Abstract
Purpose
Soil organic nitrogen (SON) biochemistry trends in paddy soils are poorly understood on a long-term scale.
Methods
To explore the effect of land use on SON sequestration, SON and amino acid (AA) fractions were investigated in soil profiles comprising recent and buried paddy soil (BPS) and buried non-paddy soils (BNS). Two ancient paddy soils from Chuodun ruin site, China, were distinguished based on colour and rice phytolith abundance. 14C abundance in soil organic carbon was used to estimate the age of carbonized rice and ancient paddy soil via a liquid scintillation analysis method, dating to 3800–5500 and 960–4000 BC.
Results
The proportions of D-AAs and acidic AAs in BPS, up to 6.13% and 7.73%, respectively, were higher than those in modern paddy soils. D-alanine (and the D-/L- ratio), aspartate, and glutamate increased with soil depth in BPS, and the amount of D-aspartate was linearly and significantly positively correlated with soil depth (p < 0.05). Based on phytolith stability and abundance, the N sequestration rate (NSR: residual N content as a proportion of initial N content) was proposed to indicate the residual N content varied with time. The NSR was estimated as 10.8–91.2% in BPS with a phytolith stability factor of 0.5–0.9.
Conclusion
These data suggest that intermittent continuous high-intensity rice cultivation could increase soil N sequestration potential over the long term, and that N sequestration is not only associated with AA aging in the organic N fraction, but also with biogeochemical processes in BPS and paddy management. In addition, high-intensity rice cultivation can increase N loss risks, and in turn result in large fluctuations in N sequestration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrogen (N), an essential element for plant growth and development, is mainly present in soil in the form of organic matter such as peptides and amino acids (Jones and Kielland 2012), in addition to some amino sugars, nucleic acids, and alkaloids (Knicker 2010). Soil organic N (SON) accounts for approximately ≥ 95% of total soil N (Schulten and Schnitzer 1997). Any change in SON amount and turnover could impact the soil N cycling and N2O emission rates (Farzadfar et al. 2021; van Groenigen et al. 2015). Previous studies have revealed that SON is a potential key source of N during rice cultivation (Hirzel et al. 2012). Investigating N storage and use dynamics over the long term could enhance our understanding of N cycling dynamics in paddy fields and paddy soil biogeochemistry.
Archaeological and archaeobotanical studies have identified prehistoric (Neolithic age) paddy soils at the Chuodun site in the Yangtze Delta, China (Cao et al. 2006; Li et al. 2007), which have provided case studies of sequences of up to four buried ancient and modern paddy soils (MPS) in two adjacent soil pedons, with a focus on combustion-derived organic matter (Cao et al. 2006), and, in the present case, soil organic N dynamics over a long-term scale.
SON fractions are diverse, including ammonium-N (NH4+-N), amino acid N (AAN), amino sugar N (ASN), unknown hydrolyzed N (UHN), and non-hydrolyzed N (NHN) (Bremner 1965). AAN accounts for 33.1–41.7% of hydrolyzed N, while ASN and NH4+-N account for 4.5–7.4% and 18.0–32.0% of hydrolyzed N, respectively (Sowden et al. 1977). NH4+-N is generally considered a temporary N pool harboring a high amount of easily mineralized organic N, and AAN acts primarily as a transitional N pool, so both NH4+-N and AAN are potential indicators of soil N supply potential (Lü et al. 2013). ASN content is significantly correlated with soil N supply potential and is often applied as an indicator of microbial processes during soil N cycling (Wang et al. 2010; He et al. 2011). UHN, which includes heterocyclic N, products of soil humification processes and immobilized NH4+-N not released by partial hydrolysis, is the main contributor to soil labile organic N (Kelley and Stevenson 1995). NHN is often closely associated with UHN, a compound containing heterocyclic N, such as proteins (Stevenson 1982); therefore, NHN forms humic substances with stable structures (Leinweber and Schulten 1998; Piper and Posner 1972). Sealed-tube hydrolysis is one of the techniques that has been applied in SON fraction analyses (Stevenson 1982). After hydrolysis, different fractions of organic N can be extracted for use in SON trend and long-term N-use effectiveness assessments. The method applied in the present study provides a quantitative estimate of the different organic N fractions and soil N supply potential (Wang et al. 2017; Pan et al. 2022).
SON fractionation and distribution are key factors influencing soil N availability. Soil N-use effectiveness decreases following long-term paddy rice cultivation, although total soil N contents remain almost constant (Olk et al. 1996). In addition, SON mineralization appears to be inhibited under flooded conditions (Olk et al. 2007), and AAN contents can be highly correlated with mineralizable soil N potential due to the higher contents of hydrolysable amino acids (AAs) in the topsoil than in the subsoil (Gotoh et al. 1986). Binding of amide N to the aromatic ring (Schmidt-Rohr et al. 2004), and the resistance of D-alanine in the bacterial cell wall to chemical degradation, limits further decomposition of organic N (Amelung et al. 2006). Thus, it is assumed that biogeochemical processes under long-term rice cultivation will lead to AA aging (Houtermans et al. 2017), particularly the formation of D-AAs as markers of SON aging (Amelung et al. 2008). Overall, our understanding of SON and AA fractionation and distribution in paddy soils under long-term rice cultivation remains limited.
Here, for the first time, the distribution of organic N is explored in modern to ancient paddy soil horizons in two adjacent soil profiles. We hypothesized that N sequestration will be affected by the intermittent and continuous rice cultivation in a long term. The aim of this study was to investigate the temporal variability of organic N and AAs in buried paddy soils (BPS) and buried non-paddy soil (BNS), which could provide insights into SON, N sequestration, and AA dynamics under intermittent and continuous rice cultivation and organic N management.
2 Materials and methods
2.1 Study site description
Chuodun site (31°24′12″N, 120°50′1.5″E) is located in the east of the Taihu lake alluvial plain at 3 to 4 m above sea level (Fig. 1). Northern subtropical mixed forests with broadleaf deciduous and evergreen trees characterize the natural vegetation of the Taihu Lake watershed. Long-term human activities have considerably modified the pristine vegetation, and most of the natural forests in the densely populated areas have been cleared. The northern part of Chuodun site is at a low altitude, and therefore, was reclaimed for rice cultivation. Chuodun site, considered an ancient paddy soil, was excavated and identified in 2003 (Cao et al. 2006).
Pedons sampled at Chuodun excavation site in China. Pedon 01 covers three soil layers with three respective paddy soil pedocomplexes formed on loess. Pedon 03 includes four soil layers with paddy management in the topmost horizon and a relictic complex, paddy B horizon at 50–60 cm. Most horizons formed in pedon 03 have been under non-paddy management
2.2 Soil sampling and classification
At the Chuodun excavation site, two soil profiles of the ancient paddy fields were sampled, including P01, from the northeastern part of Unit VI of the site, and P03, 30 m southeast of P01 (Fig. 1). The boundary of each rice field was confirmed based on the abundance of carbonized rice and distribution of ridges and ditches, as well as the plant opal content. Rice cultivation intensity was classified based on phytolith abundance in soils. Soils with > 7000 pellets of rice phytoliths g−1 soil were defined as intensively cultivated paddy soils (modified from Hiroshi 1976 and Cao et al. 2006), whereas those with < 7000 pellets of rice phytoliths g−1 soil were defined as weakly cultivated soils, i.e. non-paddy soils (Table 1). Soil ages were estimated based on radiocarbon data and archaeological context (Cao et al. 2006; Zheng et al. 2009). The description and sampling of soil pedons were carried out according to the WRB soil classification (FAO 2006). Pedons at P01 and P03 were both sampled from the surface to the parent material layer derived from aeolian Xiashu loess (Yang et al. 2007). Detailed sampling information is presented in Table 1.
The top horizons of both pedons under study have been under paddy management recently and were a dark brown colour. However, management history differed in the subsoil horizons. P01 consisted of soil material likely formed under continuous paddy management, as indicated by high rice phytolith abundance. The modern topsoil reached a 42 cm depth and was formed on a paddy pedocomplex developed between 960 and 4000 BC (Tables 1 and 2). From the 100 to 160 cm depth, a second buried pedocomplex was observed, which was differentiated into three humus-rich buried topsoil horizons (Ah) underlain by a mottled subsoil horizon (Bw), and formed on loess substrate (Fig. 1; Table 1).
In the P03 pedon, paddy management started relatively recently, since rice phytolith abundance was generally < 7000 pellets g−1 soil from the B horizon, at a depth of 23–50 cm (Table 1). However, four pedocomplexes that developed between phases of flooding and sediment burial could be distinguished. Layer 1 is a recent paddy soil and developed to a thickness of 50 cm, including a humus-rich Ah horizon, a dense plough pan, and two subsoil horizons with redoximorphic features (Table 1; Fig. 1). Layer 2, identified in the 50–60 cm depth, was most likely a fragment of a B horizon under paddy management, based on the number of rice phytoliths. Layer 3 was dated to the Majiabang culture (3800 to 5000 BC) and includes A and B horizons differentiated by colour, which formed under non-paddy management (Table 1; Fig. 1). Layer 4 is soil formed on the parent material (loess) and potentially has never been under any management since no phytoliths were observed.
An examination of both pedons revealed that redoximorphic features are typical in paddy soils. The “paddy” pedon, P01, exhibited more gleyic features (Fig. 1, Table 1), which could be attributed to its frequent submergence.
2.3 Soil physicochemical properties and organic nitrogen fraction analysis
Soil pH was measured in a soil mixture (2.5:1 = water:soil ratio) with 1 M KCl, using a glass electrode (HANNA-PH211A). Soil organic carbon (C; SOC) was measured following organic matter oxidation using the potassium dichromate method. Total N (TN) in soil was measured using the Kjeldahl method.
Briefly, soil organic hydrolyzed N was extracted as follows: 2.5 g soil sample and 8 ml of 6 M hydrochloric acid (HCl) were mixed in ampoules, and the mixtures purged with N2 and sealed. Ampoules were then heated at 110 °C for 12 h. The hydrolyzed mixture was filtered and subsequently neutralized by NaOH addition. Different organic N fractions were distilled using a modified version of the method of Stevenson (1982) (Wang et al. 2017), with the addition of different reagents. Total hydrolyzed nitrogen (THN) was determined by pipetting 5.0 ml of neutralized acid solution into a decoction tube, adding 0.5 g of potassium sulphate mixed with a catalyst, adding 2 ml of concentrated sulphuric acid (H2SO4), and mixing well. Afterwards, the mixtures were heated to 150 °C until bubbles disappeared, followed by a gradual increase in heating temperature to 350 °C until the suspension clarified, and then maintained 350 °C heated for 1 h. After cooling, 10 ml of 10 M NaOH solution was added to the solution, using boric acid as absorbent, and distilled for approximately 4 min until the volume of distillate reached 35 ml. Afterwards, the solution was titrated with 0.0025 M H2SO4. To determine amino acid nitrogen (AAN) contents, 5 ml of neutralized acid solution was pipetted into a boiling tube, 1 ml of 0.5 M NaOH added, and then the acid solution was placed in boiling water until the volume reduced to 2–3 ml. After the acid solution had cooled, 0.50 g of citric acid and 0.1 g of ninhydrin were added, and then the mixture was placed in boiling water again, shaken several times in boiling water for 1 min, and then heated for 9 min. The distillation was started with boric acid, as the absorbent solution, for approximately 4 min, until the volume of distillate reached 35 ml. The microtitrator titrated with 0.0025 M H2SO4. To determine hydrolyzed NH4+-N) contents, 10 ml of neutralizing acid solution was aspirated into a decoction tube, 10 ml of phosphoric acid-boric acid buffer (pH 11.2) added, and distillation started, using boric acid as absorbent, for approximately 2 min, until the volume of distillate reached 20 ml. The distillate was titrated with 0.0025 M H2SO4 on a microtitrator. To determine ASN contents, 10 ml of neutralizing acid solution was aspirated into a decoction tube, and 0.07 g of magnesium oxide added and distillation started with boric acid as absorbent, for approximately 4 min until the volume of distillate reached 35 ml. Afterward, the distillate was titrated with 0.0025 M H2SO4 on a microtitrator. The result of the measurement was a combination of NH4+-N and ASN, and the ASN was calculated using the following formula:
UHN was determined by differential subtraction, as follows:
NHN was also determined by differential subtraction, as follows:
Soil amino acid (AA) contents were measured to determine the degrees of N sequestration in different rice cultivation periods. AAs were extracted and purified as follows. Two grams of 100-mesh-sieved soil samples was hydrolyzed in 6 M HCl in sealed tubes at 105 °C for 12 h with N flushed into the tubes before sealing. After hydrolysis, an internal standard solution containing 100 μg L-norvaline was added to the hydrolysate.
Purification procedures for hydrolysates were according to Amelung and Zhang (2001). Briefly, hydrolysates were dried using a rotary evaporator (< 46 °C) and re-dissolved in 0.05 M HCl. The solutions were purified using a polypropylene sample preparation column filled with 3 g Dowex 50 WX8 cation exchange resin (100–200-mesh, hydrogen form, Sigma-Aldrich, USA). After resin preparation, the AA solution was added. The impurity ions were eluted using oxalic acid, HCl, and Milli-Q water. Finally, AAs were eluted using NH3·H2O and dried in a rotary evaporator immediately. Dried samples were dissolved with 0.1 M HCl and filtered for testing. The OPA/NAC (o-phthalaldehyde and N-acetyl-L-cystein) method was used for the derivatization of AAs (Zhao and Bada 1995). The OPA/NAC reagent was prepared by mixing both 0.2 ml of OPA and NAC stock solution (50 mg/ml) in 1.6 ml of 0.4 M borate buffer (pH 9.8). Purified AA solution (0.2 ml) was mixed with 0.05 ml OPA/NAC reagent in a liquid chromatography auto-sampler vial for 15 min, with 750 ml of 50 mM NaAc (pH 5.4) added immediately to stop the derivatization. An Ultra-Fast Liquid Chromatograph system (UFLC-20, Shimadzu, Japan) with a C18 column was used for AA derivative separation. Column temperature was 30 °C and the flow rate was 1.0 ml/min. The fluorescence detection wavelengths were λex/λem = 340/450 nm. Gradient elution programs of solvent A (50 mM sodium acetate buffer, pH 5.4) and solvent B (methanol) were set as follows: 0–35 min, 10% B; 35–50 min, 22% B; 50–75 min, 40% B; 75–85 min, 45% B; 85–86 min, 75% B; 86–89 min, 10% B.
2.4 Organic nitrogen input and nitrogen sequestration rate estimation
Considering the correlation between phytolith content and plant residues in soil, initial organic N input can be estimated based on phytolith abundance, as follows:
where, INC, initial N content, is the estimated total initial organic N input to soil (g kg−1); SPC is soil phytolith content (g g−1) converted from the number of phytoliths, with a conversion factor of approximately 100,000 phytoliths for 1 g of Poaceae, according to Wang et al. (1991); NCR, N content of rice plants is the average N content of the reference rice plant (g kg−1), which is 4.5 g kg−1 in the present study, according to Yang et al. (2021); PSF, phytolith stability factor, is the residual ratio of phytolith after a long term geochemical process, which is 0.8–1 (PSF = 1 means all phytoliths are found in the soil, smaller values indicate more phytolith loss), according to related studies (Meunier et al. 1999; Parr and Sullivan 2005; Song et al. 2016). In addition, some studies suggest that phytoliths are nearly immobile or have little potential to migrate in the soil (Fisher et al. 1995; Piperno 2006a, b). In contrast, it has been suggested that bioturbation is the main cause of phytolith translocation (Farmer et al. 2005; Humphreys et al. 2003; Runge 1999). Fishkis et al. (2010) found that the transport distance of phytolith in two German forest soils was 3.86 cm year−1 (silty loam soil) and 3.99 cm year−1 (loamy sand soil), respectively. The soil texture in this study is in loamy and clay (Table 1). Hence, we can reasonably hypothesized that the long-term tillage and drainage of the rice field would lead phytolith loss to a greater degree than in the natural ecosystem; accordingly, the phytolith stability factor in the present study was adjusted to 0.5–0.9.
The N sequestration rate (NSR) under continuous high-intensity rice cultivation was calculated using following equation:
where RNC is residual N content as a proportion of INC (%).
The N sequestration rate (NSR) was fitted with an exponential decay equation:
where N0 is the initial N content (g kg−1); Ne is the equilibrium constant (g kg−1); D is the decay index (g year−1).
2.5 Statistical analysis
Data were analyzed in IBM SPSS Statistics 26 (IBM Corp., Armonk, NY, USA). Fitting and graphing were performed using Excel 2016 (Microsoft Corp., Redmond, WA, USA) and Origin Pro 2022 (OriginLab Corporation, Northampton, MA, USA). The least significant difference (Duncan) test was used to test for significant differences between treatments (p < 0.05).
3 Results
3.1 Soil physicochemical properties
The basic properties of the soil profiles are shown in Table 2. pH ranged from 4.94 to 5.24 in the modern topsoil, but it ranged from 5.82 to 6.36 in the modern subsoil. In the soil profile, pH remained relatively stable in buried ancient soils (P01: 100–116 cm, 5.75–5.90; P03: 50–88 cm, 5.94–6.17). In the modern topsoil, SOC content was in the 5.85–11.9 g kg−1 range, and dropped to 4.22–5.71 g kg−1 in the modern subsoil. In P01, there were two buried pedocomplexes with significantly elevated SOC contents (9.9–12.9 g kg−1), in addition to high C/N ratios (11.7–14.4; Table 2, 100–160 cm) and TN contents (0.73–1.02; Table 2, 100–160 cm). In P03, SOC and TN contents decreased with an increase in depth, although there were three buried layers. However, similar to in P01, the older pedocomplex included a topsoil fragment with enhanced SOC, at 8 g kg−1, a C/N of 11.5 and 0.7 g kg−1 TN (Table 2; layer 4, 88–103 cm).
3.2 Distribution of soil organic nitrogen fractions
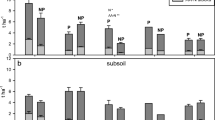
Both THN and NHN in P01 and P03 exhibited decreasing trends with an increase in depth. THN in P01 ranged from 0.89 to 0.24 g kg−1, and THN in P03 ranged from 1.03 to 0.19 g kg−1. THN maintained a stable status in the Majiabang culture layer (Fig. 2a: 100–160 cm; Fig. 2b: 60–88 cm). NHN decreased from 0.36 to 0.05 g kg−1 in P01, and from 0.44 to 0.01 g kg−1 in P03. In the Majiabang culture layer, NHN increased with an increase in cultivation years (Fig. 2a: 5000–3800 BC; Fig. 2b: 5500–3800 BC). The hydrolysis rate of P01 decreased from 71.0% in the MPS (Fig. 2a: 0–15 cm) to 33.2% in the Majiabang culture layer (Fig. 2a: 100–116 cm). In addition, the hydrolysis rate in P03 varied between the MPS (Fig. 2b: 0–13 cm) and the Majiabang culture layer (P03: 70–88 cm). Hydrolysis rate increased with an increase in depth in the parent soil material (PSM) layer, and the highest hydrolysis rate was observed in the PSM layer, reaching 84.0% (Fig. 2a: 174–200 cm) and 94.8% (Fig. 2b: 130–155 cm) in P01 and P03, respectively.
Both the content and proportion of AAN decreased gradually from the MPS to the bottom layer, and remained stable in the Majiabang culture layer (Fig. 3a: 100–160 cm; Fig. 3b: 60–88 cm). AAN content decreased from 0.36 to 0.02 g kg−1, from 0.37 to 0.01 g kg−1, respectively. ASN trends were similar to those of AAN, decreasing from 0.08 g kg−1 in the MPS to 0.00 g kg−1 in the Majiabang culture layer (Fig. 3a: 150–160 cm), and from 0.07 g kg−1 in the MPS to 0.01 g kg−1 in the PSM layer (Fig. 3b: 103–130 cm). NH4+-N showed a fluctuating decrease, from 0.26 g kg−1 in MPS to 0.16 g kg−1 in the Maqiao culture layer, from 0.28 g kg−1 MPS to 0.12 in PSM, respectively. Furthermore, UHN contents decreased gradually from the MPS (0.19–0.04 g kg−1) to the Majiabang culture layer (0.31–0.05 g kg−1) (Fig. 3a, b).
3.3 Differences in soil organic nitrogen fractions under different rice cultivation intensities
THN, AAN, UHN, and NHN in BPS (phytolith > 7000 pellet g−1 soil) were 0.34, 0.04, 0.07, and 0.40 g kg−1, respectively, and higher than those in BNS (phytolith < 7000 pellet g−1 soil; mean value: 0.30, 0.02, 0.05, and 0.28 g kg−1, respectively; Table 3). In addition, NH4+-N and ASN contents in BNS (0.22 g kg−1 and 0.01 g kg−1) were higher than in BPS (0.21 g kg−1 and 0.01 g kg−1). Conversely, BPS had lower organic N fractions than MPS, excluding in the case of NHN, whereas the organic N fractions of BPS were all higher than those of the PSM.
The proportion of THN in BPS was lower than those in BNS, MPS, and PSM (Fig. 4a), and there was a marked increase in the proportion of AAN in MPS compared to that in BPS (Fig. 4b). The proportion of NH4+-N in BNS was higher than that in BPS. The proportions of AAN and NH4+-N in BPS were higher than those in PSM.
3.4 Composition and D-/L- ratio of amino acids in buried paddy soil
AA compositions in MPS and BPS are shown in Figs. 5a and 6a. Total AA contents decreased with an increase in soil depth, and decreased from 17.7 to 0.67 μmol g−1; however, an increase occurred (5.07 μmol g−1) in the Song Dynasty BPS layer (42–57 cm). D-AA contents were lower than L-AA contents in all soil profiles. In addition, D-AA contents were the highest in the surface layer and decreased with an increase in depth; however, the overall decreasing trend was more gradual than that of L-AA, and the contents (0.20 μmol g−1) were higher in the Song Dynasty in BPS layer. However, D-AA contents increased, from 2.06 to 6.13%, whereas L-AA contents decreased, from 89.8 to 73.5%, with an increase in soil depth (Fig. 5b). Further analysis of the trends of differently charged AAs (Acidic AA, Neutral AA, and Alkaline AA) showed that the contents of all the differently charged AAs decreased with an increase in soil depth (Fig. 6a); however, the contents increased in the Song Dynasty BPS layer. The MPS (0–15 cm) had the highest proportion of neutral AAs and the lowest proportion of alkaline AAs, whereas the Song Dynasty BPS (42–57 cm) had the highest proportion of acidic AAs (Fig. 6b).
Both D-aspartate and D-alanine contents in addition to their D/L ratios increased with an increase in soil depth (Fig. 7). In addition, both D-glutamate proportion and its D/L ratio showed maximum values (0.28% and 0.06) in the 42–57 cm soil layer; however, D-glutamate was not observed in the 57–75 cm soil layer in P01.
4 Discussion
4.1 Distribution characteristics of soil basic properties in buried ancient layers
According to the archaeological diagnosis and 14C analyses, the buried ancient soils in Chuodun site could be dated back to 4000 BC, the Majiabang culture period (Neolithic age, Cao et al. 2006; Zheng et al. 2009). Up to four pedogenetic phases were observed, which were potentially separated by flooding, sedimentation, or erosion events. Hence, we distinguished P01 into a layer from the Mid Majiabang Culture (3800–5000 years BC) formed on loess, which was likely cut by a sedimentation event, leading to the formation of a 60-cm-thick second layer of the Song Dynasty, and the Maqiao Culture (960–3800 years BC). Another sedimentation event followed afterward, which developed the recent soil. Based on the considerable number of phytoliths, > 100,000 pellets g−1, all P01 layers had been under paddy management, whereas, in P03, exclusively non-paddy-managed soil relicts could be observed. Accordingly, we could compare paddy and non-paddy horizons to elucidate the millennial-scale dynamics of organic N fractions under rice agriculture.
High SOC and TN contents in buried P01 layers not only indicated notable preservation of cultivated soils but also could be attributed to typically observed increases in C and N under oxygen-depleted paddy management (Kukal et al. 2009; Houtermans et al. 2017). In a comparison of “paddy” P01 to “non-paddy” P03, the latter had less SOC and TN in buried horizons, although burial conditions after sedimentation events were comparably favourable for organic matter preservation. As reported earlier, SOC in the ancient paddy soils was present to a large extent in aromatic forms, and thus was a rather stable C form (Cao et al. 2006), unveiling charred material as the major organic matter constituent. Because prehistoric farmers adopted the “ploughing with fire and weeding with water” agricultural management practice, burned rice straw remained on the field and increased carbonized SOM, and the practice has remained widespread in Chinese rice cropping systems in recent times (Cao et al. 2006). Hence, SOC and N stored in the buried non-paddy layers were relatively easily decomposable. A significant proportion of SOC is derived from rice straw combustion, which is consistent with black C analysis results, which have revealed that up to 30% of SOC could be attributed to BC in Chinese paddy crop rotation systems (Cao et al. 2006).
4.2 Distribution characteristics of organic nitrogen in buried paddy soils
The contents of different hydrolyzed organic N fractions decreased with an increase in depth, which is similar to the results of previous studies on other soil types (Dick 1983; Franzluebbers and Stuedemann 2009). THN content increased slightly in the ancient paddy soil layer (P01: 42–57 cm; P03: 50–60 cm). This may be due to organic N accumulation in the surface layer of the BPS, which has a relatively slow organic matter decomposition rate (Neue et al. 1997; Tong et al. 2009). We also observed that BPS had the lowest hydrolysis rate (Fig. 2a, 100–150 cm, from 33.22 to 37.22%). BPS had the highest NHN content (0.40 g kg−1; 52.09%), which may be related to the anaerobic environment under long-term rice cultivation that reduces the organic matter decomposition rate and the plough pan formed by tillage. Wang et al. (2017) reported that NHN content increased with an increase in rice cultivation duration in a reclaimed coastal tidal flat. In the present study, BPS layers showed evidence of high-intensity human rice cultivation in 5000–3800 B.C. (Table 1, 100–160 cm, 19,678–105,159 rice phytolith pellets). In addition, BPS layers retained high amounts of rice roots and plant residues (Table 2), which may be due to the high amounts of organic matter entering the soil and being sequestered or fixed during rice cultivation. According to Ren et al. (2016), the main source of NHN is humic substances imported into soil, and it is a stable component of humus. In addition, N fixation has been associated with rice roots, at about 1–7 kg N ha−1 per rice season (Roger and Ladha 1990). Since NHN is difficult to mineralize, it is more likely to be stored in the soil (Tian et al. 2017). By comparing the N mineralization rates between BPS and MPS in Chodun site in a 103-d incubation experiment, Lu et al. (2009) reported that the cumulative N mineralization in BPS was much lower than that in MPS. NHN, a difficult-to-use organic N, usually exists in the form of heterocyclic N or aromatic and heterocyclic compounds (Ma et al. 2021), which are very stable forms of organic N and, therefore, can serve as fixed-storage N pools.
THN content was stabilized gradually in the bottom layer. AAN showed a decreasing trend with increase in depth, whereas NH4+-N was maintained at a high level, indicating that AAN transfer to the bottom layer was difficult, whereas NH4+-N was more likely to diffuse to the bottom layer. Hence, organic N accumulation was likely to be accelerated following long-term rice cultivation; in particular, AAN and NH4+-N contents increased significantly in BPS compared to in BNS. Such shifts in soil properties due to rice cultivation influence organic N sequestration. Under rice cultivation, the soil N pool gradually increases, and hydrolytic organic N and other organic N fractions have exponential growth trends similar to that of total N (Wang et al. 2017). Furthermore, high N fertilizer and organic manure inputs (Li et al. 2010), nutrient from rice plant residue (Lal 2002), low rates of C and N decomposition under flooding conditions (Neue et al. 1997), increase in abundance of N-fixing microorganisms in paddy soils (Bannert et al. 2011), and formation of dense plough pans (Huang et al. 2015) could all reduce N leaching. In addition, flooded rice cultivation conditions may increase pH in acidic soil and decrease pH in alkaline soil (Sahrawat 2005). Such shifts in soil pH also enhance or minimize soil organic N accumulation. The potential for organic N sequestration in soil is not only influenced by pH (maximum at pH 4–5, decreasing at pH 15) but is also associated with clay (relationship with mineral surface) and ion contents (Shen 1999). Typically, AAN and UHN are more stable than NH4+-N and ASN, and the rates of accumulation of stable fractions are higher than those of labile fractions (Mulvaney et al. 2001). However, our results were not consistent with the above findings. According to our results, AAN and UHN were more unstable than NH4+-N and ASN. In addition, AAN had a broader content range than UHN (AAN: 0.04–0.22 g kg−1; UHN: 0.07–0.18 g kg−1, Table 3). The observation could be attributed to an increase in SON-synthesizing microbial communities (Bannert et al. 2011; Nemergut et al. 2007). Additionally, the increase in the labile N pool could be due to the slow mineralization and decomposition rates of the stable N pool, resulting in increases in all organic N fractions (Rovira and Vallejo 2002). In particular, AAN transformation plays a more important role in N sequestration than UHN.
4.3 Amino acids as indicators of nitrogen aging in buried paddy soils
AAN decomposition plays a major role in N conservation in the course of N cycling (Macdonald et al. 2014). In P01, layers at 42–57 cm and 100–160 cm are the buried ancient paddy soil, where the total amino acids accumulated slightly due to rice cultivation (Cao et al. 2006). In the present study, L-type amino acids were the major components of total amino acids, similar to previous studies (Roth et al. 2013; Pan et al. 2022). This is mainly due to soil amino acids being synthesized by most organisms mainly as L-type amino acids. In the present study, L-type amino acids were predominant in all layers of paddy soils, whereas D-type amino acid contents were significantly lower (Fig. 5a). However, L-type amino acids can be transformed into D-type amino acids gradually and slowly (Penkman 2017), so that D-type amino acids increase gradually with an increase in cultivation years (Fig. 5b). D-type amino acids are essential for the characterization of peptidoglycan and vary among bacteria; therefore, differences in the distributions of different D-type amino acids could also reflect differences in soil microbial communities (Radkov and Moe 2014).
Cell walls of microorganisms typically have high proportions of alanine, aspartate, and glutamate, which also represent major sources of amino acids in the soil (Friedel and Scheller 2002; Yu et al. 2002). In the present study, D-alanine, D-aspartate, and D-glutamate contents increased with an increase in depth in the soil layer. In addition, ratio of D-aspartate content to the total amino acid content exhibited a significant positive linear correlation with depth, indicating that aspartate contents could be used to identify the depth of the identified paddy soil, and suggesting relatively slow rates of degradation of organic matter containing D-aspartate in the BPS. Some studies have reported D-alanine and D-glutamate as potential ideal markers of bacterial N sequestration (Amelung 2003; Kimber and Hare 1992). In the present study, the highest proportions of D-glutamate out of the total amino acid contents were observed in the 42–57-cm buried soil layer, whereas the highest proportions of D-alanine out of the total amino acid content were observed in the 57–75-cm buried soil layer (Fig. 7). The difference could be attributed to varied synthesis trends by different microbial groups in the soil. D-alanine is an essential component of peptidoglycan in the extracellular and periplasmic space, and it is deposited following cell death (Amelung et al. 2008). The D-/L- ratio of amino acids can reveal sample history, based on capacity to be relatively stable over long time scales (Schultz and Moini 2003). Similarly, we observed that the D-/L- ratios of the more ancient BPS were all higher than those of MPS (Fig. 7), which also indicates that BPS have more stable N and sequester N in the form of AAN.
Amino acids can be grouped according to their relative charge, including acidic amino acids: aspartate, glutamate; alkaline amino acids: lysine, arginine, histidine; and neutral amino acids: all other amino acids (Fischer et al. 1998). In the present study, with an increase in soil depth, the proportions of neutral amino acids decreased whereas the proportions of alkaline and acidic amino acids first increased and then stabilized, which is consistent with the findings of Belluomini et al. (1986), who observed that the proportions of amino acid types in six samples from a Pliocene–Pleistocene clay sequence had regular distribution across the series, with neutral amino acids being the most abundant, and basic and acidic amino acids accounting for approximately 24% and 11% of the total amino acids, respectively. The trend could be related to shifts in soil particle structure. Keil et al. (1998) also confirmed that alkaline amino acids are easily lost in sand particles and easily conserved in clay particles. Following long-term paddy soil cultivation, a plough substrate is formed, and clay content in the soil usually increases below the plough substrate (Table 2), which increases the proportion of alkaline content in BPS. This may be responsible for the increase and sequestration of N in clay soils after long-term rice cultivation (Nakahara et al. 2016).
4.4 Effect of continuous high-intensity rice cultivation on soil nitrogen sequestration
According to our results, continuous high-intensity rice cultivation increased soil N accumulation, and N content trends were consistent with rice cultivation intensity. Similarly, according to Lu et al. (2009), increased N contents under high-intensity rice cultivation could be due to biological N2 fixation. Organic materials such as straw and residues enter the soil during paddy management under high-intensity rice cultivation (Cao et al. 2006). Although organic matter input may increase soil N supply, it also causes N immobilization (Toriyama et al. 2020). Straw burning followed by flooding was used extensively in Neolithic rice cultivation (Gu 1998; Cao et al. 2006), which may have increased N losses in the period (Bird et al. 2001). We used the number of rice phytoliths to estimate N sequestration rate and found that an increase in rice cultivation intensity decreased N sequestration rate (Fig. 8). Numerous studies have confirmed that paddy fields have high N losses during rice cultivation, despite high N fertilizer input (Galloway et al. 2008; Ju et al. 2009). In the present study, the initial N content in BPS was estimated to be 13.1–23.6 g kg−1 and N sequestration rate was estimated 10.8–91.2% (Table 4), when rice phytolith loss rate was assumed to be < 50% (usually considered to be < 20% (Song et al. 2016); therefore, we suggest that there is a positive correlation between phytolith abundance and N sequestration potential. Zhao et al. (2016) suggested that the phytolith content could be increased by adding N. However, Tan et al. (2021) reported a negative correlation between phytolith abundance and total N, and concluded that soil nutrient availability varies at large spatial scales. Consequently, our results demonstrated that soil N sequestration potential under continuous high-intensity rice cultivation is influenced not only by organic matter and nutrient inputs but also by N loss intensity from paddy fields.
5 Conclusion
In the present study, the excavation of two soil pedons at the Chuodun archaeological site in China facilitated comparison of NSR in paddy cultivated buried soil horizons that dated back to the Neolithic age. As expected, the AAN status was a key factor controlling N transformation, and the increase in D-AAs, D/L ratio of AAs, and alkaline AAs provided evidence of N aging and storage in BPS. According to the results of the present study, continuous high-intensity rice cultivation not only enhanced NSR but also increased N loss rates. Hence, N sequestration dynamics under rice cultivation is associated with organic matter input and field management. Strategies of enhancing soil N sequestration potential over the long term should be investigated further.
Data availability
Data are available upon request to the corresponding author (Shunyao Zhuang).
Abbreviations
- AAN:
-
Amino acid nitrogen
- ASN:
-
Amino sugar nitrogen
- NHN:
-
Non-hydrolyzed nitrogen
- NH4 +-N:
-
Ammonium-nitrogen
- UHN:
-
Unknown hydrolyzed nitrogen
- THN:
-
Total hydrolyzed nitrogen
- BPS:
-
Buried paddy soil
- BNS:
-
Buried non-paddy soil
- MPS:
-
Modern paddy soil
- PSM:
-
Parent soil material
- INC:
-
Initial nitrogen content
- NCR:
-
Nitrogen content of rice plants
- SPC:
-
Soil phytolith content
- NSR:
-
Nitrogen sequestration rate
- PSF:
-
Phytolith stability factor
- SON:
-
Soil organic nitrogen
- SOC:
-
Soil organic carbon
- TN:
-
Total nitrogen
References
Amelung W (2003) Nitrogen biomarkers and their fate in soil. J Plant Nutr Soil Sci 166:677–686. https://doi.org/10.1002/jpln.200321274
Amelung W, Brodowski S, Sandhage-Hofmann A, Bol R (2008) Combining biomarker with stable isotope analyses for assessing the transformation and turnover of soil organic matter. Adv Agron 100:155–250. https://doi.org/10.1016/S0065-2113(08)00606-8
Amelung W, Zhang X (2001) Determination of amino acid enantiomers in soils. Soil Biol Biochem 33:553–562. https://doi.org/10.1016/S0038-0717(00)00195-4
Amelung W, Zhang X, Flach KW (2006) Amino acids in grassland soils: climatic effects on concentrations and chirality. Geoderma 130:207–217. https://doi.org/10.1016/j.geoderma.2005.01.017
Bannert A, Kleineidam K, Wissing L, Mueller-Niggemann C, Vogelsang V, Welzl G, Cao Z, Schloter M (2011) Changes in diversity and functional gene abundances of microbial communities involved in nitrogen fixation, nitrification, and denitrification in a tidal wetland versus paddy soils cultivated for different time periods. Appl Environ Microb 77:6109–6116. https://doi.org/10.1128/AEM.01751-10
Belluomini G, Branca M, Calderoni G, Schnitzer M (1986) Distribution and geochemical significance of amino acids and amino sugars in a clay suite of the pliocene-pleistocene age from central Italy. Org Geochem 9:127–133. https://doi.org/10.1016/0146-6380(86)90102-6
Bird JA, Horwath WR, Eagle AJ, van Kessel C (2001) Immobilization of fertilizer nitrogen in rice: effects of straw management practice. Soil Sci Soci Am J 65:1143–1152. https://doi.org/10.2136/sssaj2001.6541143x
Bremner JM (1965) Organic forms of nitrogen. Madision, American Society of Agronomy, pp 1238–1255
Cao ZH, Ding JL, Hu ZY, Knicker H, Kögel-Knabner I, Yang LZ, Yin R, Lin XG, Dong YH (2006) Ancient paddy soils from the Neolithic age in China’s Yangtze River Delta. Naturwissenschaften 93:232–236. https://doi.org/10.1007/s00114-006-0083-4
Dick WA (1983) Organic carbon, nitrogen, and phosphorus concentrations and pH in soil profiles as affected by tillage intensity. Soil Sci Soci Am J 47:102–107. https://doi.org/10.2136/sssaj1983.03615995004700010021x
FAO (2006) World reference base for soil resources, 2006: a framework for international classification, correlation and communication. Food and Agriculture Organization of the United Nations, Rome
Farmer VC, Delbos E, Miller JD (2005) The role of phytolith formation and dissolution in controlling concentrations of silica in soil solutions and streams. Geoderma 127:71–79. https://doi.org/10.1016/j.geoderma.2004.11.014
Farzadfar S, Knight JD, Congreves KA (2021) Soil organic nitrogen: an overlooked but potentially significant contribution to crop nutrition. Plant Soil 462:7–23. https://doi.org/10.1007/s11104-021-04860-w
Fischer WN, André B, Rentsch D, Krolkiewicz S, Tegeder M, Breitkreuz K, Frommer WB (1998) Amino acid transport in plants. Trends Plant Sci 3:188–195. https://doi.org/10.1016/S1360-1385(98)01231-X
Fisher RF, Newell Bourne C, Fisher WF (1995) Opal phytoliths as an indicator of the floristics of prehistoric grasslands. Geoderma 68:243–255. https://doi.org/10.1016/0016-7061(95)00044-9
Fishkis O, Ingwersen J, Lamers M, Denysenko D, Streck T (2010) Phytolith transport in soil: a field study using fluorescent labelling. Geoderma 157:27–36. https://doi.org/10.1016/j.geoderma.2010.03.012
Franzluebbers AJ, Stuedemann JA (2009) Soil-profile organic carbon and total nitrogen during 12 years of pasture management in the southern piedmont USA. Agr Ecosyst Environ 129:28–36. https://doi.org/10.1016/j.agee.2008.06.013
Friedel JK, Scheller E (2002) Composition of hydrolysable amino acids in soil organic matter and soil microbial biomass. Soil Biol Biochem 34:315–325. https://doi.org/10.1016/S0038-0717(01)00185-7
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR., Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892. https://www.science.org/doi/10.1126/science.1136674
Gotoh S, Araragi M, Koga H, Ono SI (1986) Hydrolyzable organic forms of nitrogen in some rice soil profiles as affected by organic matter application. Soil Sci Plant Nutr 32:535–550. https://doi.org/10.1080/00380768.1986.10557536
Gu JX (1998) Preliminary study of Neolithic Age rice culture at CaoXie-Shan site. Southeast Culture 3:43–45 (in Chinese)
He HB, Li XB, Zhang W, Zhang XD (2011) Differentiating the dynamics of native and newly immobilized amino sugars in soil frequently amended with inorganic nitrogen and glucose. Eur J Soil Sci 62:144–151. https://doi.org/10.1111/j.1365-2389.2010.01324.x
Hiroshi F (1976) Fundamental studies in plant opal analysis: on the silica bodies of motor cell of rice plants and their relatives and the method of quantitative analysis. Archaeol Natural Sci 9:15–29 (in Chinese)
Hirzel J, Cordero K, Fernández C, Acuña J, Sandoval M, Zagal E (2012) Soil potentially mineralizable nitrogen and its relation to rice production and nitrogen needs in two paddy rice soils of Chile. J Plant Nutr 35:396–412. https://doi.org/10.1080/01904167.2012.639920
Houtermans M, Lehndorff E, Utami SR, Said-Pullicino D, Romani M, Koelbl A, Kaiser K, Cao ZH, Amelung W (2017) Nitrogen sequestration under long-term paddy management in soils developed on contrasting parent material. Biol Fert Soils 53:837–848. https://doi.org/10.1007/s00374-017-1223-z
Huang LM, Thompson A, Zhang GL, Chen LM, Han GZ, Gong ZT (2015) The use of chronosequences in studies of paddy soil evolution: a review. Geoderma 237:199–210. https://doi.org/10.1016/j.geoderma.2014.09.007
Humphreys GS, Hart DM, Simons NA, Field RJ (2003) Phytoliths as indicator of process in soils. Papers from a Conference held at the ANU, August 2001, Canberra Australia. Phytolith and Starch Research in the Australian–Pacific–Asian Regions: the State of the Art: Terra Australia, 19, pp 93–104
Jones DL, Kielland K (2012) Amino acid, peptide and protein mineralization dynamics in a taiga forest soil. Soil Biol Biochem 55:60–69. https://doi.org/10.1016/j.soilbio.2012.06.005
Ju XT, Xing GX, Chen XP, Zhang SL, Zhang LJ, Liu XJ, Cui ZL, Yin B, Christie P, Zhu ZL, Zhang FS (2009) Reducing environmental risk by improving N management in intensive Chinese agricultural systems. P Natl Acad Sci USA 106:3041–3046. https://doi.org/10.1073/pnas.0813417106
Keil RG, Tsamakis E, Giddings JC, Hedges JI (1998) Biochemical distributions (amino acids, neutral sugars, and lignin phenols) among size-classes of modern marine sediments from the Washington coast. Geochim Cosmochim Ac 62:1347–1364. https://doi.org/10.1016/S0016-7037(98)00080-5
Kelley KR, Stevenson FJ (1995) Forms and nature of organic N in soil. Fert Res 42:1–11. https://doi.org/10.1007/BF00750495
Kimber RW, Hare PE (1992) Wide range of racemization of amino acids in peptides from human fossil bone and its implications for amino acid racemization dating. Geochim Cosmochim Ac 56:739–743. https://doi.org/10.1016/0016-7037(92)90094-Y
Knicker H (2010) “Black nitrogen” – an important fraction in determining the recalcitrance of charcoal. Org Geochem 41:947–950. https://doi.org/10.1016/j.orggeochem.2010.04.007
Kukal SS, Rehana R, Benbi DK (2009) Soil organic carbon sequestration in relation to organic and inorganic fertilization in rice–wheat and maize–wheat systems. Soil till Res 102:87–92. https://doi.org/10.1016/j.still.2008.07.017
Lal R (2002) Soil carbon sequestration in China through agricultural intensification, and restoration of degraded and desertified ecosystems. Land Degrad Dev 13:469–478. https://doi.org/10.1002/ldr.531
Leinweber P, Schulten HR (1998) Nonhydrolyzable organic nitrogen in soil size separates from long-term agricultural experiments. Soil Sci Soc Am J 62:383–393. https://doi.org/10.2136/sssaj1998.03615995006200020014x
Li CH, Zhang GY, Yang LZ, Lin XG, Hu ZY, Dong YH, Cao ZH, Zheng YF, Ding JL (2007) Pollen and phytolith analyses of ancient paddy fields at Chuodun Site, the Yangtze River Delta. Pedosphere 17:209–218. https://doi.org/10.1016/S1002-0160(07)60027-7
Li Z, Liu M, Wu X, Han F, Zhang T (2010) Effects of long-term chemical fertilization and organic amendments on dynamics of soil organic C and total N in paddy soil derived from barren land in subtropical China. Soil till Res 106:268–274. https://doi.org/10.1016/j.still.2009.12.008
Lu J, Hu Z, Xu Z, Cao Z, Zhuang S, Yang L, Lin X, Dong Y, Yin R, Ding J, Zheng Y (2009) Effects of rice cropping intensity on soil nitrogen mineralization rate and potential in buried ancient paddy soils from the Neolithic Age in China’s Yangtze River Delta. J Soil Sediment 9:526–536. https://doi.org/10.1007/s11368-009-0138-1
Lü H, He H, Zhao J, Zhang W, Xie H, Hu G, Liu X, Wu Y, Zhang X (2013) Dynamics of fertilizer - derived organic nitrogen fractions in an arable soil during a growing season. Plant Soil 373:595–607. https://doi.org/10.1007/s11104-013-1824-8
Ma F, Wang Y, Yan P, Wei F, Duan Z, Yang Z, Liu J (2021) Effect of cotton residues incorporation on soil properties, organic nitrogen fractions, and nitrogen-mineralizing enzyme activity under long-term continuous cotton cropping. PeerJ 9:11053. https://doi.org/10.7717/peerj.11053
Macdonald BC, Farrell M, Tuomi S, Barton PS, Cunningham SA, Manning AD (2014) Carrion decomposition causes large and lasting effects on soil amino acid and peptide flux. Soil Biol Biochem 69:132–140. https://doi.org/10.1016/j.soilbio.2013.10.042
Meunier JD, Colin F, Alarcon C (1999) Biogenic silica storage in soils. Geology 27:835–838. https://doi.org/10.1130/0091-7613(1999)027%3c0835:BSSIS%3e2.3.CO;2
Mulvaney R, Khan S, Hoeft R, Brown H (2001) A soil organic nitrogen fraction that reduces the need for nitrogen fertilization. Soil Sci Soc Am J 65:1164–1172. https://doi.org/10.2136/sssaj2001.6541164x
Nakahara S, Zou P, Ando H, Fu J, Cao Z, Nakamura T, Sugiura Y, Watanabe A (2016) Stability of soil organic matter accumulated under long-term use as a rice paddy. J Geophys Res Biogeo 121:67–77. https://doi.org/10.1002/2015JG003104
Nemergut DR, Anderson SP, Cleveland CC, Martin AP, Miller AE, Seimon A, Schmidt SK (2007) Microbial community succession in an unvegetated, recently deglaciated soil. Microb Ecol 53:110–122. https://doi.org/10.1007/s00248-006-9144-7
Neue H, Gaunt J, Wang Z, Becker-Heidmann P, Quijano C (1997) Carbon in tropical wetlands. Geoderma 79:163–185. https://doi.org/10.1016/S0016-7061(97)00041-4
Olk DC, Cassman KG, Randall EW, Kinchesh P, Sanger LJ, Anderson JM (1996) Changes in chemical properties of organic matter with intensified rice cropping in tropical lowland soil. Eur J Soil Sci 47:293–303. https://doi.org/10.1111/j.1365-2389.1996.tb01403.x
Olk DC, Samson MI, Gapas P (2007) Inhibition of nitrogen mineralization in young humic fractions by anaerobic decomposition of rice crop residues. Eur J Soil Sci 58:270–281. https://doi.org/10.1111/j.1365-2389.2006.00836.x
Pan JH, Wang J, Zhuang SY (2022) Amino acid nitrogen trends in paddy soils under :long-term rice cultivation in southeast coast of China. Catena 212:106044. https://doi.org/10.1016/j.catena.2022.106044
Parr JF, Sullivan LA (2005) Soil carbon sequestration in phytoliths. Soil Biol Biochem 37:117–124. https://doi.org/10.1016/j.soilbio.2004.06.013
Penkman K (2017) Amino acid racemization. Encyclopedia. Geoarchaeol 16:14–15
Piper TJ, Posner AM (1972) Humic acid nitrogen. Plant Soil 36:595–598. https://doi.org/10.1007/BF01373509
Piperno DR (2006a) Phytoliths: a comprehensive guide for archaeologists and paleoecologists. AltaMira press, Lanham MD
Piperno DR (2006b) Quaternary environmental history and agricultural impact on vegetation in Central America. Ann MO Bot Gard 93:274–296. https://doi.org/10.3417/0026-6493(2006)93[274:QEHAAI]2.0.CO;2
Radkov AD, Moe LA (2014) Bacterial synthesis of D-amino acids. Appl Microbiol Biot 98:5363–5374. https://doi.org/10.1007/s00253-014-5726-3
Roger PA, Ladha JK (1990) Estimation of biological N2 fixation and its contribution to nitrogen balance in wetland rice fields. In: Trans 14th International Congress of Soil Science, Kyoto, Japan, pp 128–133
Roth PJ, Lehndorff E, Hahn A, Frenzel P, Amelung W (2013) Cycling of rice rhizodeposits through peptide-bound amino acid enantiomers in soils under 50 and 2000 years of paddy management. Soil Biol Biochem 65:227–235. https://doi.org/10.1016/j.soilbio.2013.05.026
Rovira P, Vallejo VR (2002) Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: an acid hydrolysis approach. Geoderma 107:109–141. https://doi.org/10.1016/S0016-7061(01)00143-4
Ren C, Sun P, Kang D, Zhao F, Feng Y, Ren G, Han X, Yang G (2016) Responsiveness of soil nitrogen fractions and bacterial communities to afforestation in the Loess Hilly Region (LHR) of China. Sci Rep 6:28469. https://doi.org/10.1038/srep28469
Runge F (1999) The opal phytolith inventory of soils in central Africa —quantities, shapes, classification, and spectra. Rev Palaeobot Palyno 107:23–53. https://doi.org/10.1016/S0034-6667(99)00018-4
Sahrawat K (2005) Fertility and organic matter in submerged rice soils. Curr Sci 88:735–739. http://oar.icrisat.org/id/eprint/3948
Schultz CL, Moini M (2003) Analysis of underivatized amino acids and their D/L-enantiomers by sheathless capillary electrophoresis/electrospray ionization-mass spectrometry. Anal Chem 75:1508–1513. https://doi.org/10.1021/ac0263925
Schulten HR, Schnitzer M (1997) The chemistry of soil organic nitrogen: a review. Biol Fert Soils 26:1–15. https://doi.org/10.1007/s003740050335
Schmidt-Rohr K, Mao JD, Olk D (2004) Nitrogen-bonded aromatics in soil organic matter and their implications for a yield decline in intensive rice cropping. P Natl Acad Sci USA 101:6351–6354. https://doi.org/10.1073/pnas.0401349101
Shen YH (1999) Sorption of natural dissolved organic matter on soil. Chemosphere 38:1505–1515. https://doi.org/10.1016/S0045-6535(98)00371-3
Song Z, Mcgrouther K, Wang H (2016) Occurrence, turnover and carbon sequestration potential of phytoliths in terrestrial ecosystems. Earth-Sci Rev 158:19–30. https://doi.org/10.1016/j.earscirev.2016.04.007
Sowden FJ, Chen Y, Schnitzer M (1977) The nitrogen distribution in soils formed under widely differing climatic conditions. Geochim Cosmochim Ac 41:1524–1526. https://doi.org/10.1016/0016-7037(77)90257-5
Stevenson FJ (1982) Nitrogen—organic forms. Page AL (ed). Methods of soil analysis part2 chemical and microbiological properties. Madison: America Society of Agronomy, pp 625–641
Tan L, Fan X, Yan G, Peng M, Zhang N, Ye M, Gao Z, Song A, Nikolic M, Liang Y (2021) Sequestration potential of phytolith occluded carbon in China’s paddy rice (Oryza sativa L.) systems. Sci Total Environ 774:145696. https://doi.org/10.1016/j.scitotenv.2021.145696
Tian J, Wei K, Condron LM, Chen Z, Xu Z, Chen L (2017) Effects of elevated nitrogen and precipitation on soil organic nitrogen fractions and nitrogen-mineralizing enzymes in semi-arid steppe and abandoned cropland. Plant Soil 417:217–229. https://doi.org/10.1007/s11104-017-3253-6
Tong C, Xiao H, Tang G, Wang H, Huang T, Xia H, Keith SJ, Li Y, Liu S, Wu J (2009) Long-term fertilizer effects on organic carbon and total nitrogen and coupling relationships of C and N in paddy soils in subtropical China. Soil till Res 106:8–14. https://doi.org/10.1016/j.still.2009.09.003
Toriyama K, Amino T, Kobayashi K (2020) Contribution of fallow weed incorporation to nitrogen supplying capacity of paddy soil under organic farming. Soil Sci Plant Nutr 66:133–143. https://doi.org/10.1080/00380768.2020.1716389
van Groenigen J, Huygens D, Boeckx P, Kuyper TW, Lubbers IM, Rütting T, Groffman PM (2015) The soil N cycle: new insights and key challenges. Soil 1:235–256. https://doi.org/10.5194/soil-1-235-2015
Wang JS, Stewart JR, Khan SA, Dawson JO (2010) Elevated amino sugar nitrogen concentrations in soils: a potential method for assessing N fertility enhancement by actinorhizal plants. Symbiosis 50:71–76. https://doi.org/10.1007/s13199-009-0038-6
Wang J, Zhuang SY, Zhu ZL (2017) Soil organic nitrogen composition and mineralization of paddy soils in a cultivation chronosequence in China. J Soil Sediment 17:1588–1598. https://doi.org/10.1007/s11368-016-1629-5
Wang YJ, Lv HY, Heng P, Cang SX, Feng ZJ (1991) Study of plant opaline silicas and its preliminary application to quaternary geology in China. Marine Geol Quaternary Geol 13:113–124 (in Chinese)
Yang X, Liu Y, Li C, Song Y, Zhu H, Jin X (2007) Rare earth elements of aeolian deposits in Northern China and their implications for determining the provenance of dust storms in Beijing. Geomorphology 87:365–377. https://doi.org/10.1016/j.geomorph.2006.10.004
Yang X, Yang P, Lou J, Zhang Y, Ye S, Mao X, Chen J, Stephan MH (2021) Grain yield, plant nitrogen content and nitrogen use efficiency as affected by controlled-release urea and straw biochar in a rice field. J Plant Nutr 45:1393–1402. https://doi.org/10.1080/01904167.2021.2014884
Yu Z, Zhang Q, Kraus TEC, Dahlgren RA, Anastasio C, Zasoski RJ (2002) Contribution of amino compounds to dissolved organic nitrogen in forest soils. Biogeochemistry 61:173–198. https://doi.org/10.1023/A:1020221528515
Zhao M, Bada JL (1995) Determination of α-dialkylamino acids and their enantiomers in geological samples by high-performance liquid chromatography after derivatization with a chiral adduct of o-phthaldialdehyde. J Chromatogr A 690:55–63. https://doi.org/10.1016/0021-9673(94)00927-2
Zhao Y, Song Z, Xu X, Liu H, Wu X, Li Z, Guo F, Pan W (2016) Nitrogen application increases phytolith carbon sequestration in degraded grasslands of north China. Ecol Res 31:117–123. https://doi.org/10.1007/s11284-015-1320-0
Zheng Y, Sun G, Qin L, Li C, Wu X, Chen X (2009) Rice fields and modes of rice cultivation between 5000 and 2500 BC in east China. J Archaeol Sci 36:2609–2616. https://doi.org/10.1016/j.jas.2009.09.026
Acknowledgements
The authors thank the individuals who helped with sample collection and analysis. The authors would like to thank Associate Professor Peter Almond from Lincoln University (NZ) for the constructive comments on the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (41671296) and the National Key R & D Program of China (2016FYE0112700).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Zhaoliang Song
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Amino acid N more unstable in paddy soil than in non-paddy soil
• D-AAs and acidic AAs higher in buried paddy soil than modern paddy soil
• D- and D-/L-alanine, aspartate, and glutamate increased in buried paddy soil
• D-aspartate content linearly and significantly positively correlated with soil depth
• Continuous high-intensity rice cultivation could increase N sequestration potential
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, J., Wang, J. & Zhuang, S. Soil organic nitrogen fraction and sequestration in a buried paddy soil since the Neolithic age. J Soils Sediments 23, 2021–2036 (2023). https://doi.org/10.1007/s11368-023-03442-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03442-w