10″) to C-2″, C-3″, and C-4″, together with a NOE correlation between H-1″ (δH 3.08) and H-10″ assigned the structure of a geranyl moiety (Fig. 2a). The flavanone skeleton was established by the HMBC correlations from an aromatic proton H-6 (δH 5.95) to C-4a, C-5, C-7, and C-8, from hydroxyl proton 5-OH (δH 12.09) to C-4a, C-5, and C-6, from H-2′ to C-2, C-4′, and C-6′, and from H-3 (δH 2.69) to C-4. The geranyl side chain was attached to C-8, which was established by the HMBC correlations from H-1″ to C-7, C-8, and C-8a. The structure of ring B was determined as 3′,4′-dihydroxyphenyl on the basis of the comparison of the 13C NMR chemical shifts reported in the literature [16]. Although the proton signals for H-5′ (δH 6.72) and H-6′ (δH 6.72) were completely overlapped in DMSO-d6, they were slightly differentiated in CD3OD, and the ortho coupling constants (8.3 Hz) of H-5′ (δH 6.78)/H-6′ (δH 6.80) and the meta coupling constants (1.5 Hz) of H-2′ (δH 6.92)/H-6′ confirmed the 3′,4′-dihydroxyphenyl structure. The absolute configuration at C-2 was unambiguously determined to be S by the negative Cotton effect around 290 nm [17] (Fig. 2b). Yang et al. [16] previously pointed out that the assignment of the substitution pattern in the dihydroxy aromatic ring requires careful analyses of 1H and 13C NMR data and revised the structures of 28 compounds with 3,5-dioxygenated aromatic rings. During this study, we found that the reported 3′,5′-dihydroxyphenyl structures of three flavanones, 5,7,3′,5′-tetrahydroxyflavanone [18], schizolaenone C [19], and xeractinol [20], should be corrected to the 3′,4′-dihydroxyphenyl structures (Fig. 3), which were ultimately identified to be (–)-eriodictyol [21], diplacone [22], and taxifolin 6-C-glucoside [23], respectively.
The molecular formula of 2 was determined to be C25H28O6. Comparison of the 1H NMR spectra of 1 and 2 indicated their structural similarity (Table 2). The 1H NMR spectrum of 2 showed mutually coupled aromatic protons at δH 7.28 (H-2′ and H-6′) and δH 6.86 (H-3′ and H-5′), indicating the presence of a p-substituted phenyl group. The HMBC correlations from H-2′/H-6′ to a methine carbon at δC 78.6 (C-2) and an oxygenated aromatic carbon at δC 156.3 (C-4′) and from H-3′/H-5′ to an aromatic carbon at δC 130.6 (C-1′) assigned a hydroxyl group substituted at C-4′ in the aromatic ring B of the flavanone. Detailed analysis of the 1D and 2D NMR spectra of 2 indicated the 5,7,4′-trihydroxy flavanone with a modified geranyl side chain bound to C-8. The 1H NMR spectrum showed the presence of an E-olefin (JH-5″,H-6″ 15.0 Hz) at C-5″ (δH 5.54, δC 124.8)/C-6″ (δH 5.60, δC 139.5). The HMBC correlation from H-8″/H-9″ (δH 1.28) to C-6″ and C-7″ (δC 70.9) confirmed the position of a hydroxyl group at C-7″. Finally, the planar structure of 2 was determined and the absolute configuration at C-2 was determined to be S by the negative Cotton effect at 290 nm in ECD spectroscopy (Fig. 4) [17].
Compound 3 had the molecular formula C25H28O6. The 1H and 13C NMR spectra of 2 and 3 implied a difference in the modified geranyl side chain. HMBC correlations from a methyl at δH 1.69 (Me-9″) to an oxymethine carbon at δC 75.8 (C-6″) and olefin carbons at δC 147.2 (C-7″) and δC 111.1 (C-8″) assigned the planar structure of 3. The 2S configuration of 3 was determined by the ECD spectrum (Fig. 5a) [17]. Detailed analysis of the 13C NMR spectrum of 3 showed two sets of slightly shifted signals at δC 35.82/35.87 (C-4″) and δC 75.76/75.80 (C-6″), indicating that 3 was an epimeric mixture at C-6″. Purification by HPLC with a chiral-phase column furnished two equivalent peaks (Fig. 5b), and thereby 3 was determined to be an epimeric mixture of 6″R- and 6″S-3 in the ratio 1:1.
The molecular formula of 4 was determined to be C25H28O6 from the HRESIMS. In the 13C NMR spectrum of 4, most carbons showed sets of slightly shifted chemical shifts in the ratio 1:1 (Table 3), and 4 was indicated to be a mixture of stereoisomers. 1D and 2D NMR spectroscopic data showed one carbonyl carbon at δC 203.74/203.78, four oxygenated aromatic carbons at δC 139.40/139.45 (C-3), δC 143.06/143.07 (C-4), δC 165.3 (C-2′), and δC 163.27/163.28 (C-4′), 12 sp2 carbons at δC 127.96/127.98 (C, C-1), δC 118.88/118.93 (CH, C-2), δC 114.69/114.71 (CH, C-5), δC 121.26/121.29 (CH, C-6), δC 113.62/113.64 (C, C-1′), δC 103.6 (CH, C-3′), δC 107.9 (CH, C-5′), δC 132.21/132.25 (CH, C-6′), δC 119.7 (CH, C-1″), δC 129.64/129.71 (CH, C-2″), δC 146.99/147.07 (C, C-7″), and δC 111.44/111.57 (CH2, C-8″), two methyls at δC 29.15/29.29 (C-9″) and δC 26.25/26.41 (C-10″), four methylenes at δC 39.64/39.69 (C-α), 26.58/26.63 (C-β), δC 36.68/36.88 (C-4″), and δC 17.51/17.59 (C-5″), one oxymethine at δC 75.87/75.90 (C-6″), and one quaternary carbon at δC 78.51/78.63 (C-3″). COSY correlations revealed five spin systems H-5 (δH 6.71)/H-6 (δH 6.60), H-α (δH 3.07)/H-β (δH 2.96), H-5′ (δH 6.30)/H-6′ (δH 7.48), H-1″ (δH 6.50)/H-2″ (δH 5.58), and H-5″ (δH 1.61)/H-6″ (δH 4.05) (Fig. 6a). A dihydrochalcone skeleton was confirmed by the HMBC correlations from H-5 to C-1 and C-3, from H-6 to C-2 and C-4, from H-5′ to C-1′ and C-3′, from H-6′ to C-2′, C-4′, and a carbonyl carbon, from H-β to C-1, C-2, and C-6, from H-α to a carbonyl carbon (Fig. 6a). The remaining structure was elucidated from the 1D and 2D NMR spectroscopic data. A coupling constant of 10.0 Hz between olefin protons H-1″ and H-2″ confirmed the Z-olefin configuration. HMBC correlations from H-1″ to C-1, C-3, and C-3″, from H-2″ to C-2 and C-3″, from H-6″ to C-4″, C-5″, C-7″, and C-8″, from Me-10″ (δH 1.36) to C-2″, C-3″, and C-4″, from Me-9″ (δH 1.68) to C-6″, C-7″, and C-8″ established the planar structure of 4. As explained above, the 13C NMR spectrum showed 4 to be a mixture of two stereoisomers. In addition, no Cotton effect in the ECD spectroscopy indicated that 4 had a racemic nature. Taken together, 4 appeared to be a mixture of four stereoisomers. HPLC analysis with a chiral-phase column showed four peaks with nearly equal peak areas. Thus, 4 was confirmed to be a mixture of four stereoisomers at C-3″ and C-6″ (Fig. 6b).
Study on the absolute configurations of four known geranyl flavonoids (5–8)
Four known geranyl flavonoids 5–8 were obtained from A. communis in this study. However, as there were no previous descriptions of the stereochemistries of 7 and 8 [14, 15], we investigated their absolute configurations. The specific rotations of 7 and 8 were nearly zero (7, − 0.7; 8, + 0.4), and no obvious Cotton effects were observed in the ECD spectra. Resolution of 7 by HPLC with a chiral-phase column successfully afforded equivalent amounts of (+)-7 \( ([\alpha ]^{ 20}_{\text{D}} + 40) \) and (–)-7 \( ([\alpha ]^{ 20}_{\text{D}} - 4 5) \), and thus 7 was concluded to be a racemic mixture (Fig. 7a). The ECD spectrum of (+)-7 showed positive Cotton effects around 220 and 275 nm, and (−)-7 showed opposite Cotton effects to (+)-isomer (Fig. 7b). A Cotton effect due to the styrene chromophore is expected to be observed around 270 nm [24, 25]. Thus, the positive Cotton effect around 270 nm of (+)-7 indicated a 3″S configuration. To confirm this result, the ECD spectrum of S-chromenone (9) was calculated and a positive Cotton effect was observed at 270 nm (Fig. 7b). Compound 8 was separated by HPLC with a chiral-phase column to afford (+)- and (–)-isomers in the ratio 1:1 (Fig. 8a). The absolute configurations of (+)-and (−)-isomers were determined to be 3″S and 3″R, respectively, from their ECD spectra (Fig. 8b).
Biological activities of 1–8
The biological activities of 1–6, (+)- and (–)-7, and (+)- and (–)-8 were evaluated with our in-house screening including cytotoxicity, antimicrobial activities, inhibitory activity of the cholesterol ester accumulation in macrophages, inhibitory activity of the RANKL-induced formation of multinuclear osteoclasts, and inhibitory activities of the ubiquitin–proteasome system (proteasome, E1, Ubc13 (E2)–Uev1A interaction, p53–Mdm2 (E3) interaction, and USP7). Among them, 1, 4, 5, and 6 inhibited USP7 activity with 76, 92, 42, and 94% inhibition, respectively, at 1 μM, and the IC50 values of 1, 5, and 6 were 0.26, 1.2, and 0.094 μM, respectively. In addition, 1, 5, and 6 inhibited the chymotrypsin-like activity of the proteasome by 77, 24, and 67%, respectively, at 1 μM. This study showed that these geranyl flavonoids inhibited the enzymatic activity of USP7 and the proteasome at relatively low concentrations. Flavonoids are highly reactive and are known to inhibit various enzymatic activities including the proteasome [26, 27]. Further study is needed to reveal the relationship between structures of geranyl flavonoids and inhibitory activities of USP7 and the proteasome.
Conclusion
Four new geranyl flavonoids were obtained from the leaves of A. communis collected in Indonesia along with four known congeners. The chemical structures were elucidated by analyses of NMR and MS spectroscopic data together with the calculated ECD spectra. During the structure elucidation, we found that the reported structures for 5,7,3′,5′-tetrahydroxyflavanone, schizolaenone C, and xeractinol were incorrect and the same as those of (–)-eriodictyol, diplacone, and taxifolin 6-C-glucoside, respectively. The geranyl flavonoids obtained in this study inhibited the activities of USP7 and the chymotrypsin-like activity of the proteasome. Although an inhibitory effect of flavonoids on the proteasome has already been reported, this is the first report of geranyl flavonoids that inhibited USP7 and the proteasome. Among the geranyl flavonoids tested in this experiment, the USP7 inhibitory activity of 6 (IC50 value, 0.094 μM) was 55 times more potent than the commercially available positive control, P5091 (IC50 value, 5.2 μM). Ubiquitin-dependent protein degradation is a promising target for cancer drug discovery, and our study showed the potential of geranyl flavonoids as inhibitors of the ubiquitin–proteasome system.
Experimental
General experimental procedures
Optical rotations were measured on a JASCO DIP-1000 polarimeter in MeOH. UV spectra were measured on a JASCO V-550 spectrophotometer in MeOH. ECD spectra were measured on a JASCO J-820 spectropolarimeter in MeOH. IR spectra were recorded on a Perkin Elmer Frontier FT-IR spectrophotometer. 1H and 13C NMR spectra were recorded on a Bruker Avance III 600 NMR spectrometer. HRESIMS spectra were measured on a Bruker BioTOF and impact II mass spectrometer. The preparative HPLC system comprised a Waters 515 HPLC pump, Waters 2489 UV/visible detector, and Pantos Unicorder U-228. Silica gel-precoated plates (TLC Silica gel 60F254, Merck) were used for TLC detection, and spots were visualized using UV light (254 and/or 365 nm) and p-anisaldehyde reagent.
Plant material
Leaves of A. communis (15P099, fresh weight 169 g) were collected at Sam Ratulangi University, Kampus Bahu, Indonesia, in 2015 and soaked in EtOH immediately.
Extraction and separation
Leaves of A. communis (fresh weight, 169 g) were extracted with EtOH. Half of the extract was partitioned between EtOAc and H2O. The EtOAc fraction was partitioned between n-hexane and 90% MeOH–H2O. The 90% MeOH–H2O layer (1.1 g) was concentrated in vacuo and subjected to silica gel column chromatography with a stepwise gradient elution using n-hexane/EtOAc (2:1), CH2Cl2/MeOH (19:1 and 9:1), and CH2Cl2/MeOH/H2O (6:4:1) to afford 10 fractions. A portion (30 mg) of Fr. 2 (130 mg), eluted with n-hexane/EtOAc (2:1), was applied to gel filtration HPLC (Asahipak GS-310P, Asahi Chemical Industry Co., Ltd., 21.5 × 500 mm) with n-hexane/EtOAc (2:1) and HPLC (Inertsil Diol, GL Sciences, 20 × 250 mm) with n-hexane/EtOAc (2:1) to afford 7 (6.9 mg). A portion (30 mg) of Fr. 3 (90 mg), eluted with n-hexane/EtOAc (2:1), was applied to gel filtration HPLC (Asahipak GS-310P, 21.5 × 500 mm) with CH2Cl2/MeOH (10:1) to yield 5 (10.4 mg). Fr. 4 (64 mg), eluted with CH2Cl2/MeOH (19:1), was subjected to gel filtration HPLC (Asahipak GS-310P, 21.5 × 500 mm) with CH2Cl2/MeOH (4:1) followed by HPLC (Inertsil Diol, 20 × 250 mm) with n-hexane/EtOAc (1:1) to afford 1 (2.5 mg) and 6 (2.2 mg). The remaining half of the EtOH extract was subjected to solvent partitioning followed by silica gel column chromatography in a similar manner to that described above and afforded 10 fractions. Fr. 1 (140 mg), eluted with CH2Cl2/MeOH (19:1), was applied to gel filtration HPLC (Asahipak GS-310P, 21.5 × 500 mm) with MeOH to afford nine fractions. Frs. 6 (13 mg) and 7 (14 mg) were combined and subjected to normal phase HPLC (COSMOSIL 5SL-II, Nacalai Tesque Inc., 10 × 250 mm) with n-hexane/CH2Cl2/MeOH (20:19:1) followed by ODS HPLC (COSMOSIL 5C18-AR-II, Nacalai Tesque Inc., 10 × 250 mm) eluted with 70% MeOH–H2O to afford 2 (0.5 mg), 3 (0.8 mg), and 4 (1.7 mg). Fr. 5 (12 mg) was purified by normal phase HPLC (COSMOSIL 5SL-II, 10 × 250 mm) with n-hexane/CH2Cl2/MeOH (20:19:1) and ODS HPLC (COSMOSIL π-Nap, Nacalai Tesque Inc., 10 × 250 mm) with 70% MeOH–H2O to afford 8 (1.5 mg). Separation of 3 (0.8 mg) by HPLC using a chiral-phase column (CHIRALCEL OJ-H, Daicel, 4.6 × 250 mm) with n-hexane/EtOH (4:1) afforded two equivalent amounts of diastereomers (0.4 mg each). Analysis of 4 (10 μg) under the same conditions revealed four peaks. Resolution of 7 (2.4 mg) and 8 (1.5 mg) was carried out by the same column with n-hexane/2-propanol (4:1) to yield (+)- (1.1 mg)/(–)-7 (1.1 mg) and (+)- (0.4 mg)/(–)-8 (0.6 mg), respectively.
Compound 1: a yellow amorphous solid. \( [\alpha ]^{ 2 1}_{\text{D}} {-} 1 9 \) (c = 2.1, MeOH). UV λ max (MeOH) nm (log ε): 292 (3.9) nm. IR (film) υmax 3198, 2923, 2854, 1634, 1602, 1520, 1436, 1378, 1342, 1264, 1180, 1072, 1114, 1072, 1022, 1000, 820, 781, 550 cm−1. 1H and 13C NMR data (DMSO-d6), see Tables 1 and 2. HRESIMS m/z 423.1837 [M–H]− (calcd for C25H27O6, 423.1813).
Compound 2: a yellow amorphous solid. \( [\alpha ]^{ 2 5}_{\text{D}} {-} 20 \) (c = 0.4, MeOH). UV λmax (MeOH) nm (log ε): 292 (4.1) nm. IR (film) υmax 3210, 2925, 1635, 1600, 1519, 1436, 1343, 1267, 1170, 1073, 1023, 834 cm−1. 1H and 13C NMR data (CDCl3), see Tables 1 and 2. HRESIMS m/z 447.1773 [M + Na]+ (calcd for C25H28NaO6, 447.1778).
Compound 3: a yellow amorphous solid. \( [\alpha ]^{ 2 5}_{\text{D}} {-} 1 4 \) (c = 0.4, MeOH). UV λmax (MeOH) nm (log ε): 294 (3.9) nm. IR (film) υmax 3210, 2924, 1634, 1600, 1518, 1435, 1342, 1267, 1170, 1073, 1023, 833 cm−1. 1H and 13C NMR data (CDCl3), see Tables 1 and 2. HRESIMS m/z 447.1774 [M + Na]+ (calcd for C25H28NaO6, 447.1778).
Compound 4: a yellow amorphous solid. \( [\alpha ]^{ 2 1}_{\text{D}} + 7. 2 \) (c = 1.9, MeOH). UV λ max (MeOH) nm (log ε): 326 (3.6), 276 (4.1), 218 (4.3) nm. IR (film) υmax 3252, 2926, 1719, 1627, 1494, 1443, 1365, 1239, 1207, 1133, 1022, 987, 902, 802, 724, 614, 563 cm−1. 1H and 13C NMR data (CDCl3), see Table 3. HRESIMS m/z 447.1777 [M + Na]+ (calcd for C25H28NaO6, 447.1778).
Conformational analysis and ECD calculation of S-9
This experiment was conducted as previously reported [28]. Conformational analysis was performed with Spartan’16 (Ver. 1.0.0; Wavefunction Inc., Irvine, CA), and ECD calculation was performed with Gaussian09 (Revision D.01; Gaussian, Wallingford, CT, USA) [29] at the B3LYP/TZVP level. No wavelength correction was needed.
USP7 inhibition assay
This assay was performed as previously reported [30]. P5091 (Abcam, Inc.) was used as positive control and its IC50 value was 5.2 μM.
Proteasome inhibition assay
This experiment was performed as previously reported [31]. MG132 (Peptide Institute, Inc.) was used as positive control and its IC50 value was 0.037 μM.
References
Harvey AL, Edrada-Ebel R, Quinn RJ (2015) The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 14:111–129
Rates SM (2001) Plants as source of drugs. Toxicon 39:603–613
Jagtap UB, Bapat VA (2010) Artocarpus: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 129:142–166
Baliga MS, Shivashankara AR, Haniadka R, Dsouza J, Bhat HP (2011) Phytochemistry, nutritional and pharmacological properties of Artocarpus heterophyllus Lam (jackfruit): a review. Food Res Int 44:1800–1811
Hakim A (2010) Diversity of secondary metabolites from genus Artocarpus (Moraceae). Nusant Biosci 2:146–156
Hsu CL, Shyu MH, Lin JA, Yen GC, Fang SC (2011) Cytotoxic effects of geranyl flavonoid derivatives from the fruit of Artocarpus communis in SK-Hep-1 human hepatocellular carcinoma cells. Food Chem 127:127–134
Suhartati T, Achmad SA, Aimi N, Hakim EH, Kitajima M, Takayama H, Takeya K (2001) Artoindonesianin L, a new prenylated flavone with cytotoxic activity from Artocarpus rotunda. Fitoterapia 72:912–918
Kuete V, Kamga J, Sandjo LP, Ngameni B, Poumale HM, Ambassa P, Ngadjui BT (2011) Antimicrobial activities of the methanol extract, fractions and compounds from Ficus polita Vahl (Moraceae). BMC Complement Altern Med 11:6
Bourjot M, Apel C, Martin MT, Grellier P, Nguyen V, Guéritte F, Litaudon M (2010) Antiplasmodial, antitrypanosomal, and cytotoxic activities of prenylated favonoids isolated from the stem bark of Artocarpus styracifolius. Planta Med 76:1600–1604
Hsu CL, Chang FR, Tseng PY, Chen YF, El-Shazly M, Du YC, Fang SC (2012) Geranyl flavonoid derivatives from the fresh leaves of Artocarpus communis and their anti-inflammatory activity. Planta Med 78:995–1001
Wei BL, Weng JR, Chiu PH, Hung CF, Wang JP, Lin CN (2005) Antiinflammatory flavonoids from Artocarpus heterophyllus and Artocarpus communis. J Agric Food Chem 53:3867–3871
Shirataki Y, Endo M, Yokoe I, Komatsu M (1983) Studies on the constituents of Sophora species. XVIII. Constituents of the root of Sophora tomentosa L. Chem Pharm Bull 31:2859–2863
Shirataki Y, Yokoe I, Endo M, Komatsu M (1985) Determination of C-6 or C-8 substituted flavanone using 13C-1H long range coupling and the revised structures of some flavanones. Chem Pharm Bull 33:444–447
McLean S, Reynolds WF, Tinto WF, Chan WR, Shepherd V (1996) Complete 13C and 1H spectral assignments of prenylated flavonoids and a hydroxy fatty acid from the leaves of caribbean Artocarpus communis. Magn Reson Chem 34:719–722
Wang Y, Xu K, Lin L, Pan Y, Zheng X (2007) Geranyl flavonoids from the leaves of Artocarpus altilis. Phytochemistry 68:1300–1306
Yang YN, Zhu H, Chen Z, Liu F, An YW, Feng ZM, Jiang JS, Zhang PC (2015) NMR spectroscopic method for the assignment of 3,5-dioxygenated aromatic rings in natural products. J Nat Prod 78:705–711
Slade D, Ferreira D, Marais JPJ (2005) Circular dichroism, a powerful tool for the assessment of absolute configuration of flavonoids. Phytochemistry 66:2177–2215
Hashmi MA, Khan A, Ayub K, Farooq U (2014) Spectroscopic and density functional theory studies of 5,7,3′,5′-tetrahydroxyflavanone from the leaves of Olea ferruginea. Spectrochim Acta Part A Mol Biomol Spectrosc 128:225–230
Murphy B, Cao S, Norris A, Miller J, Ratovoson F, Andriantsiferana R, Rasamison V, Kingston D (2006) Cytotoxic compounds of Schizolaena hystrix from the madagascar rainforest. Planta Med 72:1235–1238
Dokkedal AL, Lavarda F, Santos LC, Vilegas W (2007) Xeractinol: a new flavanonol C-glucoside from Paepalanthus argenteus var. argenteus (Bongard) Hensold (Eriocaulaceae). J Braz Chem Soc 18:437–439
Hori K, Satake T, Saiki Y, Tanaka N, Murakami T, Chen CM (1988) Chemical and chemotaxonomical studies of filices. LXXIV. The novel flavanone glycosides of Pyrrosia linearfolia (HOOK.) CHING. Yakugaku Zasshi 108:417–421
Phillips WR, Baj NJ, Gunatilaka AAL, Kingston DGI (1996) C-Geranyl compounds from Mimulus clevelandii. J Nat Prod 59:495–497
Mbafor JT, Fomum ZT, Promsattha R, Sanson DR, Tempesta MS (1989) Isolation and characterization of taxifolin 6-C-glucoside from Garcinia epunctata. J Nat Prod 52:417–419
Choi H, Hwang H, Chin J, Kim E, Lee J, Nam SJ, Lee BC, Rho BJ, Kang H (2011) Tuberatolides, potent FXR antagonists from the Korean marine tunicate Botryllus tuberatus. J Nat Prod 74:90–94
Kikuchi T, Mori Y, Yokoi T, Nakazawa S, Kuroda H, Masada Y, Kitamura K, Kuriyama K (1983) Structure and absolute configuration of sargatriol, a new isoprenoid chromenol from a brown alga, Sargassum tortile C. Agardh. Chem Pharm Bull 31:106–113
Chang TL (2009) Inhibitory effect of flavonoids on 26S proteasome activity. J Agric Food Chem 57:9706–9715
Chen D, Landis-Piwowar KR, Chen MS, Dou QP (2007) Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res 9:R80
Kagiyama I, Kato H, Nehira T, Frisvad JC, Sherman DH, Williams RM, Tsukamoto S (2016) Taichunamides: prenylated indole alkaloids from Aspergillus taichungensis (IBT 19404). Angew Chem Int Ed 55:1128–1132
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, Revision D.01. Gaussian Inc., Wallingford
Tanokashira N, Kukita S, Kato H, Nehira T, Angkouw ED, Mangindaan REP, Voogd NJD, Tsukamoto S (2016) Petroquinones: trimeric and dimeric xestoquinone derivatives isolated from the marine sponge Petrosia alfiani. Tetrahedron 72:5530–5540
Noda A, Sakai E, Kato H, Losung F, Mangindaan REP, Voogd NJD, Yokosawa H, Tsukamoto S (2015) Strongylophorines, meroditerpenoids from the marine sponge Petrosia corticata, function as proteasome inhibitors. Bioorg Med Chem Lett 25:2650–2653
Acknowledgements
This work was supported by JSPS KAKENHI Grants 26305005 (S.T.) and The Program for Promoting the Enhancement of Research Universities, MEXT (H.K.). We thank V. H. Bahihi and S. Puasa of Sam Ratulangi University for collection and identification of the plant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inoue, M., Hitora, Y., Kato, H. et al. New geranyl flavonoids from the leaves of Artocarpus communis. J Nat Med 72, 632–640 (2018). https://doi.org/10.1007/s11418-018-1192-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-018-1192-z