Abstract
To investigate if andrographolide impairs cholestatic liver injury. All rats were randomly divided into six groups—(1) control (n = 6), (2) control + 200 mg/kg andrographolide (n = 6), (3) alpha-naphthylisothiocyanate (ANIT)-control (n = 6), (4) ANIT + 50 mg/kg andrographolide (n = 6), (5) ANIT + 100 mg/kg andrographolide (n = 6), and (6) ANIT + 200 mg/kg andrographolide (n = 6). We gavaged 50 mg/kg ANIT to mimic cholestatic liver injury in rats. Seven days after treatment, all the rats were killed. Serum biochemistry and hepatic histopathological assays were performed to evaluate liver injury. We observed that 200 mg/kg andrographolide significantly decreased the level of alanine transaminase, aspartate aminotransferase, alkaline phosphatase, γ-glutamyltranspeptidase, total bilirubin, and total bile acid in the blood. It also markedly decreased hepatic interleukin-6 and tumor necrosis factor α. Furthermore, 200 mg/kg andrographolide significantly decreased malondialdehyde but increased superoxide dismutase, glutathione, and erythrocyte glutathione peroxidase. Moreover, 200 mg/kg andrographolide effectively increased the accumulation of sirtuin 1 and nuclear erythroid 2-related factor-2. It also attenuated the level of nuclear factor kappa-light-chain-enhancer of activated B and cyclooxygenase-2. These data suggest that andrographolide may impair cholestatic liver injury via anti-inflammatory and anti-oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholestatic liver injury is caused by the accumulation of toxic bile acids in the liver [1,2,3,4,5]. Usually, cholestatic liver injury can lead to liver fibrosis, cirrhosis, and failure [6]. Unfortunately, effective treatment for cholestatic liver injury has not been established [7]. Thus, there is a need to develop novel therapeutics to impair cholestatic liver injury.
It has been proved that inflammation is involved in cholestatic liver injury [8,9,10]. Li et al. reported that activation nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) could promote the accumulation of several proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF- α) [11]. In addition, cyclooxygenase-2 (COX-2) also plays an important role in chronic liver inflammation. Lee et al. demonstrated that COX-2 activation resulted in the nuclear translocation of NF-κB [12]. Recently, some studies suggested that inhibition of IL-6, TNF-α, and COX-2 could impair cholestatic liver injury [13, 14].
Generally, oxidative stress can enhance cells to release IL-6, TNF-α and reactive oxygen species (ROS) [15]. Rudraiah et al. suggested that activation of nuclear erythroid 2-related factor-2 (Nrf2), a master defense against hepatotoxicity, could counteract oxidative stress [16]. Additionally, it is reported that sirtuin 1 (SIRT1) could impair liver injury by inhibiting inflammation and reducing the level of NF-κB [17, 18]. This suggests that up-regulating expression of SIRT1 may be a potential target for cholestatic liver injury.
Andrographolide is the major bioactive component of Andrographis paniculata [19, 20] (Fig. 1). Several studies demonstrated that andrographolide could attenuate diseases via several functions, such as anti-inflammatory and anti-oxidative activities [21,22,23,24]. Previous studies reported that andrographolide inhibited inflammation by attenuating the accumulation of COX-2, IL-6, and TNF-α [25, 26]. In addition, andrographolide could activate Nrf2 to inhibit the accumulation of NF-κB [27]. This suggests andrographolide is a promising medicine to treat inflammatory-related disease. Thus, the aim of this study was to evaluate the benefit of andrographolide in cholestatic liver injury.
Materials and methods
Chemicals
Andrographolide (purity ≥ 99%) was obtained from Dalian Meilun Biotechnology Co., Ltd (Meilun, code MB6935, China). Alpha-naphthylisothiocyanate (ANIT) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (Aladdin, code N137779, China). Carboxymethylcellulose sodium (CMC-Na) was purchased from Sinopharm Chemical Reagent Co., Ltd (Sinopharm, code 30036328, China).
Animals
Male Sprague–Dawley (SD) rats weighing 200 ± 20 g were obtained from the laboratory animal center of Dalian Medical University. These rats were kept in a standard environment with a 12-h light: dark cycle and a relative temperature (25 °C ± 2 °C). Each rat was allowed free access to food and water. Animal experiments were performed according to the National Institutes of Health Guidelines, which were approved by the Animal Experimentation Ethics Committee of Dalian Medical University (Approved No. SCXK-2008-0002).
The animals were divided into six groups—(1) control (n = 6), (2) control + 200 mg/kg andrographolide (n = 6), (3) alpha-naphthylisothiocyanate (ANIT)-control (n = 6), (4) ANIT + 50 mg/kg andrographolide (n = 6), (5) ANIT + 100 mg/kg andrographolide (n = 6), (6) ANIT + 200 mg/kg andrographolide (n = 6). We gavaged 50 mg/kg ANIT to mimic cholestatic liver injury in rats on day 4 after treatment. Seven days after treatment, all the animals were killed. The blood samples were immediately separated by centrifugation at 4 °C for 10 min to obtain serum samples. All samples were stored at − 20 °C until use. The right lobe of the liver was obtained for histological studies and other liver tissues were stored at − 80°C.
Serum biochemical analysis
The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transferase (γ-GGT), total bilirubin (TBIL), and total bile acid (TBA) were assayed using the commercial kits from Nanjing Jiancheng Bioengineering Institute (Jiancheng, China; ALT, code C009-2; AST, code C010-2; ALP, code A 059-2; γ-GGT code C017-2; TBIL, code C019-1; TBA, code E003-2).
Histomorphology
Liver tissue was excised and fixed in 4% paraformaldehyde overnight at 4 °C and embedded in paraffin after dehydration in an ethyl alcohol series. The paraffin-embedded sections, 4–5 μm per specimen, were prepared and stained with hematoxylin–eosin (HE staining). Each sample was identified by microscope (Leica, code DM4000B, Germany) and evaluated with 200 × magnification.
Determination of hepatic IL-6 and TNF-α, MDA, SOD, GSH, GSH-PX
To evaluate the level of IL-6 and TNF-α, MDA, SOD, GSH, GSH-PX in the liver, 0.1 g liver tissue was completely homogenized and centrifuged at 4 °C for 20 min. The supernatant was collected carefully and the optical density was measured under a specific wavelength by a Microplate Reader (Perkin Elmer, USA). The following kits, purchased from Nanjing Jiancheng Bioengineering Institute, were used to measure distinct parameters—IL-6 (code H007, 450 nm), TNF-α (code H052, 450 nm), MDA, (code A003-1, 550 nm), SOD (code A001-1, 550 nm), GSH (code A006-1, 420 nm), and GSH-PX (code A005, 420 nm).
Quantitative real-time polymerase chain reaction (RT-PCR)
RNA was isolated from the frozen liver samples (0.05 g) of each rat with the help of Trizol reagent protocols (TaKaRa, code 9108, China). Messenger RNA (mRNA) optical density was measured at 260 nm by a spectrophotometer. RNA (2 μg) was then reverse-transcribed into cDNA by PrimeScript® Buffer 2 reagent (TaKaRa, code RR047A, China). QPCR was performed using the RT Primer Mix kit (TaKaRa, code RR420A, China). The primers used are shown in Table 1. S1RT1 mRNA and Nrf2 mRNA expression were calculated using the ΔΔCt method. Beta-actin (β-actin) was normalized as an endogenous reference control.
Western blotting
Rat liver samples (0.02 g) were homogenized in ice-cold lysis buffer. The samples were centrifuged at 4 °C for 10 min and the supernatant was subsequently collected. The concentration of the protein was evaluated using the BCA protein assay kit (Beyutime Biotech, code P0010, China). Liver proteins were separated in 10% SDS-PAGE gel and then transferred onto a nitrocellulose membrane. Western blot assay was performed using the rabbit anti-NF-κB antibody (Abcam, code ab16502, UK, diluted 1:1,000), anti-COX2 antibody (Abcam, code ab15191, UK, diluted 1:1,000), anti-SIRT1 antibody (Abcam, code ab110304, UK, diluted 1:1,000), anti-Nrf2 antibody (Abcam, code ab31163, UK, diluted 1:1,000) and the mouse anti-β-actin antibody (Beyutime Biotech, code AF0003, China, diluted 1:1,000), and then incubated overnight at 4 °C. On the second day, the membranes were washed with TBST followed by incubation in secondary antibody for 1 h at room temperature. Subsequently, the membranes were visualized using the Bio-Rad ChemiDoc XRS+ imaging system (USA). The quantification analyses of Western blot were analyzed with the Image J software program (NIH).
Statistical analysis
The Graphpad Prism software program, version 5.0, was used for statistical analysis and figure drawing. All data were expressed as mean ± standard deviation (SD). The significance of differences among groups was calculated by one-way analysis of ANOVA. A P value < 0.05 was considered to be statistically significant.
Result
Andrographolide impairs cholestatic liver injury
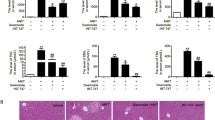
Compared with the control group, 200 mg/kg andrographolide could not significantly alter the level of ALT, AST, ALP, γ-GGT, TBIL and TBA in healthy rats; however, ANIT significantly increased the above-mentioned parameters (*P < 0.05, Fig. 2). Interestingly, in the andrographolide (100 mg/kg, 200 mg/kg) treatment group, the level of ALT, AST, ALP, γ-GGT, TBIL, and TBA was significantly (#P < 0.05, Fig. 2) decreased when compared with the rats in the ANIT-control group. However, 50 mg/kg andrographolide failed to show significant differences in ALT, ALP, TBIL, and TBA when compared to rats in the ANIT-control group.
Effects of andrographolide on serum biochemistry in ANIT-induced cholestasis rats. Alpha-naphthylisothiocyanate (ANIT)-induced liver injury rats were treated with 50 mg/kg, 100 mg/kg, and 200 mg/kg andrographolide. The following liver function parameters were assayed—a alanine aminotransferase (ALT); b aspartate aminotransferase (AST); c alkaline phosphatase (ALP); d γ-glutamyl transferase (γ-GGT); e total bilirubin (TBIL); f total bile acid (TBA). Data are expressed as the mean ± SD (n = 6 in each group). *P < 0.05 compared with the normal group; #P < 0.05 compared with the ANIT-control group
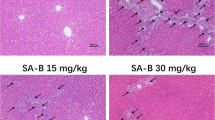
The histological examination for necrosis and inflammation are shown in Fig. 3. The control group presented a normal cellular structure with central veins, hepatocytes and hepatic plates (Fig. 3a). Consistent with serum biochemical analysis, 200 mg/kg andrographolide alone also failed to alter the histomorphology, when compared to the control group (Fig. 3b). However, the ANIT-control group and 50 mg/kg andrographolide treatment group exhibited acute severe inflammatory cellular infiltration and hepatic necrosis (Fig. 3c, d). Compared with the ANIT-control group, 100 mg/kg andrographolide treatment moderately reduced the severity of hepatic damage (Fig. 3e) and 200 mg/kg andrographolide treatment markedly reduced the severity of inflammatory and hepatic damage (Fig. 3f).
Andrographolide inhibits inflammation
As depicted in Fig. 4a, b, 200 mg/kg andrographolide alone failed to change the level of IL-6 and TNF-α, when compared to rats in the control group. However, compared to the control group, ANIT significantly increased the level of IL-6 and TNF-α (*P < 0.01, Fig. 4a, b). Interestingly, 50 mg/kg, 100 mg/kg and 200 mg/kg andrographolide could significantly (#P < 0.05, Fig. 4) inhibit ANIT-induced IL-6 and TNF-α, when compared to the ANIT-control group.
Effects of andrographolide on serum interleukin-6 (IL-6), tumor necrosis factor α (TNF-α) and hepatic nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) and cyclooxygenase-2 (COX-2) in ANIT-induced cholestasis rats. a Effect of andrographolide on serum IL-6; b effect of andrographolide on serum TNF-α; c effect of andrographolide on the expression of NF-κB and COX-2; d protein quantification of hepatic NF-κB and COX-2. All data are expressed as mean ± SD (n = 6 in each group). *P < 0.05 compared with the normal group; #P < 0.05 compared with the ANIT-control group
In addition, we investigated the expression of NF-κB and COX-2 in the liver (Fig. 4c, d). Andrographolide (200 mg/kg) failed to change the expression of NF-κB and COX-2 in healthy rats; however, ANIT significantly increased the accumulation of these two parameters (*P < 0.05, Fig. 4c, d). Interestingly, 50 mg/kg, 100 mg/kg, or 200 mg/kg andrographolide significantly attenuated the level of NF-κB and COX-2 in ANIT-treated rats (#P < 0.05, Fig. 4c, d).
Andrographolide inhibits oxidative stress
As shown in Fig. 5, compared to the control group, rats in the ANIT group showed a significant increase in the level of MDA and a significant decrease in hepatic SOD, GSH, and GSH-PX level (*P < 0.05). However, 100 mg/kg and 200 mg/kg andrographolide significantly impaired the ANIT-induced MDA and increased the level of SOD, GSH, and GSH-PX, when compared to the ANIT-control group (#P < 0.05). Unfortunately, 50 mg/kg andrographolide only modestly enhanced the accumulation of SOD, GSH and GSH-PX.
Effects of andrographolide on hepatic malondialdehyde (MDA), superoxide dismutase (SOD), glutathione (GSH) and erythrocyte glutathione peroxidase(GSH-Px) in ANIT-induced cholestasis rats. a MDA, b SOD, c GSH and d GSH-Px. All data are expressed as mean ± SD (n = 6 in each group). *P < 0.05 compared with the normal group; #P < 0.05 compared with the ANIT-control group
Andrographolide induces the accumulation of SIRT1 and Nrf2
We evaluated the hepatic SIRT1 and Nrf2 mRNA levels in the rats. The data demonstrated that SIRT1 and Nrf2 mRNAs were significantly (*P < 0.05) decreased in the ANIT-control group when compared to the control group (Fig. 6a, b). In addition, 200 mg/kg andrographolide alone failed to change the expression of SIRT1 and Nrf2 mRNAs. However, 50 mg/kg, 100 mg/kg, or 200 mg/kg andrographolide could significantly (#P < 0.05) increase the level of SIRT1 and Nrf2 mRNAs in the ANIT-treated rats, when compared to the ANIT-control group (Fig. 6a, b).
Effects of andrographolide on hepatic sirtuin 1 (SIRT1) and nuclear erythroid 2-related factor-2 (Nrf2) expression in ANIT-induced cholestasis rats. a Effect of andrographolide on hepatic SIRT1 mRNA expression; b effect of andrographolide on hepatic Nrf2 mRNA expression; c effect of andrographolide on hepatic SIRT1 and Nrf2 protein expression; d effects of andrographolide on hepatic SIRT1 and Nrf2 relative expression. All data are expressed as mean ± SD (n = 6 in each group). *P < 0.05 compared with the normal group; #P < 0.05 compared with the ANIT-control group
In addition, we evaluated hepatic SIRT1 and Nrf2 in the protein level. Consistent with the mRNA level, ANIT significantly (*P < 0.05) decreased the expression of SIRT1 and Nrf2 when compared to the control group. However, 50 mg/kg, 100 mg/kg, or 200 mg/kg andrographolide could significantly (#P < 0.05) increase the level of SIRT1 and Nrf2 protein in the ANIT-treated rats.
Discussion
Cholestasis is a common symptom of liver injuries, which can lead to acute liver toxicity, fibrosis, liver failure and even death [28]. Unfortunately, there is no standard pharmacological therapy for patients with cholestatic liver injury [19]. The present study demonstrated that andrographolide could impair ANIT-induced cholestatic liver injury. In addition, we found andrographolide could impair inflammation and oxidative stress.
Andrographolide, isolated from Andrographis paniculata, exhibits several activities, such as anti-bacteria, anti-inflammation, and immune suppression [19, 20, 29,30,31]. Thus, we speculated that andrographolide could impair cholestatic liver injury. We evaluated liver injury biomarkers (ALT, AST, ALP, γ-GGT, TBIL and TBA) in andrographolide-treated rats. We observed that 200 mg/kg andrographolide could significantly attenuate cholestatic liver injury in the ANIT-induced rat model. In addition, the benefit was proved by the histopathological image.
Inflammatory reaction has been shown to play a prominent role in cholestatic liver injury [12]. In the present study, we found ANIT could increase the level of several inflammatory markers, such as IL-6 and TNF-α. However, andrographolide significantly decreased the level of ANIT-induced IL-6 and TNF-α. In addition, several studies reported that TNF-α and IL-6 could activate NF-κB, which causes inflammation [32]. In the present study, we observed that andrographolide could decrease the level of NF-κB in ANIT-induced liver injury rats. Interestingly, we also found that andrographolide reduced the accumulation of COX-2, another pro-inflammatory factor that increases the nuclear translocation of NF-κB [12]. Thus, andrographolide may have inhibited the expression of NF-κB via reducing IL-6, TNF-α, and COX-2.
It is generally accepted that oxidative stress is involved in liver injury [33]. Yan et al. reported that the hepatic level of MDA was increased in ANIT-induced cholestatic liver injury [7]. In addition, several studies demonstrated that SOD, GSH, and GSH-PX have an anti-oxidant effect in all living cells [7, 30, 33, 34]. Consistent with these studies, we found ANIT significantly enhanced the accumulation of MDA but decreased the levels of SOD, GSH and GSH-PX. Interestingly, andrographolide could significantly attenuate the level of MDA and promoted SOD, GSH, and GSH-PX in ANIT-treated rats. These results suggest that andrographolide may impair cholestatic liver injury by attenuating oxidative stress.
It has been proved that SIRT1 and Nrf2 are two core players in liver physiology and a therapeutic target against hepatic inflammation [16,17,18]. It is reported that SIRT1 could attenuate oxidative stress to inhibit inflammation [1, 35]. Nrf2, a transcription factor, can inhibit the expression of many anti-oxidative stress genes, such as HO-1 and MSP23 [36]. In addition, several studies demonstrated that SIRT1 attenuated oxidative stress-induced injury by increased Nrf2 expression [1, 37]. Therefore, we evaluated the level of SIRT1 and Nrf2 in the present study. We observed that andrographolide could induce the accumulation of hepatic SIRT1 and Nrf2. Thus we speculated that andrographolide impaired oxidative stress-induced liver injury via up-regulating SIRT1 and Nrf2.
Conclusion
In conclusion, the present study found that andrographolide can attenuate inflammation and oxidative stress to impair cholestatic liver injury. In addition, andrographolide induces the accumulation of SIRT1 and Nrf2, two core inhibition regulators of inflammation and cholestatic liver injury.
References
Yu L, Liu X, Yuan Z, Li X, Yang H, Yuan Z, Sun L, Zhang L, Jiang Z (2017) SRT1720 alleviates ANIT-induced cholestasis in a mouse model. Front Pharmacol 8:256
Kang YZ, Sun XY, Liu YH, Shen ZY (2015) Autoimmune hepatitis-primary biliary cirrhosis concurrent with biliary stricture after liver transplantation. World J Gastroenterol 21:2236–2241
Liu J, Lu YF, Zhang Y, Wu KC, Fan F, Klaassen CD (2013) Oleanolic acid alters bile acid metabolism and produces cholestatic liver injury in mice. Toxicol Appl Pharmacol 272:816–824
Feng C, Li WJ, He RH, Sun XW, Wang G, Wang LQ (2018) Impacts of different methods of conception on the perinatal outcome of intrahepatic cholestasis of pregnancy in twin pregnancies. Sci Rep 8:3985
Riverso M, Chang M, Soldevila-Pico C, Lai J, Liu X (2018) Histologic characterization of kratom use-associated liver injury. Gastroenterol Res 11:79–82
Wu JS, Li YF, Li YY, Dai Y, Li WK, Zheng M, Shi ZC, Shi R, Wang TM, Ma BL, Liu P, Ma YM (2017) Huangqi decoction alleviates alpha-naphthylisothiocyanate induced intrahepatic cholestasis by reversing disordered bile acid and glutathione homeostasis in mice. Front Pharmacol 8:938
Yan JY, Ai G, Zhang XJ, Xu HJ, Huang ZM (2015) Investigations of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic against alpha-naphthylisothiocyanate-induced cholestatic liver injury in rats. J Ethnopharmacol 172:202–213
Arauz J, Zarco N, Hernandez-Aquino E, Galicia-Moreno M, Favari L, Segovia J, Muriel P (2017) Coffee consumption prevents fibrosis in a rat model that mimics secondary biliary cirrhosis in humans. Nutr Res 40:65–74
Wang L, Wu G, Wu F, Jiang N, Lin Y (2017) Geniposide attenuates ANIT-induced cholestasis through regulation of transporters and enzymes involved in bile acids homeostasis in rats. J Ethnopharmacol 196:178–185
Dai M, Hua H, Lin H, Xu G, Hu X, Li F, Gonzalez FJ, Liu A, Yang J (2018) Targeted metabolomics reveals a protective role for basal PPARalpha in cholestasis induced by alpha-naphthylisothiocyanate. J Proteome Res 17(4):1500–1508
Li YF, Wu JS, Li YY, Dai Y, Zheng M, Zeng JK, Wang GF, Wang TM, Li WK, Zhang XY, Gu M, Huang C, Yang L, Wang ZT, Ma YM (2017) Chicken bile powder protects against alpha-naphthylisothiocyanate-induced cholestatic liver injury in mice. Oncotarget 8(57):97137–97152
Lee TY, Chang HH, Wen CK, Huang TH, Chang YS (2014) Modulation of thioacetamide-induced hepatic inflammations, angiogenesis and fibrosis by andrographolide in mice. J Ethnopharmacol 158(Pt A):423–430
Gehrke N, Nagel M, Straub BK, Worns MA, Schuchmann M, Galle PR, Schattenberg JM (2018) Loss of cellular FLICE-inhibitory protein promotes acute cholestatic liver injury and inflammation from bile duct ligation. Am J Physiol Gastrointest Liver Physiol 314(3):G319–G333
Chavez E, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Castro-Sanchez L, Salazar EP, Moreno MG, Muriel P (2010) Antifibrotic and fibrolytic properties of celecoxib in liver damage induced by carbon tetrachloride in the rat. Liver Int 30(7):969–978
Yao H, Xu Y, Yin L, Tao X, Xu L, Qi Y, Han X, Sun P, Liu K, Peng J (2017) Dioscin protects ANIT-induced intrahepatic cholestasis through regulating transporters, apoptosis and oxidative stress. Front Pharmacol 8:116
Rudraiah S, Moscovitz JE, Donepudi AC, Campion SN, Slitt AL, Aleksunes LM, Manautou JE (2014) Differential Fmo3 gene expression in various liver injury models involving hepatic oxidative stress in mice. Toxicology 325:85–95
Tsai MS, Lee PH, Sun CK, Chiu TC, Lin YC, Chang IW, Chen PH, Kao YH (2018) Nerve growth factor upregulates sirtuin 1 expression in cholestasis: a potential therapeutic target. Exp Mol Med 50(1):e426
Abd El Motteleb DM, Ibrahim I, Elshazly SM (2017) Sildenafil protects against bile duct ligation induced hepatic fibrosis in rats: potential role for silent information regulator 1 (SIRT1). Toxicol Appl Pharmacol 335:64–71
Cabrera D, Wree A, Povero D, Solis N, Hernandez A, Pizarro M, Moshage H, Torres J, Feldstein AE, Cabello-Verrugio C, Brandan E, Barrera F, Arab JP, Arrese M (2017) Andrographolide ameliorates inflammation and fibrogenesis and attenuates inflammasome activation in experimental non-alcoholic steatohepatitis. Sci Rep 7(1):3491
Li M, Zhang T, Zhu L, Wang R, Jin Y (2017) Liposomal andrographolide dry powder inhalers for treatment of bacterial pneumonia via anti-inflammatory pathway. Int J Pharm 528(1–2):163–171
Feng B, Zhang Q, Wang X, Sun X, Mu X, Dong H (2017) Effect of andrographolide on gene expression profile and intracellular calcium in primary rat myocardium microvascular endothelial cells. J Cardiovasc Pharmacol 70(6):369–381
Ambili R, Janam P, Saneesh Babu PS, Prasad M, Vinod D, Anil Kumar PR, Kumary TV, Asha Nair S, Radhakrishna Pillai M (2017) An ex vivo evaluation of the efficacy of andrographolide in modulating differential expression of transcription factors and target genes in periodontal cells and its potential role in treating periodontal diseases. J Ethnopharmacol 196:160–167
Wu KC, McDonald PR, Liu J, Klaassen CD (2014) Screening of natural compounds as activators of the keap1-nrf2 pathway. Planta Med 80(1):97–104
Panraksa P, Ramphan S, Khongwichit S, Smith DR (2017) Activity of andrographolide against dengue virus. Antiviral Res 139:69–78
Yang Y, Yan H, Jing M, Zhang Z, Zhang G, Sun Y, Shan L, Yu P, Wang Y, Xu L (2016) Andrographolide derivative AL-1 ameliorates TNBS-induced colitis in mice: involvement of NF-small ka, CyrillicB and PPAR-gamma signaling pathways. Sci Rep 6:29716
Ding Y, Shi C, Chen L, Ma P, Li K, Jin J, Zhang Q, Li A (2017) Effects of andrographolide on postoperative cognitive dysfunction and the association with NF-kappaB/MAPK pathway. Oncol Lett 14(6):7367–7373
Tan WSD, Liao W, Zhou S, Wong WSF (2017) Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochem Pharmacol 139:71–81
Wang T, Zhou ZX, Sun LX, Li X, Xu ZM, Chen M, Zhao GL, Jiang ZZ, Zhang LY (2014) Resveratrol effectively attenuates alpha-naphthyl-isothiocyanate-induced acute cholestasis and liver injury through choleretic and anti-inflammatory mechanisms. Acta Pharmacol Sin 35(12):1527–1536
Nie X, Chen SR, Wang K, Peng Y, Wang YT, Wang D, Wang Y, Zhou GC (2017) Attenuation of innate immunity by andrographolide derivatives through NF-kappaB signaling pathway. Sci Rep 7(1):4738
Chen HW, Huang CS, Li CC, Lin AH, Huang YJ, Wang TS, Yao HT, Lii CK (2014) Bioavailability of andrographolide and protection against carbon tetrachloride-induced oxidative damage in rats. Toxicol Appl Pharmacol 280(1):1–9
Yan J, Xie G, Liang C, Hu Y, Zhao A, Huang F, Hu P, Liu P, Jia W, Wang X (2017) Herbal medicine Yinchenhaotang protects against alpha-naphthylisothiocyanate-induced cholestasis in rats. Sci Rep 7(1):4211
Peng S, Hang N, Liu W, Guo W, Jiang C, Yang X, Xu Q, Sun Y (2016) Andrographolide sulfonate ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-kappaB pathways. Acta Pharm Sin B 6(3):205–211
Cao WR, Ge JQ, Xie X, Fan ML, Fan XD, Wang H, Dong ZY, Liao ZH, Lan XZ, Chen M (2017) Protective effects of petroleum ether extracts of Herpetospermum caudigerum against alpha-naphthylisothiocyanate-induced acute cholestasis of rats. J Ethnopharmacol 198:139–147
Chen KL, Bi KS, Han F, Zhu HY, Zhang XS, Mao XJ, Yin R (2015) Evaluation of the protective effect of Zhi-Zi-da-Huang decoction on acute liver injury with cholestasis induced by alpha-naphthylisothiocyanate in rats. J Ethnopharmacol 172:402–409
Yang H, Bi Y, Xue L, Wang J, Lu Y, Zhang Z, Chen X, Chu Y, Yang R, Wang R, Liu G (2015) Multifaceted Modulation of SIRT1 in cancer and inflammation. Crit Rev Oncog 20(1–2):49–64
Ishii T, Itoh K, Takahashi S et al (2000) Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 275:16023–16029
Xue F, Huang JW, Ding PY, Zang HG, Kou ZJ, Li T, Fan J, Peng ZW, Yan WJ (2016) Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav Brain Res 309:1
Acknowledgements
This study was partly supported by the National Natural Science Foundation of China (no. 81473504) and the China Scholarship Council (no. 201708080032).
Author information
Authors and Affiliations
Contributions
PG and ZW carried out the research; LW, FC, LZ, PL and WL provided Figs. 1, 2 and 4; XD and YS provided Fig. 3; HB was responsible for the reagents and animals; LW and FC wrote the paper. All of the authors listed have revised and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Cao, F., Zhu, Ll. et al. Andrographolide impairs alpha-naphthylisothiocyanate-induced cholestatic liver injury in vivo. J Nat Med 73, 388–396 (2019). https://doi.org/10.1007/s11418-018-01275-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-018-01275-3