Abstract
Purpose
Many eutrophic lakes are located in regions where lakes become ice-covered during the winter. This study aimed to find out if phosphorus (P) could be withdrawn from such lakes by utilizing the wintertime accumulation of P to the near-bottom water.
Methods
Data for water quality and sediment characteristics were collected from two eutrophic boreal lakes with tube samplers and sediment corers. Diffusion rates of P across the sediment-water interface (SWI) and within the active sediment layers, and potential export of P via wintertime withdrawal were calculated.
Results
In the stratifying Lake Kymijärvi, P concentration in the near-bottom water reached 66 µg L−1 and P diffusion across SWI in the hypoxic area 5.4 mg m−2 d−1. In the shallow Lake Savijärvi, maximum P concentration was 78 µg L−1 but P diffusion rate only 0.34 mg m−2 d−1. In Kymijärvi, the concentrations of Fe and Mn in the sediment were high relative to P. In Savijärvi, sediment P was bound to clay minerals and calcium carbonates, while Fe was bound in sulfides.
Conclusion
In Kymijärvi, a theoretical14.3% reduction in epilimnetic TP concentration could be achieved in 20 years with 20 L s−1 winter withdrawal. In Savijärvi, 10 L s−1 withdrawal could theoretically cause a 5.8% reduction in TP concentration in 5 years, but the low P diffusion rate across SWI, and the low discharge of the lake may limit P removal. In Kymijärvi, where summertime withdrawal is already applied, additional winter withdrawal could accelerate lake recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The circulation of phosphorus (P) from the bottom sediment to the overlying water, i.e., internal P loading, is a major factor that delays the recovery of eutrophic lakes after the external loading of P has been reduced (Søndergaard et al. 2005; Welch and Cooke 2005). The cycling of P is of special importance, because in most lakes it is the main nutrient that limits the growth of primary producers (Schindler 2012). Therefore, any process that accelerates the flux of P from the sediment to the water column can maintain the eutrophic state and prevent or delay the transition to the often desired less productive state. Mechanisms that enhance circulation of sediment P include for instance diffusion and sediment resuspension (Tammeorg et al. 2015).
For the restoration of eutrophic lakes, numerous methods have been developed to reduce the internal P loading from the sediment. Some of the methods aim to retain P in the sediment and prevent the flux of P between the sediment and the water column (oxygenation, aeration, many chemical treatments) (Beutel and Horne 1999; Cooke et al. 2005; Huser et al. 2016). Another strategy is to remove nutrients from the lake ecosystem (dredging, fish removal, hypolimnetic withdrawal) (Van der Does et al. 1992; Cooke et al. 2005; Boros 2022).
Hypolimnetic withdrawal (HW) is a method that utilizes the diffusive flux of sediment P for restoration purposes. In this method, P-rich water is removed near the bottom of lake deeps by gravity, pumping or siphoning (Cooke et al. 2005; Nürnberg 2007). Such water withdrawal can reduce the epilimnetic P concentration of eutrophic lakes when used for lengthy time periods (Nürnberg 2007). A limitation of the method is that during the open water season it can be used only in lakes that are deep enough for thermal stratification, i.e., in lakes where dissolved P accumulates in the hypolimnion during the summer (Nürnberg 2020). In lakes that do not stratify, such accumulation does not take place.
Ice cover is common in lakes situated in high latitudes in both hemispheres. In the Northern Hemisphere, the southern boundary for regular seasonal ice cover follows approximately 45°N (Kirillin et al. 2012; Leppäranta 2015). Numerous ice-covered lakes are anthropogenically eutrophic (e.g., Schindler and Smol 2006; Schroth et al. 2015). Winter ice cover is also commonly observed in high altitude lakes, including those impacted by human activities. Under ice cover conditions, dissolved P often accumulates in near-bottom water layers if oxygen depletion takes place (Schroth et al. 2015; Joung et al. 2017). This raises the question whether withdrawal of P together with near-bottom water could be used as a restoration tool for shallow eutrophic lakes during winter under the ice cover. Additionally, for deeper stratifying lakes, under-ice withdrawal would extend the annual operational time of the method and thus possibly speed up the restoration process. Therefore, in this study the accumulation of P during the ice-covered period was examined in two boreal lakes in Finland. One of the study lakes is thermally stratified during the summer, while the other is a shallow polymictic lake. The diffusion rate of P from sediment to water was studied to investigate whether the wintertime diffusion rate is high enough to allow effective withdrawal of P with near-bottom water. The content of some key elements in the sediment and sediment porewater was examined to clarify the factors contributing to the P release rate.
2 Materials and methods
2.1 Study lakes
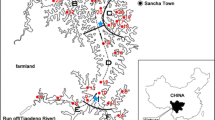
The study was conducted in Lake Kymijärvi (60° 57´N, 25° 48° E) and in Lake Savijärvi (60° 21´N, 25° 20° E) in southern Finland (Fig. 1). Kymijärvi is a dimictic lake (area 6.5 km2; mean depth 2.6 m, max depth 10.1 m) that was becaming eutrophic during the 1960s and 1970s due to heavy external nutrient loading. The present external P loading of Kymijärvi is below the critical loading of the lake as defined by the model of Vollenweider (1976), but internal loading is intensive and cyanobacterial blooms are still common. In the stratifying parts of the lake, situated in the Myllypohja basin (area 0.9 km2, max depth 8.8 m) hypolimnetic anoxia is an annual phenomenon during the summer and P accumulates in the hypolimnion during the summer stratification period in June–September, with near-bottom total P (TP) concentration often exceeding 200 µg L−1 (Silvonen et al. 2021). The summertime concentration of dissolved P in the sediment porewater (10 cm sediment depth) exceeds 5 mg L−1. The diffusion rate of P from the sediment in the stratifying areas (> 4 m depth) varies during the stratification period between 1‒3 mg P m−2 d−1 at 5 m depth, between 3‒9 mg P m−2 d−1 at 7 m depth and between 4.8‒7.5 mg P m−2 d−1 at 8.8 m depth (Silvonen et al. 2021). In Myllypohja basin, HW with a closed-circuit system was started in 2019 to improve the water quality (Silvonen et al. 2022). The hypolimnetic water is pumped through a withdrawal pipe into a mixing well of a treatment unit. In the well, water is aerated which facilitates the oxidation of reduced substances, including dissolved ferrous iron that is very abundant in the near-bottom water of Myllypohja basin. The oxidized iron forms a precipitate and binds dissolved P (Silvonen et al. 2022). The precipitate is captured in the treatment unit by sand filtration, after which the water flows back into Myllypohja basin via a wetland and an adjacent channel (Silvonen et al. 2024). The withdrawal in Myllypohja started with a low pumping rate (5–10 L s−1), but the rate will be elevated, which is facilitated by increasing the area of the sand filter.
Savijärvi is a small and shallow lake (area 0.40 km2, mean depth 1.6 m, max depth 2.6 m) that does not stratify during the open water season. It is eutrophic, and the total P concentration of the water is usually > 150 µg L−1 (Lappalainen et al. 2016). Intensive cyanobacterial blooms are an annual phenomenon. During the ice-covered period, anoxic conditions and consequent fish kills are common (Lappalainen et al. 2016). P diffusion from the sediments of Savijärvi has not been previously studied.
2.2 Sampling and analyses
In both lakes, sampling was conducted twice during the ice-covered period; in January and in March 2019 (Kymijärvi 23 January and 27 March, Savijärvi 25 January and 29 March). In Kymijärvi, the samples were taken from the Myllypohja basin, which shows stable stratification during the summer (Silvonen et al. 2021). In Savijärvi, sampling was conducted at the deepest part of the lake. On both sampling days and in both lakes, vertical profiles of water column temperature and dissolved oxygen (DO) were measured from surface to bottom with a YSI-6600V2 CTD-sonde (YSI Corporation, Yellow Springs, OH, USA). Additionally, two replicate water samples for P analysis were taken from each meter from the surface to the proximity of the bottom with a Limnos tube sampler (h = 1 m, V = 7.5 L). Samples for total phosphorus (TP) were stored in 250 mL polyethylene bottles. The bottles were filled up to avoid interference with oxygen, and from each bottle a subsample was swiftly filtered in the field with a syringe filter (pore size 0.25 μm) into a separate polyethylene bottle for soluble reactive P (SRP) analysis. Subsamples for SRP were preserved with 4 M H2SO4 (1 mL per 100 mL of sample). Both TP and SRP concentrations were measured spectrophotometrically using Lachat Quickchem 8500 Flow Injection Analysis System.
For sediment pore water analyses, a sediment core was taken from each lake in January and March with a HTH gravity corer (tube diameter 9 cm). Porewater samples were extracted from the sediment cores at 1 cm intervals using Rhizon filters (Rhizosphere Research Products, Netherlands; pore size 0.2 μm) and vacuum tube (Seeberg-Elverfeldt et al. 2005; Silvonen et al. 2021). The concentrations of iron (Fe), manganese (Mn), calcium (Ca) and sulfur (S) in the pore water were analyzed with ICP-OES (Inductively coupled plasma - optical emission spectrometry, Thermo iCAP 6000) and the concentration of SRP with Lachat Quickchem 8500. The pore water samples were not treated for sulfide preservation, hence the elemental S of the pore water samples in this study primarily indicates sulfate (SO4) and is hereafter referred to as such. The active sediment layer – i.e., the sediment layer from which P is diffused upwards towards the surface (Lin et al. 2017) – was determined for both lakes as the depth of the highest concentration peak of SRP in the pore water profiles.
Additionally, a sediment core was taken from each lake during the January sampling campaign for solid phase analysis. The cores were sliced at 1 cm resolution down to 30 cm depth and oven-dried. Water content was calculated from weight loss on drying. The organic fraction of the sediment was determined by loss on ignition at 550 °C (2 h). Additionally, to analyze the total HNO3-soluble contents of P, Fe, Mn, Ca and S in the sediment solid phase, subsamples were digested with boiling concentrated 65% HNO3 for 30 min according to a modified version of US EPA protocol 3050 for solid wastes. Digests were diluted to 1 M HNO3 and analyzed by ICP-OES. Protocol 3050 is considered to extract the majority of the reactive phases of these elements from sediment samples, including organic matter, oxide, sulfide and carbonate minerals, although recovery of elements is < 100% (Kimbrough and Wakakuwa 1989) due to partial insolubility of some phases in HNO3.
2.3 Estimation of P diffusion rates
The diffusion rates of P across the sediment-water interface (SWI) and within the active sediment layers were calculated according to Berner (1980):
where Φ is sediment porosity, Dsed is the diffusion coefficient of orthophosphate and dc/dx the concentration gradient between the sediment pore water and the overlying water column (dx = 0.5 cm), and:
where Di = temperature-adjusted diffusion coefficient of H2PO4 (cm2 s−1) (Lewandowski and Hupfer 2005)
Sarazin et al. (1995)
where Ws = dry mass of the sediment, Wt = total mass of the sediment.
The potential export of P via wintertime withdrawal from the study lakes was calculated based on the observed hypolimnetic P concentrations assuming a 60-d annual withdrawal period (February–March). The 60-d period was used in the calculations to account for possible future reductions in ice-cover duration. In the present climate, the average length of the ice season in the lakes of southern Finland is 4–5 months, and the 60-d assumption holds even when the predicted reductions of the ice-cover period are taken into account (Leppäranta 2010; Gebre et al. 2014). In the calculations, the average TP concentration observed at 6‒8 m depth was used for Kymijärvi. This was because the depths > 8 m represent only c. 4% of the area of the basin. Therefore, the average P concentration at 6‒8 m depth gives a best estimate of the quality of the withdrawn water, with a possible small contribution of higher-TP water from closer to the sediments. In Savijärvi, the average P concentration at 0‒2 m was used. In the calculations, TP concentration observed in late January was used for the first 30 d period and TP concentration observed in late March for the second 30 d period. The predicted effect of P removal on the TP concentrations of the epilimnion (TP change, %) was calculated with different withdrawal discharges (5‒50 L s−1) and duration of withdrawal (5‒30 years) using the empirical relationship established by Nürnberg (2020):
where total areal TP export = TP removed from the lake (mg m−2) by withdrawal during the entire restoration period.
3 Results
3.1 Water quality
Both lakes showed normal inverse temperature stratification under the ice cover. At 1 m depth, the temperature increased between January and March by 2.1 °C in Kymijärvi and by 1.4 °C in Savijärvi (Table 1). In Kymijärvi, DO concentration was > 5 mg L−1 throughout the water column in January, but in March hypoxic conditions (< 1 mg L−1) prevailed below 4 m depth (Table 1). In Savijärvi, DO concentration was ≤ 1 mg L−1 throughout the water column in January, whereas in March hypoxic conditions occurred only below 1.5 m (Table 1). Water pH was between 6.5‒7.0 in both lakes throughout the water column, with the exception of the uppermost 1 m in Kymijärvi in March, where pH reached 7.5. The concentration of TP was below 20 µg L−1 in Kymijärvi at all sampling depths in January, whereas in March it reached 66 µg L−1 near the bottom (Fig. 2). The concentration of SRP remained below 10 µg L−1 throughout the water column on both sampling dates. In Savijärvi, TP was 48‒53 µg L−1 in January and 66‒78 µg L−1 in March, while SRP was also relatively low as it remained below 12 µg L−1 both in January and March (Fig. 2).
3.2 Sediment pore water
In the sediment pore water, the highest concentrations of SRP were observed in both lakes in March at 5‒15 cm sediment depth (Fig. 3). In Kymijärvi, the highest concentration was 3.77 mg L−1 at 7 cm depth and in Savijärvi 1.88 mg L−1 at 11 cm depth. Therefore, the active sediment layer in Kymijärvi was determined as 0‒7 cm in Kymijärvi and as 0‒11 cm in Savijärvi.
In the surface sediment (1 cm depth) of Kymijärvi, SRP concentration was 1.22 mg L−1 in January and 0.91 mg L−1 in March (Fig. 3). In Savijärvi, the surface sediment concentration was 0.03 mg L−1 in January and 0.07 mg L−1 in March. Similar to the concentrations of SRP, the concentrations of Fe and Mn in the sediment pore water of Kymijärvi were lowest at the sediment surface (Fig. 3). The highest pore water concentrations of Fe (> 35 mg L−1) and Mn (> 5 mg L−1) in Kymijärvi were observed at 3 cm depth in January and at 7 cm depth in March. In Savijärvi, the concentrations of Fe and Mn also followed the profile of SRP, but the concentrations were considerably lower than in Kymijärvi (max. Fe 9.07 mg L−1, Mn 0.95 mg L−1). Ca and SO4-S profiles in the pore water were similar in the two lakes. In both lakes, the concentration of Ca was lowest and the concentration of SO4-S highest at the surface, with the exception of a SO4-S peak at 6–8 cm depth in the sediment of Savijärvi. In Kymijärvi, Ca concentration fluctuated between 15.6‒35.6 mg L−1 and SO4-S concentration between 0.23‒3.85 mg L−1 (Fig. 3). In Savijärvi, Ca concentration ranged between 9.81‒28.93 mg L−1 and SO4-S concentration between 0.23‒2.56 mg L−1 (Fig. 3).
3.3 Sediment composition and dry matter quality
The water content of sediment in Kymijärvi was 92.3% at 0‒5 cm, 88.6% at 5‒10 cm and 86.5% at 10‒15 cm sediment depth. In Savijärvi, the corresponding values were 97.1%, 95.0% and 93.8%, respectively. The organic fraction of the sediment in Kymijärvi was 19.5% at the surface, declining towards deeper layers (e.g., 12‒13% at 20‒25 cm depth) and showing a slight increase at 25‒30 cm depth (Fig. 4). In Savijärvi, the organic fraction of the sediment was 45.5% at the surface, decreasing constantly downward to below 25% at 25‒30 cm depth (Fig. 4). Total HNO3-extractable P content of sediment dry matter in Kymijärvi varied between 0.94‒1.79 mg g−1, being highest at the sediment surface and lowest at 20 cm depth (Fig. 5). In Savijärvi, P content varied between 0.79‒2.23 mg g−1 with the highest values observed at the surface. The lowest concentration was found at 29 cm sediment depth (Fig. 5). The concentration of total HNO3-extractable Fe fluctuated between 43.18‒53.46 mg g−1 in Kymijärvi and between 31.43‒45.57 mg g−1 in Savijärvi. Also the concentrations of total HNO3-extractable Mn were higher in Kymijärvi (0.98‒1.74 mg g−1) than in Savijärvi (0.46‒0.58 mg g−1). On the other hand, contents of HNO3-extractable Ca and S were higher in Savijärvi (average Ca: 5.56 mg g−1, S: 9.25 mg g−1), than in Kymijärvi (Ca: 4.37 mg g−1, S: 3.95 mg g−1). In Savijärvi, the vertical fluctuation of S closely followed the vertical Fe profile (Fig. 5).
3.4 Diffusion rate and effects of winter withdrawal
The diffusion rate of SRP across the SWI in Kymijärvi was 5.4 mg m−2 d−1 in January and 4.0 mg m−2 d−1 in March (Table 2). In Savijärvi, the diffusion rates were considerably lower, being 0.09 mg m−2 d−1 in January and 0.34 mg m−2 d−1 in March. Also within the active sediment layer, the diffusion rates were higher in Kymijärvi (> 0.5 mg m−2 d−1) than in Savijärvi (< 0.5 mg m−2 d−1) (Table 2).
With a withdrawal rate of 5 L s−1, the theoretical amount of P removed from Kymijärvi during the winter is so small even during 30 years’ pumping (26.8 kg) that practically no effect on epilimnetic TP concentration is predicted (Table 3). With a withdrawal rate of 10 L s−1, the amount of P removed from Kymijärvi would vary from 8.9 to 53.6 mg m−2 assuming a withdrawal duration of 5 to 30 years (Table 3). According to Nürnberg’s (2020) equation, such a rate of removal would not significantly affect the epilimnetic TP concentration of the lake water during the first 20 years of withdrawal (Table 3). However, with a 30-year duration of withdrawal at 10 L s−1, a 10.1% reduction in the epilimnetic TP concentration is predicted. With 30 L s−1 withdrawal rate, the effect on water TP concentration appears in less than 10 years and 20.1% reduction in epilimnetic TP concentration will be achieved in 20 years. With withdrawal rates of 40‒50 L s−1, a reduction exceeding 30% can be achieved in 30 years (Table 3). In Savijärvi, the theoretical effects of winter withdrawal are stronger than in Kymijärvi. Withdrawal at 10 L s−1 would cause a 5.8% reduction in TP concentration already in 5 years (with P removal of 39.7 mg m−2). With withdrawal rates > 30 L s−1, a 20% reduction in TP concentration would be achieved in 5 years and > 45% reduction in 30 years (Table 3).
4 Discussion
4.1 Factors regulating P diffusion and hypolimnetic P concentration in winter
Silvonen et al. (2021) showed that during the summer stratification, the diffusion rate of P across the SWI varied between 7‒9 mg m−2 d−1 in the deepest part of Kymijärvi, while the maximum winter value observed in the present study was 5.4 mg m−2 d−1. There are two main reasons for the lower values in wintertime. First, elevated pore water concentrations in summer are regulated by higher bacterial activity at higher temperatures and by higher supply of organic matter (Eckerrot and Pettersson 1993; Consiorczyk et al. 1997), both of which enhance mineralization of organic matter and chemical reactions liberating SRP (Gudasz et al. 2010; Bergström et al. 2010; Lu et al. 2016; Zhao et al. 2024). During the summer, the pore water SRP concentration in Kymijärvi approached 6 mg L−1 (Silvonen et al. 2021), while it remained below 4 mg L−1 during the winter. Higher pore water concentrations yield a stronger concentration gradient across the SWI, which speeds up diffusive fluxes. Second, diffusion itself is a temperature-dependent process, and seasonally lowest P release rates are thus usually found during winter (Lewandowski and Hupfer 2005; Kowalczewska-Madura and Goldyn 2009; James 2017). Thus, the low winter temperatures contributed to the low wintertime diffusion rates in Kymijärvi compared with the summertime estimates. Such seasonal variation in pore water chemistry, with highest P concentration in late summer and lowest during the winter, is common in lake sediments (Eckerrot and Pettersson 1993; Amirbahman et al. 2013). However, even at temperatures 2‒4 °C, diffusive P release rate from the sediment can exceed 10 mg m−2 d−1 if anoxic conditions prevail (Penn et al. 2000; Kowalczewska-Madura and Goldyn 2009). Due to the slower diffusion, the wintertime concentration of P in the hypolimnion of Kymijärvi was lower than during the summer stratification, when it reaches 200‒300 µg L−1 (Silvonen et al. 2021). Additionally, the shorter hypoxic period during the winter study (ca. 1.5 months) compared with summer (ca. 3 months) contributed to the lower overall P accumulation in the hypolimnion during the winter.
In Kymijärvi, a strong coupling exists between the cycling of P and Fe and Mn oxides (Silvonen et al. 2021). Relatively high Fe/P ratios in the sediment lead to a high sorption potential of P under oxic conditions (e.g., Boström et al. 1982) but the reduction of oxides under conditions of low redox potential leads to P release. The elevated concentrations of Fe and Mn in the pore water coincident with high concentrations of SRP confirm the reduction of Fe and Mn oxides in the sediment column (Froelich et al. 1979) and release of associated P. The maximum SRP concentration was however slightly deeper in the sediment than the maximum Fe and Mn concentrations, suggesting that also direct mineralization from organic matter contributed to the liberation of SRP for instance through sulfate reduction and methanogenesis (Reed et al. 2011; Egger et al. 2016). Release of hydrogen sulfide and sulfidization of oxide minerals following sulfate reduction can also accelerate P liberation in the deepest part of Kymijärvi (Carignan and Tessier 1988; Silvonen et al. 2021). Active sulfate reduction is confirmed by the concave profile of porewater SO4-S, indicating consumption with increasing depth in the sediment column (e.g., Kristensen 2000).
In Savijärvi, the pore water SRP concentration was lower and concentration gradient weaker than in Kymijärvi, which explained the lower diffusion estimates. Especially the low SRP concentration of the surface sediment of Savijärvi contributed to the low diffusion rate. In the uppermost 10 cm of the sediment, the pore water concentrations of dissolved P, Fe and Mn were considerably lower in Savijärvi than in Kymijärvi, implying less active release of P from reduction of oxide minerals. It is also notable that the depth profile of solid-phase P at Savijärvi is similar to the profile of Ca, while the Fe profile appears more similar to that of S. Savijärvi is situated in the region of southern Finland that was submerged below pro-glacial water bodies during the retreat of the Fennoscandian ice sheet and its catchment is dominated by clay-rich soils derived from aquatic sediments (Hagman 2011). Thus, P cycling in the sediments of Savijärvi is probably less dominated by association with Fe and Mn oxides than in Kymijärvi and may include additional fractions from the clay soil mineralogy, such as detrital apatite, or P bound to non-reducible oxides. At the same time, the overall content of reactive Fe is lower and the similarity of the Fe and S profiles suggests a significant proportion of reactive Fe is permanently bound in sulfides (Lehtoranta et al. 2008; Egger et al. 2016). Hypoxic periods do not therefore affect the cycling of P in Savijärvi as strongly as in Kymijärvi. The concentration of organic matter in the surface sediment of Savijärvi was higher than in Kymijärvi and therefore mineralization of organic matter is probably relatively a more important process contributing to P cycling in Savijärvi than in Kymijärvi.
Over the course of the winter, P diffusion rate across the SWI decreased in Kymijärvi and increased in Savijärvi. In Kymijärvi, this is explained by the slower upward diffusion rate of P within the active sediment layer (0‒7 cm) compared with diffusion at SWI, which led to the depletion of P at the SWI as winter progressed. Consequently, SRP concentration in the surface sediment pore water decreased during the winter. In Savijärvi, P diffusion rate across the SWI increased because diffusion within the active sediment layer was equally fast or faster than at the SWI, and thus supplied SRP to the surface sediment. As a result, SRP at the sediment surface increased during the winter. However, the concentration of SRP in the near-bottom water simultaneously decreased. This was in line with the finding that the oxygen conditions in Savijärvi improved from January to March, which was most likely due to the oxygen production by algae growing on the sediment surface (Carlton and Wetzel 1988). Indeed, benthic algae in the sediment surface of Savijärvi were visually observed during sampling, and benthic microflora can considerably affect redox-related phosphorus release from the sediment by oxygen production (Carlton and Wetzel 1988; Zhang et al. 2013). Moreover, their uptake of SRP can further decrease the near-bottom P concentrations. This provides an explanation for the seemingly contradictory observations from porewater and water column data in this lake.
4.2 Potential of winter water withdrawal in lake restoration
In both lakes, under-ice hypoxia is a frequent phenomenon (Hagman 2011; Järveläinen et al. 2016), therefore accumulation of P in deeper water layers is expected to occur annually and our calculations of potential P removal are valid scenarios for the future effects of withdrawal on in-lake P cycling. When considering the possibilities to improve the water quality of the study lakes with wintertime withdrawal, it must however be considered that possible withdrawal outflow rates depend on the withdrawal method applied. With conventional withdrawal, where the hypolimnetic water is led downstream, the discharge is limited by the discharge of the target lake, because the water level must not decrease due to withdrawal (Cooke et al. 2005; Silvonen et al. 2021). With closed-circuit withdrawal, the natural outflow rate does not restrict withdrawal outflow rate, because the water is returned to the same lake (Silvonen et al. 2021, 2024). Assuming conventional withdrawal and a 30% withdrawal rate of the natural outflow (Silvonen et al. 2021), a 15 L s−1 withdrawal rate is possible in Kymijärvi where the natural outflow rate is at least 50 L s−1 even during the driest months of the year (Korkiakoski 2011). During the first 10‒15 years, such wintertime withdrawal rate would have small effect on the TP concentration of the lake epilimnion but could lead to > 10% reduction in the concentration in 25‒30 years. With closed-circuit withdrawal and 45 L s−1 rate, in contrast, a 15% reduction in TP concentration would be reached in 10 years. For closed-circuit withdrawal, it must be taken into account that the circuit does not retain phosphorus with 100% efficiency. Silvonen et al. (2024) reported a 78% total P retention for the Kymijärvi withdrawal circuit (sand filter + wetland), suggesting that the amounts of P removed from the lake during the winter by closed-circuit system are overestimated in the present study by c. 20%. This is however compensated for by the duration of the winter withdrawal, which can usually be longer that the 60 days used in the calculations. Moreover, the duration of the summer stratification period will probably increase due to climate change (Arvola et al. 2010), which facilitates a longer withdrawal period during the summer. With a 45 L s−1 withdrawal rate, 74 kg of P (82.2 mg m−2) can be annually removed from the Myllypohja basin during summer stratification (Silvonen et al. 2021). Such P removal should result in 49.4% reduction in epilimnetic TP within 10 years. If such effort is complemented with annual 60-d wintertime withdrawal, the reduction of TP concentration in Myllypohja basin would increase by 1.3%.
In Savijärvi, which has a mean outflow rate of 28 L s−1 (Hagman 2011), the withdrawal rate with conventional withdrawal should be considerably lower than in Kymijärvi. With 5 L s−1 withdrawal rate, > 25 years of wintertime withdrawal would be required for 20% reduction in TP concentration. With closed-circuit withdrawal and a 25 L s−1 rate, a 19.0% reduction in TP concentration could be achieved in 5 years and a 34.8% reduction in 15 years. However, it is noteworthy that due to the shallowness and polymictic nature of Savijärvi, the near-bottom water and the surface layers of the water column are in more immediate and continuous interaction than in the deep areas of stratifying lakes. This means that the effects of P removal from the near-bottom water in Savijärvi could affect the TP concentrations in the photic zone within a shorter period than is estimated with Nürnberg’s (2020) empirical relationship regression, which is based on a dataset of deeper lakes. Further studies are needed to investigate the potential of withdrawal in non-stratifying lakes deeper than Savijärvi (and hence with a greater distance between the sediment and the productive layer).
Because the diffusion of P across the SWI is considerably lower during winter than in summer, there is technically a risk that diffusion cannot match the P removal rate by withdrawal, in which case P concentration the near-bottom water could decrease, leading to decreased P removal efficiency. In Kymijärvi, a 0.6 ha sediment area would be needed for the wintertime diffusion to complement the daily removal of P with a 10 L s−1 withdrawal rate. With a 45 L s−1 withdrawal rate, a 2.7 ha sediment area would be needed. Hence, these are the minimum areas that the withdrawal pipe should affect. Although the horizontal extent of the effect of water withdrawal has not been modeled in this study, the stated areas are plausible because the withdrawal pipe is at the deepest point of the stratifying area, and gravitational transport of sediment from the surrounding areas takes place. Silvonen et al. (2021) estimated that HW in Kymijärvi affects the hypolimnetic water at depth zones > 7 m, covering 11 ha. Thus, dilution will probably not restrict the efficiency of winter HW in Kymijärvi.
With lower P diffusion rates in Savijärvi, 26.5 ha of sediment would be required to complement 10 L s−1 withdrawal and 119.1 ha to complement 45 L s−1 withdrawal. Thus, dilution of the near-bottom water TP seems more likely than in Kymijärvi, potentially reducing the efficiency of winter withdrawal in Savijärvi. Dilution theoretically accelerates P diffusion across the SWI via effect on the concentration gradient, but the effect is minor compared with the effect of pore water P concentration (Silvonen et al. 2021). On the other hand, sediment resuspension can also take place under the ice cover and together with diffusion this feeds the near-bottom waters with P (Niemistö and Horppila 2007). The higher contribution of non-SRP to TP concentrations in Savijärvi, compared with Kymijärvi, suggests that resuspension was more intensive in Savijärvi. This was in line with the higher water content and organic fraction of the surface sediment in Savijärvi, indicating looser sediment structure compared with Kymijärvi. Thus, resuspension could in the long run compensate for the dilution of withdrawn water, especially in Savijärvi, via withdrawal of particulate P.
5 Conclusions
In Kymijärvi, P diffusion across the SWI was lower during the winter than during summer, which was mainly due to lower temperatures and supply of organic matter, limiting the microbial P liberation and dissolution of Fe-hydroxides into the porewater, thus resulting in a weaker diffusive P gradient at the sediment-water interface. In Savijärvi, despite the higher degree of eutrophication, diffusive flux of sedimentary P was lower than in Kymijärvi, because the proportion of redox-dependent P in the sediment was low. Due to higher P concentrations in the near-bottom water of Savijärvi, P export via withdrawal, and thus the effectiveness of the method, could in theory be higher in Savijärvi than in Kymijärvi. However, due to low diffusion rates, the maintenance of such high P removal rate in Savijärvi over the entire winter withdrawal period would require a very large effective area of the withdrawal pipe. The problem could possibly be avoided by using multiple withdrawal pipes (Nürnberg 2007). The low outflow rate of Savijärvi limits the possible withdrawal rate by leading the water downstream. Closed-circuit under-ice withdrawal with a high flow rate could improve the water quality of Savijärvi in the long run if dilution of the near-bottom water due to withdrawal can be avoided. In Kymijärvi, where summertime withdrawal is already applied, additional winter withdrawal could slightly accelerate lake recovery regardless of the application.
In all, the impact of winter P withdrawal on the epilimnetic water quality of eutrophic lakes does not seem strong, and lengthy periods of time are required for detectable effects. Effective restoration of a disturbed ecosystem is, however, a long-term enterprise, which can take longer than the period of degradation (e.g. Lake 2013). Therefore, any acceleration of lake recovery is welcome. Winter withdrawal can thus be worthwhile especially in stratified lakes, where summer withdrawal is already performed. In such cases, additional cost and effort caused by wintertime withdrawal would probably be low. Moreover, in many eutrophic lakes hypolimnetic P concentrations are considerably higher than those observed in the present study. For instance, in Lake Vesijärvi situated nearby Kymijärvi, TP concentrations > 1000 µg L−1 are found in near-bottom water layers during the winter (KVVY Tutkimus Oy 2023). Lake-specific benefits and costs of winter withdrawal will determine if wintertime withdrawal can be regarded as a feasible strategy, and preliminary studies are needed for each lake before any decisions on the restoration strategy.
Availability of data
Data are available from the corresponding author upon reasonable request.
References
Amirbahman A, Lake BA, Norton SA (2013) Seasonal phosphorus dynamics in the surficial sediment of two shallow temperate lakes, a solid-phase and pore-water study. Hydrobiologia 701:65–77
Arvola L, George G, Livingstone DM, Järvinen M, Blenckner T, Dokulil MT, Jennings E, Nic Aonghusa C, Nõges P, Nõges T, Weyhenmeyer G (2010) The impact of changing climate on the thermal characteristics of lakes. In: George G (ed) The Impact of Climate Change on European Lakes. Springer, Dordrecht, Heidelberg, pp 85–101
Bergström I, Kortelainen P, Sarvala J, Salonen K (2010) Effects of temperature and sediment properties on benthic CO2 production in an oligotrophic boreal lake. Freshw Biol 5:1747–1757
Beutel MW, Horne AJ (1999) A review of the effects of hypolimnetic oxygenation on lake and reservoir water quality. Lake Reserv Manag 15:285–297
Berner RA (1980) Early diagenesis: A theoretical approach, No. 1. Princeton University Press
Boros G (2022) Nitrogen and phosphorus removal by fishing in a large freshwater lake (Lake Balaton, Hungary). Inland Waters 12:277–282
Boström B, Jansson M, Forsberg C (1982) Phosphorus release from lake sediments. Arch Hydrobiol Beih 18:5–59
Carignan R, Tessier A (1988) The co-diagenesis of sulfur and iron in acid lakes sediments of southwestern Quebec. Geochim Cosmochim Acta 52:1179–1188
Carlton RG, Wetzel RG (1988) Phosphorus flux from lake sediments: effect of epipelic algal oxygen production. Limnol Oceanogr 33:562–570
Consiorczyk T, Casper P, Koschel R (1997) Variations of phosphorus release from sediments in stratified lakes. Wat Air Soil Poll 99:427–434
Cooke GD, Welch EB, Peterson SA, Nichols SA (2005) Restoration and Management of Lakes and Reservoirs. Taylor and Francis, Boca Raton
Eckerrot Å, Pettersson K (1993) Pore water phosphorus and iron concentrations in a shallow, eutrophic lake – indications of bacterial regulation. Hydrobiologia 253:65–177
Egger M, Kraal P, Jilbert T, Sulu-Gambari F, Sapart CJ, Röckmann T, Slomp CP (2016) Anaerobic oxidation of methane alters sediment records of sulfur, iron and phosphorus in the Black Sea. Biogeosci 13:5333–5355
Froelich PN, Klinkhammer GP, Bender ML, Luedtke NA, Heath GR, Cullen D, Dauphin P (1979) Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagenesis. Geochim Cosmochim Acta 43:1075–1090
Gebre S, Boissy T, Alfredsen K (2014) Sensitivity of lake ice regimes to climate change in the Nordic region. Cryosphere 8:1589–1605
Gudasz C, Bastviken D, Steger K, Premke K, Sobek S, Tranvik LJ (2010) Temperature-controlled organic carbon mineralization in lake sediments. Nature 466:478–481
Hagman A-M (2011) Sipoon Savijärven kunnostussuunnitelma. Uudenmaan elinkeino- liikenne- ja ympäristökeskuksen julkaisuja 20/2011. ISBN 978-952-257-433-6
Huser BJ, Egemose S, Harper H, Hupfer M, Jensen H, Pilgrim KM, Reitzel K, Rydin E, Futter M (2016) Longevity and effectiveness of aluminum addition to reduce sediment phosphorus release and restore lake water quality. Water Res 97:122–132
James WF (2017) Diffusive phosphorus fluxes in relation to the sediment phosphorus profile in Big Traverse Bay, Lake of the Woods. Lake Reserv Manag 33:360–368
Järveläinen J, Malin I, Mäyränpää R, Kotakorpi M (2016) Kymijärven ulkoinen ravinnekuormitus. Lahden kaupunki, Tekninen ja ympäristötoimiala, Lahden ympäristöpalvelut, Report 25 p (in Finnish)
Joung D, Leduc M, Ramcharitar B, Xu Y, Isles PD, Stockwell D, Druschel GK, Manley T, Scroth AW (2017) Winter weather and lake-watershed physical configuration drive phosphorus, iron, and manganese dynamics in water and sediment of ice-covered lakes. Limnol Oceanogr 62:1620–1635
Kimbrough DE, Wakakuwa JR (1989) Acid digestion for sediments, sludges, soils, and solid wastes. A proposed alternative to EPA SW 846 Method 3050. Environ Sci Technol 23(7):898–900
Kirillin G, Leppäranta M, Terzhevik A, Granin N, Bernhardt J, Engelhardt C, Efremova T, Golosov S, Palshin N, Sherstyankin P, Zdorovennova G, Zdorovennov R (2012) Physics of seasonally ice-covered lakes: a review. Aquat Sci 74:659–682
Korkiakoski P (2011) The current state, needs and possibilities for development of the water levels and flows in lake chain from Alasenjärvi to Sylvöjärvi. Thesis, Tampere University of Technology, M. Sc
Kowalczewska-Madura K, Goldyn R (2009) Internal loading of phosphorus from sediments of Swarzędzkie Lake (Western Poland). Pol J Environ Stud 18:653–643
Kristensen E (2000) Organic matter diagenesis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. Hydrobiologia 426:1–24
KVVY Tutkimus Oy (2023) Vesijärven tila vuonna 2022. Tutkimusraportti 256/23, 75 p (in Finnish)
Lake L (2013) Resistance, resilience and restoration. Ecol Manage Restor 14:1–5
Lappalainen J, Vinni M, Malinen T (2016) Living in the edge: the fate of individually marked pike (Esox lucius) in a hyper-eutrophic lake with frequent winter hypoxia. J Freshwat Ecol 31:509–519
Lehtoranta J, Ekholm P, Pitkänen H (2008) Eutrophication-driven sediment microbial processes can explain the regional variation in phosphorus concentrations between Baltic Sea sub-basins. J Mar Syst 74:495–504
Leppäranta M (2010) Modelling the formation and decay of lake ice. In: George G (ed) The Impact of Climate Change on European Lakes. Springer, Dordrecht, Heidelberg, pp 63–83
Leppäranta M (2015) Freezing of lakes and the evolution of their ice-cover. Springer, Heidelberg, New York
Lewandowski J, Hupfer M (2005) Effect of macrozoobenthos on two-dimensional small-scale heterogeneity of pore water phosphorus concentrations in lake sediments, a laboratory study. Limnol Oceanogr 50:1106–1118
Lin J, Sun Q, Ding S, Wang D, Wang Y, Chen M, Shi L, Fan X, Tsang DCW (2017) Mobile phosphorus stratification in sediment by aluminum immobilization. Chemosphere 186:644–651
Lu J, Yang J, Xu K, Hao J, Li Y-Y (2016) Phosphorus release from coprecipitants formed during orthophophate removal with Fe(III) salt coagulation: effects of pH, Eh, temperature and aging time. J Environ Chem Eng 4:3322–3329
Niemistö J, Horppila J (2007) The contribution of ice cover to sediment resuspension in a shallow temperate lake – Possible effects of climate change on internal nutrient loading. J Environ Qual 36:1318–1323
Nürnberg GK (2007) Lake responses to long-term hypolimnetic withdrawal treatments. Lake Reserv Manag 23:388–409
Nürnberg GK (2020) Hypolimnetic withdrawal as a lake restoration technique: determination of feasibility and continued benefits. Hydrobiologia 847:4487–4501
Penn MR, Auer MT, Doerr SM, Driscoll CT, Brooks CM, Effler SW (2000) Seasonality in phosphorus release rates from the sediments of a hypertrophic lake under a matrix of pH and redox conditions. Can J Fish Aquat Sci 57:1033–1041
Reed DC, Slomp CP, Gustafsson BG (2011) Sedimentary phosphorus dynamics and the evolution of bottom-water hypoxia: A coupled benthic-pelagic model of a coastal system. Limnol Oceanogr 56:1075–1092
Sarazin G, Gaillard JF, Philippe L, Rabouille C (1995) Organic matter mineralization in the pore water of a eutrophic lake (Aydat Lake, Puy de Dôme, France). Hydrobiologia 315:95–118
Schindler DW (2012) The dilemma of controlling cultural eutrophication of lakes. Proc Royal Soc B 279:4322–4333
Schindler DW, Smol JP (2006) Cumulative effects of climate warming and other human activities on freshwaters of Arctic and Subarctic North America. Ambio 35:160–168
Schroth AW, Giles CD, Isles PDF, Xu Y, Perzan Z, Druschel GK (2015) Dynamic coupling of iron, manganese, and phosphorus behavior in water and in sediment of shallow ice-covered eutrophic lakes. Environ Sci Technol 49:9758–9767
Seeberg-Elverfeldt J, Schlüter M, Feseker T, Kölling M (2005) Rhizon sampling of porewaters near the sediment-water interface of aquatic systems. Limnol Oceanogr Methods 3:361–371
Silvonen S, Niemistö J, Csibrán A, Torma P, Krámer T, Nurminen L, Jilbert T, Horppila J (2021) A biogeochemical approach to evaluate the optimization and effectiveness of hypolimnetic withdrawal. Sci Total Environ 755(Part 2):143202
Silvonen S, Niemistö J, Myyryläinen J, Kinnunen O, Huotari S, Nurminen L, Horppila J, Jilbert T (2022) Extracting phosphorus and other elements from lake water: chemical processes in a hypolimnetic withdrawal and treatment system. Water Res 218:118507
Silvonen S, Nurminen L, Horppila J, Niemistö J, Jilbert T (2024) Closed-circuit withdrawal and treatment: impact on effluent discharge on epilimnetic P and N concentrations. Limnology 25:87–95
Søndergaard M, Jensen JP, Jeppesen E (2005) Seasonal response of nutrients to reduced phosphorus loading in 12 Danish lakes. Freshw Biol 50:1605–1615
Tammeorg O, Laugaste R, Haldna M, Horppila J, Niemistö J (2015) Importance of diffusion and resuspension for the phosphorus cycling during the growing season in the large and shallow Lake Peipsi. Hydrobiologia 760:133–144
Van der Does J, Verstraelen P, Boers P, Van Roeste J, Roijackers R, Moser G (1992) Lake Restoration with and without dredging of phosphorus-enriched upper sediment layers. Hydrobiologia 233:197–210
Welch EB, Cooke GD (2005) Internal loading in shallow lakes: importance and control. Lake Reserv Manag 21:209–217
Vollenweider RA (1976) Advances in defining critical loading levels for phosphorus in lake eutrophication. Mem Ist Ital Idrobiol 33:53–83
Zhang X, Liu Z, Gulati RD, Jeppesen E (2013) The effect of benthic algae on phosphorus exchange between sediment and overlying water in shallow lakes: a microcosm study using 32P as a tracer. Hydrobiologia 710:109–116
Zhao S, Hermans M, Niemistö J, Jilbert T (2024) Elevated internal phosphorus loading from shallow areas of eutrophic boreal lakes: Insights from porewater geochemistry. Sci Total Environ 907:167950
Acknowledgements
Jan Weckström is acknowledged for help in field sampling.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). This study was funded by Maa- ja vesitekniikan tuki ry (project 39220).
Author information
Authors and Affiliations
Contributions
The authors designed the study together. Juha Niemistö and Soila Silvonen were responsible for field sampling. Tom Jilbert and Juha Niemistö were responsible for the laboratory analyses. Juha Niemistö and Soila Silvonen performed the data analyses. Soila Silvonen and Jukka Horppila wrote the first draft and all authors have read and accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Alexander Koiter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Silvonen, S., Niemistö, J., Jilbert, T. et al. Wintertime diffusion of sedimentary phosphorus – implications for under-ice phosphorus removal from eutrophic lakes. J Soils Sediments 24, 2522–2534 (2024). https://doi.org/10.1007/s11368-024-03838-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-024-03838-2