Abstract
Purpose
Biochar was used widely to deal with cadmium (Cd) pollution in soils. An indoor incubation experiment to investigate the effects of biochar on the structure and Cd-related functions of soil microbial communities under different concentration of Cd stress.
Materials and methods
A 180-day soil incubation experiment was performed by applying rice husk biochar (BC). At different time periods, Cd forms, chemical properties, and enzyme activities in soils were determined, and the effects of BC on the structure and function of microbial communities in soils with various Cd concentrations were explored by high-throughput sequencing.
Results and discussion
Compared to CK, BC significantly facilitated the conversion of bioavailable Cd to residual Cd, especially in medium Cd-contaminated soil where the residual Cd content increased by 76.35%. Biochar also significantly enhanced pH, soil organic matter, cation exchange, available phosphorus, rapidly available potassium, and catalase activities, except for ammonium nitrogen content and sucrase activities. PCoA and PERMANOVA revealed that incubation time and pollution levels significantly affected bacterial community structure, whereas pollution level was the only variable to significantly influence fungal community structure. According to co-occurrence network analysis, BC addition increased the microbial association in light and heavy Cd-contaminated soils but inhibited in medium Cd-contaminated soil. Moreover, the Cd-related functions of the microbial community predicted by PICRUSt2 suggested that BC significantly affected Cd transport-related functions in microbial communities, with higher Cd-contaminated soils showing more prominent expression of functions related to Cd transport. Pseudomonadota may have played an important role in the efflux of Cd, while Chloroflexota, Bacillota, and Actinomycetota participated in microbial detoxification and Acidobacteriaota, Armatimonadota, and Planctomycetota for Cd tolerance.
Conclusions
BC not only effectively immobilized Cd in light and medium Cd-contaminated soil but also improved three soil properties. BC had an insignificant effect on the microbial community structure in three soils but significantly affected the expression of Cd transport-related functions in the microbial communities of three soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cadmium (Cd) pollution in agricultural land has become a critical issue, resulting from prolonged and unceasing influences of mining, smelting, and sewage irrigation activities in China (Tu et al. 2020). Available Cd jeopardizes soil quality and crop yields, posing a threat to human health (Shi et al. 2019; Liu et al. 2021). In recent years, biochar has been attracting attention due to its excellent characteristics such as high-efficiency Cd stabilization, friendly environment, and improvement of soil fertility (Sun et al. 2021; Zhang et al. 2021; Xu et al. 2022).
Biochar possesses a large specific surface area as well as abundant porous structure, functional groups, and minerals (Godlewska et al. 2017; Luo et al. 2020), which can transform soil Cd speciation by either directly adsorbing soil Cd2+ or inducing changes in soil physicochemical properties and microbial communities (Chen et al. 2018a; Yang et al. 2017; Xu et al. 2022). For example, rice straw biochar application significantly increased soil pH, organic matter, cation exchange, and available potassium content in soil, which were negatively correlated with HOAc-extracted Cd (Mei et al. 2022). A meta-analysis also reported that various biochar application effectively decreased Cd availability by 41.1% on average in acidic soil (El-Naggar et al. 2022). However, due to limited adsorption sites, functional groups, and minerals of biochar as well as the various soil properties, biochar’s remediating effects in different concentration of Cd-contaminated soil would differ too. Previous studies have done a lot of work concerning the effects of biochar on Cd speciation and soil properties etc. mainly in a single level of Cd-contaminated soil, which cannot elaborate its adaptability in a wide range of Cd-contaminated soils.
On the other hand, it has been widely recognized that some Cd-resistant microbes carrying Cd-related functions could cope with Cd pollution stress via adsorption or transformation mechanisms, thereby contributing to the significant increase of residual Cd (Tu et al. 2020; Xie et al. 2021; Ma et al. 2023). Biochar can provide nutrients and suitable living space or toxic substances for soil microorganisms, which may have beneficial or detrimental effects on microorganisms, altering the diversity and structure of the microbial community (Yu et al. 2019; Zhang et al. 2021). It was reasonable to infer that soil microbes with Cd-related functions will respond accordingly following biochar application. Nevertheless, the study on the influence of biochar on soil microbial functions related to Cd transformation under the different levels of Cd pollution stress was still limited.
Rice husk represents one of the major agricultural wastes, with a global production of about 260 million tons per year (Zhang et al. 2022), which has the potential to be utilized to produce low-cost biochar. In this study, rice husk biochar (BC) was applied to three different levels of actual Cd-contaminated soils and incubated for 180 days in an indoor passivation experiment, for the purpose of the following: (1) evaluating the effects of BC on soil cadmium speciation, chemical properties, enzyme activities and microbial community structures; (2) determining the association between cadmium speciation, environmental factors, and microbial communities; (3) exploring the effect of BC on the functions related to Cd transformation in soil and identify the microbial species correlated with these functions.
2 Materials and methods
2.1 Test soils and biochar
The light (0.46 mg/kg), medium (4.18 mg/kg), and heavy (10.01 mg/kg) Cd-contaminated surface soils of agricultural land were sampled from Shaoguan City; all three soils were sandy loam according to soil texture triangle from the US department of agriculture. The soils were air-dried, ground, and sieved to determine their physicochemical properties (Table S1) and subjected to laboratory passivation incubation experiments.
The unpolluted rice husks were cleaned, dried, then crushed in a crusher, and sieved (< 0.42 mm). The rice husk powder was placed in a muffle with nitrogen gas and kept at 700 °C for 2 h. Rice husk biochar (BC) was sieved (< 0.154 mm). The biochar properties were measured as described by Xu et al. (2022) (Table S1).
2.2 Incubation experiment
Five percent of rice husk biochar was added to light (LS), medium (MS), and heavy (HS) Cd-contaminated soils, which were named treatment groups (BC). The corresponding control groups (CK) without amending biochars were set up, and the CK was the same as our previous research (Xu et al. 2022). All the treatments were in triplicate. The soils were incubated for 180 days, maintaining maximum field water holding capacity of 75%. The samples were taken, freeze-dried, and sieved at 10, 25, 40, 55, 70, 100, 130, and 180 days for the determination of soil properties.
2.3 Soil properties
Soil samples were digested with a mixture of HNO3-HF-HCl of 6:3:2 in a microwave digester to determine the total Cd content in soils. European Community Reference Bureau (BCR) method was used to extract the acid-soluble, reducible, oxidizable, and residual Cd contents of the soil independently (Rauret et al. 1999). The Cd content in the digestion solution and the BCR sequential extract were determined by ICP-MS (Perkin Elmer 600X, USA). The following methods were described by Lu (1999). Soil pH values were obtained by shaking the soil with deionized water 1:2.5 (w/v) and determined with a pH meter. SOM was measured after being oxidized using the K2Cr2O7-H2SO4 method (Wu et al. 2019). Cation exchange capacity (CEC) was determined by the spectrophotometric method. Available nitrogen (AN), phosphorus (AP), and potassium (AK) were analyzed by the universal extract-colorimetric method. Sucrase was determined using the 3,5-dinitrosalicylic acid colorimetric method, and the absorbance was measured at 508 nm, and catalase was measured via potassium permanganate titration.
2.4 High-throughput sequencing
Fresh soil samples before and after Cd immobilization (10 and 180 days) were feezed at − 80° for high-throughput sequencing. The bacterial 16S rRNA gene was amplified using the 338F/806R primer, while the fungal 18S rRNA gene using ITS1F/ITS2 primer. The amplicon libraries were sequenced using the Illumina Nova 6000 platform from Magigene Biotechnology (Guangzhou, China).
2.5 Statistical analysis
All the treatments had three replicates presented as mean ± standard deviation. One-way analysis of variance (ANOVA) was performed via SPSS 19.0, followed by Duncan’s test (P < 0.05). Principal coordinates analysis (PCoA) and Permutational multivariate analysis of variance (PERMANOVA) based on Bray–Curtis distance presented differences in microbial community structure between samples. Co-occurrence network analysis was performed by using the “wgcna” R package for correlation analysis at the OTU level and then visualized by Gephi software for presentation. The correlation between environmental factors and microbial communities were analyzed by the Mantel test. Based on the Kyoto Encyclopedia of Genomes (KEGG) and Protein Homology Group Cluster (COG) databases, a phylogenetic survey of communities was conducted by reconstructing unobserved states (PICRUSt2) to predict Cd-related functions in microbial communities (Chen et al. 2020a). Relationships between Cd-related functions and dominant bacterial phyla were analyzed by Pearson’s correlation analysis.
3 Results and discussion
3.1 Effects of biochar on soil properties
3.1.1 Cd speciation
According to the BCR method, acid-soluble and reducible were the most readily bioavailable; oxidizable was potentially toxic to organisms; residuals were generally considered to be the most stable part, which was not susceptible to transformations and migrations (Rinklebe and Shaheen 2017). Compared with CK, the changes of Cd forms showed a consistent pattern in the three soils. The content of acid-soluble and reducible Cd decreased by 0.04–0.63 mg/kg, while residual Cd increased by 0.04–0.90 mg/kg, and the oxidizable Cd did not change significantly (Fig. 1). Among them, the addition of biochar resulted in 26.79%, 27.43%, and 13.64% of acid-soluble Cd content lower than the control group in the light (LS), medium (MS), and heavy (HS) Cd-contaminated soils, respectively, but the residual Cd content increased by 19.84%, 76.35%, and 36.42%, respectively (Fig. S1). These results indicated that the immobilization of Cd by rice-husk biochar was more effective in LS and MS than HS, but the Cd in the acid-soluble state decreased in HS with little difference from that in MS (Fig. 1).
Changes in different cadmium forms in light (A), medium (B), and heavy (C) Cd-contaminated soils during incubation. Δ = content of BC-content of CK. LS, light Cd-contaminated soil; MS, medium Cd-contaminated soil; HS, heavy Cd-contaminated soil; CK, soils without treatment; BC, soils from 5% rice husk biochar treatment
Rice husk biochar could convert the acid-soluble Cd to the residual state. The main mechanisms were probably that biochar was able to adsorb Cd2+ in soil solution by using its own organic functional groups and inorganic ions (Chen et al. 2018a). At this point, Carboxyl, hydroxyl, and phenolic oxygen-containing functional groups in biochar could be used as adsorption sites to form surface complexes with Cd2+ in soil solution, reducing the mobility of Cd in soil (Derakhshan Nejad et al. 2018).
3.1.2 Chemical properties
Except for AN, the application of rice husk biochar significantly increased chemical properties such as pH, SOM, CEC, AP, and AK in three soils (Fig. 2). Specifically, compared to CK, biochar for 180 days of incubation increased the pH value in LS, MS, and HS by 1.88, 0.62, and 0.40 unit, respectively (Fig. 2A). This was probably due to the alkaline substances such as carbonates and silicates enriched in the surface of biochar (Gul et al. 2015). At the same time, the increase of pH varied as a result of the difference in buffering capacity between different soils. The increasing pH in soil could deprotonate soil colloids to promote electrostatic adsorption of Cd2+ (Xu et al. 2022). Therefore, higher pH values were more favorable for Cd immobilization in soil. Compared with CK, biochar application increased the content of SOM in LS, MS, and HS by 43.57, 32.62, and 34.35 mg/kg, respectively (Fig. 2B). This was likely due to the carbon enrichment of biochar. The high organic matter in the soil may promote soil fertility, moisture retention, and complexation reaction between Cd and oxygen-containing functional groups in soil organic matter (Wu et al. 2019). The content of organic matter in all three soils tended to decrease with increasing incubation time, which might be caused by the organic matter in the soil consumed by microorganisms for their own growth and reproduction (Ameloot et al. 2013). Compared with CK, biochar increased CEC in LS, MS, and HS by 1.31, 1.24, and 0.39 cmol+/kg, respectively (Fig. 2C). This might enhance the ion exchange between cations such as Ca2+, Mg2+, K+, and Cd2+ in the soil.
Compared to CK, the 180 days incubation with biochar increased the content of AN in LS and HS by 10.17 and 19.71 mg/kg, respectively, but slightly decreased the content of AN in MS by 2.95 mg/kg (Fig. 2D). Compared with CK, biochar increased the content of AP in LS, MS, and HS by 10.32, 12.65, and 68.63 mg/kg, respectively (Fig. 2E). Meanwhile, biochar also increased the content of AK in LS, MS, and HS by 279.12, 54.49, and 139.21 mg/kg, respectively (Fig. 2F). These results demonstrated that rice husk biochar significantly improved soil fertility, especially for AK. In addition, biochar application could also suppress heavy metal toxicity by increasing the content of soil AP and AK, which was beneficial to crop growth (Chen et al. 2020b).
3.1.3 Enzyme activity
Compared to CK, the catalase activity in LS, MS, and HS was increased to 0.04, 0.10, and 0.01 mL (0.1 mol/L KMnO4) h−1 g−1, respectively, after 180 days of incubation (Fig. 2G). The increase in catalase activity could relieve the toxic effects of hydrogen peroxide on organisms in the soil, in which it could be an oxidoreductase involving in the detoxification of heavy metals (Yang et al. 2016). Unlike catalase, biochar promoted sucrase activity in MS with an increment of 2.02 mg C6H12O6 g−1d−1, while it had no significant effect on sucrase activity in LS and even inhibited sucrase activity in HS with a decrease of 0.61 mg C6H12O6 g−1d−1 (Fig. 2H). This was probably due to that sucrase activity at its maximum in acidic soil media, whereas biochar created an alkaline condition not conducive to sucrase activity (Wang et al. 2008).
3.2 Effects of biochar on structure of microbial communities
3.2.1 Alpha diversity indexes
Before immobilization, BC had no significant effect on Chao1 and Shannon indices in bacterial and fungal communities, except for a significant increase by 1.47% of Shannon for the bacterial community in MS and a significant decrease by 15.78% of Chao1 for the fungal community in LS (Fig. 3). This indicated that BC before immobilization did not have significant effects on the microbial abundance and diversity in the three soils. With increasing incubation time, bacterial Chao1 and Shannon increased significantly in both the control and treatment groups after immobilization, but fungal Chao1 and Shannon showed a decreasing trend (Fig. 3). Generally, these groups compete for substrates and ecological niches to colonize, thus as the bacterial community increased, the fungal community was suppressed. In addition, fungi favor slightly acidic conditions, whereas most bacteria prefer neutral or slightly alkaline environments (Li et al. 2020, 2021).
In LS, bacterial Chao1 decreased by 10.5% and the Shannon index did not fluctuate much compared to CK after 180 days of biochar treatment. The Chao1 and Shannon indices of the fungal community also did not change significantly. In MS, bacterial Chao1 and Shanno increased significantly (P < 0.05) by 11.67% and 2.64%, respectively, while their variation in the fungal community was not significantly (P > 0.05) different. In HS, Chao1 and Shannon indexes in both bacterial and fungal communities did not significantly change compared to CK. These results suggest that the bacterial abundance and diversity exhibited different responses to BC in the three soils, while the fungal communities, in contrast, showed no significant responses.
3.2.2 Beta diversity
PCoA and PERMANOVA revealed that biochar treatment did not affect bacterial community structure at the phylum and genus levels, but that incubation time and pollution levels exerted significant (P < 0.05) influence (Fig. 4, Table 1). Besides, fungal community structure was only significantly (P < 0.05) influenced by pollution levels. These suggested that the structure of the fungal communities was more stable than the bacterial communities in the three soils and less susceptible to disturbance by the external environment (Zhang et al. 2021). Notably, the BC addition minimally affected the structure of the microbial communities in the three soils, this suggested that BC could immobilize Cd, improve soil properties, and minimize disturbance to the native microbial communities.
Principal coordinate analysis (PCoA) based on Bray–Curtis distance under phylum level. A Bacterial community structures in the three soils. B Fungal community structures in the three soils. L, light Cd-contaminated soil; M, medium Cd-contaminated soil; H, heavy Cd-contaminated soil; CK, soils without treatment; BC, soils from 5% rice husk biochar treatment
3.2.3 Relative abundance
Species with relative abundance ≥ 1% at the phylum level were shown in Fig. 5. The dominant bacterial phyla for all samples were Pseudomonadota, Acidobacteriaota, Bacteroidota, Chloroflexota, Bacillota, and Gemmatimonadota, which accounted for 79.64–95.07% of all phyla (Fig. 5A). Pseudomonadota are the most dominant phyla in the three soils, especially in LS. After 180 days of incubation, the percentage of Proteobacteria increased for all samples in MS but decreased in HS. BC decreased the relative abundance of Acidobacteriaota in LS and HS before immobilization but recovered with increasing incubation time. Compared to before immobilization, the percentage of Bacteroidota decreased in the three soils after immobilization, while Bacillota exhibited the opposite trend. This indicated that Bacteroidota might be advantageous in Cd-contaminated environments, which had been also reported as a typical microorganism in Cd-contaminated soils (Wang et al. 2021).
The dominant fungal phyla for all samples were Unassigned (24.73–64.26%), Ascomycota (24.05–51.85%), which occupied the majority (Fig. 5B). After immobilization, Unassigned emerged as the dominant phyla with the largest relative abundance share of all samples in MS and HS, while Ascomycota was the dominant phyla in LS. Compared to CK, BC addition led to some variation in the relative abundance of dominant phyla in the three soils, but they remained dominant among all phyla. This indicated that BC may not have exerted a substantial influence on the structure of the native microbial community in the soil (Fig. 4).
3.2.4 Microbial co-occurrence network
Microbial community co-occurrence networks were established to investigate the influence of biochar on the association of microbial communities at the OTU level before and after immobilization (Fig. 6, Table S2). The modularity indices ranged from 0.69 to 0.79 in all networks were larger than 0.5, which indicated they were highly modular (Wu et al. 2022). Moreover, the average path length ranged from 6.86 to 8.45 in all networks, showing a “small-world” characteristic, which indicated that the perturbation of the external environment would spread faster to affect the whole network and disturb the stability of the network (Dai et al. 2022). Besides, the parameters of average degree, clustering coefficient, and average path length were different according to the empirical networks and random networks, which indicated those networks were not connected randomly (Fan et al. 2018).
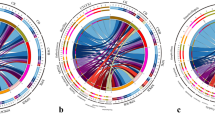
Co-occurrence networks of bacteria and fungi taxa in different treatments at OTU level in three soils. The node size is proportional to the abundance of taxa, and the nodes filled in blue are bacterial taxa, and in orange are fungal taxa. The edges are colored according to interaction types; positive correlations are labeled with blue and negative correlations are colored in red. LS, light Cd-contaminated soil; MS, medium Cd-contaminated soil; HS, heavy Cd-contaminated soil; CK, soils without treatment; BC, soils from 5% rice husk biochar treatment
The nodes and edges increased for all samples in all three soils after immobilization except for the edges in HS (Table S2), indicated that the network became correspondingly more complex with incubation time. The percentage of bacterial nodes was more than fungal nodes, and the percentage increased with increasing incubation time, which suggested the bacterial communities might play a greater role in the network. BC addition increased the proportion of positively correlated edges in LS and MS, likely to promote the synergistic interactions between microbial communities. Compared with CK, BC increased the average degree and clustering coefficient in LS by 1.09 and 0.002 and increased in HS by 18.83 and 0.07 but decreased in MS by 2.81 and 0.01, respectively (Fig. S3, Table S3). These suggested BC addition increased the microbial association in LS and HS but inhibited that in MS (Deng et al. 2012).
3.3 Correlation between environmental factors and microbial communities
According to the Mantel test, the environmental variables sucrase, AN, AP, reducible Cd, oxidizable Cd, and residual Cd were significantly correlated with the bacterial communities in LS, while pH, SOM, CEC, and AK changes caused significant variations in the structure of the fungal communities (Fig. 7A). Except for SOM, CEC, sucrase, AP, and AK, the other seven environmental variables significantly influenced the bacterial community structure in MS, while only pH changes in the fungal community were significantly correlated with their microbial structure (Fig. 7B). In HS, all environmental variables caused significant changes in bacterial and fungal community structure (Fig. 7C).
Correlations between soil properties and microbial communities in light (A), medium (B), and heavy (C) Cd-contaminated soils. LS, light Cd-contaminated soil; MS, medium Cd-contaminated soil; HS, heavy Cd-contaminated soil; the asterisks (*) represent significant differences at the level of P < 0.05; the asterisks (**) represent significant differences at the level of P < 0.01; the asterisks (***) represent significant differences at the level of P < 0.001
SOM, CEC, and AK contents were negatively correlated with acid-soluble content in LS, in contrast to a positive correlation with residual Cd content (P < 0.05) (Fig. 7A). In MS, CEC, and AK contents showed a significant negative correlation with acid-soluble Cd content, while AN content showed a significant positive correlation with it (Fig. 7B). In HS, acid-soluble Cd content was significantly negatively correlated with CEC, AN, AP, and AK contents, while significantly positively correlated with sucrase content (Fig. 7C).
3.4 Functional prediction of microbial communities
The abundance of Cd-related functions was analyzed utilizing PICRUSt2. The functions included a detoxification pump for Cd2+ (Zn2+/Cd2+-exporting ATPase), providing energy for Cd2+ to cross biofilms via ATP hydrolysis (H+-transporting ATPase), a major carrier of Cd2+ transport (Cobalt-zinc-Cd efflux system protein) and the ability to catalyze Cd2+ transport by capturing the energy generated by ATP hydrolysis using the ABC transport system (ABC-2 type transport system ATP-binding protein, ABC-2 type transport system permease protein) (Wong et al. 2009; Chen et al. 2020a; Szeri et al. 2021; Tian et al. 2022). Additionally, Cd resistance functions were also included.
In the three soils, biochar significantly affected the abundance of the function related to Cd transport (Fig. 8). Zn2+/Cd2+-exporting ATPase were at the lowest abundance levels in LS and HS before immobilization. The Zn2+/Cd2+-exporting ATPase contained two Cys residues that bind strongly to Cd2+, which could reduce the toxicity of Cd in microorganisms (Chen et al. 2018b). After 180 days of incubation, BC treatment significantly increased the abundance of Zn2+/Cd2+-exporting ATPase in HS compared to the control but inhibited in LS and MS. Besides, the abundance of the H+-transporting ATPase were not significantly different in all treatments after immobilization. The abundance of cobalt-zinc-Cd efflux system protein did not significantly change in the treatment and control groups before immobilization. BC treatment significantly increased the abundance of cobalt-zinc-Cd efflux system protein in MS and HS compared to the control after immobilization, which could be more favorable to the active Cd efflux behavior of the microbial communities in both soils (Tian et al. 2022).
Compared to CK, BC had no significant effect on the abundance of ABC-2 type transport system ATP-binding protein and ABC-2 type transport system permease protein before and after immobilization, and the abundance was higher in MS. BC significantly reduced the abundance of Cd resistance in LS and MS before immobilization. However, the abundance of Cd resistance increased substantially in the three soils after 180 days of incubation, while rice husk biochar significantly increased the abundance of Cd resistance genes in MS and HS, which was consistent with rice husk biochar significantly reducing the absolute content of acid-soluble Cd in both soils. The expression of the Cd resistance function was more prominent in medium and heavy Cd-contaminated soils. Overall, the expression of Cd transport-related genes in soil was influenced by various factors such as biochar treatment, pollution levels, and incubation time.
Furthermore, Pearson correlation was conducted to determine the relationship between Cd transport-related functions and dominated bacterial phylum in Cd-contaminated soils (Fig. S2). Chloroflexota, Bacillota, and Actinomycetota were significantly and positively correlated with the abundance of Zn2+/Cd2+-exporting ATPase, ABC-2 type transport system ATP-binding protein, and ABC-2 type transport system permease protein, which indicated that these microbial communities were stronger detoxified by themselves. Moreover, Pseudomonadota were significantly correlated with the abundance of Zn2+/Cd2+-exporting ATPase, cobalt-zinc-Cd efflux system protein, ABC-2 type transport system ATP-binding protein, and ABC-2 type transport system permease protein, especially significantly facilitating Cd efflux behaviors in microorganisms. These indicated that the majority of Cd-related functions belonged to the Actinomycetota and Pseudomonadota (Yan et al. 2020). In addition, the abundance of Cd resistance were also significantly and positively linked to Acidobacteriaota, Armatimonadota, and Planctomycetota, which indicated their possible involvement in the transformation of Cd forms in the soils as well.
4 Conclusion
Rice husk biochar (BC) was effective for Cd immobilization, especially in LS and MS. In addition to AN and sucrase, BC significantly improved soil properties such as pH, SOM, CEC, AP, AK, and catalase in all three soils. Notably, BC had no significant effect on the structure of microbial communities in the three soils, but incubation time and pollution levels had significant effects on bacterial community structure, and the structure of fungal communities was only significantly affected by pollution levels. Moreover, BC addition increased the association between microbial communities in LS and HS but inhibited in MS. At the same time, BC significantly affected the expression of Cd transport-related functions in the microbial communities of the three soils, and the expression of these functions was more prominent in the soils with heavier Cd pollution. Pseudomonadota may have played an important role in the efflux of Cd; Chloroflexota, Bacillota, and Actinomycetota in microbial detoxification; and Acidobacteriaota, Armatimonadota, and Planctomycetota in Cd tolerance. BC could not only immobilize Cd and improve soil fertility but also not significantly disturb the structure of indigenous microbial communities; this made it a better amendment agent for the treatment of Cd -contaminated soil in the future.
References
Ameloot N, Graber ER, Verheijen FGA, De Neve S et al (2013) Interactions between biochar stability and soil organisms: review and research needs. Eur J Soil Sci 64:379–390. https://doi.org/10.1111/ejss.12064
Chen D, Liu X, Bian R, Cheng K, Zhang X, Zheng J, Joseph S, Crowley D, Pan G, Li L (2018a) Effects of biochar on availability and plant uptake of heavy metals–a meta-analysis. J Environ Manage 222:76–85. https://doi.org/10.1016/j.jenvman.2018.05.004
Chen Y, Chen F, Xie M, Jiang Q, Chen W, Ao T et al (2020a) The impact of stabilizing amendments on the microbial community and metabolism in cadmium-contaminated paddy soils. Chem Eng J 395:125132. https://doi.org/10.1016/j.cej.2020.125132
Chen L, Guo L, Zhou Q, Liu M, Zhan S, Pan X, Zeng Y et al (2020b) Response of soil fertility and Cu and Cd availability to biochar application on paddy soils with different acidification levels. Biomass Convers Biorefin 12:1493–1502. https://doi.org/10.1007/s13399-020-00917-5
Chen Y, Jiang Y, Huang H, Mou L, Ru J, Zhao J, Xiao S et al (2018b) Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci Total Environ 637–638:1400–1412. https://doi.org/10.1016/j.scitotenv.2018.05.109
Dai W, Zhang P, Yang F, Wang M, Yang H, Li Z, Wang M, Liu R, Huang Y, Wu S, He G, Zhou J, Wei C et al (2022) Effects of composite materials and revegetation on soil nutrients, chemical and microbial properties in rare earth tailings. Sci Total Environ 850:157854. https://doi.org/10.1016/j.scitotenv.2022.157854
Deng Y, Jiang Y-H, Yang Y, He Z, Luo F, Zhou J et al (2012) Molecular ecological network analyses. BMC Bioinf 13:113. https://doi.org/10.1186/1471-2105-13-113
Derakhshan Nejad Z, Jung MC, Kim KH et al (2018) Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ Geochem Health 40:927–953. https://doi.org/10.1007/s10653-017-9964-z
El-Naggar A, Chen Z, Jiang W, Cai Y, Chang SX (2022) Biochar effectively remediates Cd contamination in acidic or coarse- and medium-textured soils: a global meta-analysis. Chem Eng J 442:136225. https://doi.org/10.1016/j.cej.2022.136225
Fan K, Weisenhorn P, Gilbert JA, Chu H et al (2018) Wheat rhizosphere harbors a less complex and more stable microbial co-occurrence pattern than bulk soil. Soil Biol Biochem 125:251–260. https://doi.org/10.1016/j.soilbio.2018.07.022
Rauret G, AJFL P-SN, Sahuquillo AA , Rubio R, Davidson C, Ureb A, Quevauvillerc P et al (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61. https://doi.org/10.1039/A807854H
Godlewska P, Schmidt HP, Ok YS, Oleszczuk P et al (2017) Biochar for composting improvement and contaminants reduction: a review. Bioresour Technol 246:193–202. https://doi.org/10.1016/j.biortech.2017.07.095
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H et al (2015) Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric, Ecosyst Environ 206:46–59. https://doi.org/10.1016/j.agee.2015.03.015
Li X, Wang T, Chang SX, Jiang X, Song Y (2020) Biochar increases soil microbial biomass but has variable effects on microbial diversity: a meta-analysis. Sci Total Environ 749:141593. https://doi.org/10.1016/j.scitotenv.2020.141593
Li D, Lai C, Li Y, Li H, Chen G, Lu Q (2021) Biochar improves cd-contaminated soil and lowers Cd accumulation in Chinese flowering cabbage (Brassica parachinensis L.). Soil Tillage Res 213:105085. https://doi.org/10.1016/j.still.2021.105085
Liu Q, Sheng Y, Wang W, Liu X et al (2021) Efficacy and microbial responses of biochar-nanoscale zero-valent during in-situ remediation of Cd-contaminated sediment. J Cleaner Prod 287:125076. https://doi.org/10.1016/j.jclepro.2020.125076
Lu RK (1999) Analytical methods of soil agrochemistry. Chinese Agriculture Science and Technology Press, Beijing
Luo M, Lin H, He Y, Zhang Y et al (2020) The influence of corncob-based biochar on remediation of arsenic and cadmium in yellow soil and cinnamon soil. Sci Total Environ 717:137014. https://doi.org/10.1016/j.scitotenv.2020.137014
Ma B, Wang J, Zhang L (2023) Two cadmium-resistant strains of agricultural soil effective in remediating soil cadmium pollution. J Environ Chem Eng 11:111189. https://doi.org/10.1016/j.jece.2023.111189
Mei C, Wang H, Cai K, Xiao R, Xu M, Li Z, Zhang Z, Cui J, Huang F et al (2022) Characterization of soil microbial community activity and structure for reducing available Cd by rice straw biochar and Bacillus cereus RC-1. Sci Total Environ 839:156202. https://doi.org/10.1016/j.scitotenv.2022.156202
Rinklebe J, Shaheen SM (2017) Geochemical distribution of Co, Cu, Ni, and Zn in soil profiles of fluvisols, luvisols, gleysols, and calcisols originating from Germany and Egypt. Geoderma 307:122–138. https://doi.org/10.1016/j.geoderma.2017.08.005
Shi Q, Jin X, Fan R, Xing M, Guo J, Zhang Z, Zhang J, Xu S et al (2019) Cadmium-mediated miR-30a-GRP78 leads to JNK-dependent autophagy in chicken kidney. Chemosphere 215:710–715. https://doi.org/10.1016/j.chemosphere.2018.10.019
Sun T, Xu Y, Sun Y, Wang L, Liang X, Zheng S et al (2021) Cd immobilization and soil quality under Fe-modified biochar in weakly alkaline soil. Chemosphere 280:130606. https://doi.org/10.1016/j.chemosphere.2021.130606
Szeri F, Corradi V, Niaziorimi F, Donnelly S, Conseil G, Cole SPC, Tieleman DP, van de Wetering K et al (2021) Mutagenic analysis of the putative ABCC6 substrate-binding cavity using a new homology model. Int J Mol Sci 22:6910. https://doi.org/10.3390/ijms22136910
Tian Z, Li G, Tang W, Zhu Q, Li X, Du C, Li C, Li J, Zhao C, Zhang L et al (2022) Role of Sedum alfredii and soil microbes in the remediation of ultra-high content heavy metals contaminated soil. Agric, Ecosyst Environ 339:108090. https://doi.org/10.1016/j.agee.2022.108090
Tu C, Wei J, Guan F, Liu Y, Sun Y, Luo Y et al (2020) Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ Int 137:105576. https://doi.org/10.1016/j.envint.2020.105576
Wang H, Wang G, Huang Y, Chen J, Chen M et al (2008)The effects of pH change on the activities of enzymes in an acid soil. Ecology and Environ 17:2401–2406. https://doi.org/10.16258/j.cnki.1674-5906.2008.06.056
Wang Y, Ren Q, Li T, Zhan W, Zheng K, Liu Y, Chen R et al (2021) Influences of modified biochar on metal bioavailability, metal uptake by wheat seedlings (Triticum aestivum L.) and the soil bacterial community. Ecotoxicol Environ Saf 220:112370. https://doi.org/10.1016/j.ecoenv.2021.112370
Wong CKE, Jarvis RS, Sherson SM, Cobbett CS et al (2009) Functional analysis of the heavy metal binding domains of the Zn/Cd-transporting ATPase, HMA2, in Arabidopsis thaliana. New Phytol 181:79–88. https://doi.org/10.1111/j.1469-8137.2008.02637.x
Wu C, Shi L, Xue S, Li W, Jiang X, Rajendran M, Qian Z et al (2019) Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. Sci Total Environ 647:1158–1168. https://doi.org/10.1016/j.scitotenv.2018.08.087
Wu X, Hu H, Li S, Zhao J, Li J, Zhang G, Li G, Xiu W et al (2022) Chemical fertilizer reduction with organic material amendments alters co-occurrence network patterns of bacterium-fungus-nematode communities under the wheat–maize rotation regime. Plant Soil 473:605–623. https://doi.org/10.1007/s11104-022-05314-7
Xie Y, Bu H, Feng Q, Wassie M, Amee M, Jiang Y, Bi Y, Hu L, Chen L (2021) Identification of Cd-resistant microorganisms from heavy metal-contaminated soil and its potential in promoting the growth and Cd accumulation of bermudagrass. Environ Res 200:111730. https://doi.org/10.1016/j.envres.2021.111730
Xu M, Dai W, Zhao Z, Zheng J, Huang F, Mei C, Huang S, Liu C, Wang P, Xiao R et al (2022) Effect of rice straw biochar on three different levels of Cd-contaminated soils: Cd availability, soil properties, and microbial communities. Chemosphere 301:134551. https://doi.org/10.1016/j.chemosphere.2022.134551
Yan C, Wang F, Liu H, Liu H, Pu S, Lin F, Geng H, Ma S, Zhang Y, Tian Z, Chen H, Zhou B, Yuan R et al (2020) Deciphering the toxic effects of metals in gold mining area: microbial community tolerance mechanism and change of antibiotic resistance genes. Environ Res 189:109869. https://doi.org/10.1016/j.envres.2020.109869
Yang; X, Lu K, McGrouther K, Che L, Hu G, Wang Q, Liu X, Shen L, Huang H, Ye Z, Wang2 H (2017) Bioavailability of Cd and Zn in soils treated with biochars derived from tobacco stalk and dead pigs. J Soils Sediments 17:751–762. https://doi.org/10.1007/s11368-015-1326-9
Yang J, Yang F, Yang Y, Xing G, Deng C, Shen Y, Luo L, Li B, Yuan H et al (2016) A proposal of “core enzyme” bioindicator in long-term Pb-Zn ore pollution areas based on topsoil property analysis. Environ Pollut 213:760–769. https://doi.org/10.1016/j.envpol.2016.03.030
Yu H, Zou W, Chen J, Chen H, Yu Z, Huang J, Tang H, Wei X, Gao B et al (2019) Biochar amendment improves crop production in problem soils: a review. J Environ Manage 232:8–21. https://doi.org/10.1016/j.jenvman.2018.10.117
Zhang M, Zhang L, Riaz M, Xia H, Jiang C et al (2021) Biochar amendment improved fruit quality and soil properties and microbial communities at different depths in citrus production. J Cleaner Prod 292:126062. https://doi.org/10.1016/j.jclepro.2021.126062
Zhang H, Zhang R, Li W, Ling Z, Shu W, Ma J, Yan Y (2022) Agricultural waste-derived biochars from co-hydrothermal gasification of rice husk and chicken manure and their adsorption performance for dimethoate. J Hazard Mater 429:128248. https://doi.org/10.1016/j.jhazmat.2022.128248
Funding
This research was financially supported by the Science and Technology Project of Guangzhou City, China (202103000018) and the Key-Area Research and Development Program of Guangdong Province (2019B110207001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Yuan Ge
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, M., Xiao, R., Mei, C. et al. Rice husk biochar reduces Cd availability by affecting microbial community activity and structure in Cd-contaminated soils. J Soils Sediments 24, 1764–1776 (2024). https://doi.org/10.1007/s11368-023-03711-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03711-8