Abstract
Purpose
The objective of this study was to investigate the effects of application of biochar and microbial inoculants on the bioavailability of phosphorus and potassium, tomato growth, and the bacterial community in greenhouse soil.
Materials and methods
The experiment was conducted in a greenhouse with tomato mono-cropped for 21 years at Yongqing County of Hebei Province from November 2018 to June 2019. The treatments included conventional fertilization control (CF), 2 t/ha of biochar application (B, manufactured from apricot shell), 75 L/ha of microbial inoculants application (M, containing effective strains of Bacillus megaterium and Paenibacillus mucilaginosus), the mixture of microbial inoculants and biochar application (BM).
Results
The results showed that the application of 75 L/ha microbial inoculants in greenhouse tomato could increase the yields of tomato by 23.41%, vitamin C (Vc) and soluble sugar concentrations by 14.41% and 13.62%, respectively. The microbial inoculants combined with 2 t/ha biochar enhanced the effects of microbial inoculants on the growth promotion of tomatoes. The application of microbial inoculants combined with biochar increased the P and K accumulation in tomato plants by 28.72–57.14% and 19.53–29.03%, respectively, during the whole growing stage. Moreover, the application of microbial inoculants significantly increased the relative abundance of Bacillus, Paenibacillus, and Flavobacterium and decreased the relative abundance of Acidobacterium.
Conclusions
The application of microbial inoculants improved the bioavailability of phosphorus and potassium and tomato growth by altering the composition of soil bacterial community. These results show the potential of co-application of biochar and microbial inoculants as a potential tool to sustain longer-term production of monoculture vegetable systems in greenhouses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tomato (Lycopersicon esculentum) is an annual or perennial herb of solanaceae, which is one of the most popular vegetables in the world, and its fruit is rich in lycopene, phenols, organic acids, vitamin, and many other beneficial ingredients (Cochard et al. 2022; Yagmur and Gunes 2021). In China, consecutive monoculture of tomato under greenhouse conditions is commonly adopted to obtain better economic benefits (Zheng et al. 2020). However, under conventional management practices, the long-term consecutive monoculture of tomato in greenhouses may negatively affect soil quality, resulting in poor plant growth and fruit quality, as well as intensification of pests and diseases (Hongdan et al. 2017; Zhao et al. 2019a). Therefore, it is urgent to develop sustainable and high-efficiency management strategies and improve soil quality in greenhouse vegetable production systems in order to promote the healthy development of soil and sustain long-term production.
In recent years, microbial technology has attracted great attention in the fields of agricultural production and environmental protection due to its high efficiency and environmental friendly (Singh et al. 2021). Nowadays, more and more types of functional microorganisms were identified. For example, it has been shown that Bacillus megaterium could secrete organic acids to dissolve phosphate, and a gene related to citrate synthase synthesis was found in its gene sequence (Huang et al. 2019). Gupta and Kumar (2017) have showed that the bacteria not only could dissolve phosphates by secreting organic acids but also could utilize their own functional groups to chelate metal ions in soil and further promote the release of phosphate. The bacteria secrete extracellular enzymes which play an active role in the mineralization of organophosphorus in soil (Raliya et al. 2016). At the same time, Paenibacillus mucilaginosus is well known for dissolving potassium. It has been shown that the metabolism of silicate bacteria could produce enzymes, capsular polysaccharides, and low molecular organic acids, destroy the lattice structure of potassium feldspar, and decompose and transform soil mineral potassium and immobilized potassium into available potassium that could be absorbed and utilized by plants (Sindhu et al. 2014). Paenibacillus mucilaginosus secreted capsular polysaccharides, which was a typical characteristic potassium-dissolving bacteria (Huang et al. 2019). However, the key and difficulty for these functional bacteria to play their roles are to ensure that they can stay well in soil for a long time.
Biochar is one potential amendment to improve soil properties, which is used as a soil amendment for its well-researched benefits, such as improving soil fertility and structure, promoting plant growth in agricultural production (Singh et al. 2022; Zhu et al. 2017). However, previous studies on biochar application mostly focused on its use as a soil amendment/conditioner to alter soil physical and chemical properties with less consideration given to its impact on soil biological properties, particularly with short-term application (Paz-Ferreiro et al. 2015). Moreover, biochar is rich in nutrients and characterized by high porosity on the surface area which may provide a favorable habitat for bacterial proliferation and survival (Glodowska et al. 2017). It was reported that biochar produced by slow pyrolysis of agricultural wastes significantly increased the survival of Burkholderia sp. and Bacillus sp. and stimulated seed germination, plant growth, and yields of tomato, as well as soil biological activity (Tripti et al. 2017). It is also well known that “all biochars are not created equal” and as a consequence, the effects on crops are both biochar-specific and site-specific (Mukherjee and Lal 2014). A better understanding of the complexity of these relationships requires more field studies.
Therefore, we hypothesized that (1) the pore structure of biochar provides attachment sites for the microbial inoculants applied directly to soil and then increases the soil microbial activity; (2) the combined application of the microbial inoculants together with biochar will help in promoting the functions of nutrient activation and crop growth of the strains. To test our hypothesis, a greenhouse experiment was conducted to determine: (1) the effects of co-applying biochar and microbial inoculants containing effective strains of Bacillus megaterium and Paenibacillus mucilaginosus on the availability of P and K in the soil, and the growth and development of tomato plants and (2) the response of the diversity and composition of the soil bacterial community with the co-application of biochar and the microbial inoculants. This paper will provide new insights on the development of high-efficiency environment-friendly regulation technology for intensive greenhouse vegetables production.
2 Materials and methods
2.1 Study site and soil characteristics
The experiment was conducted at Yongqing County, Langfang City, Hebei Province (39°09′01″N, 116°33′14″E), where tomato has been grown continuously (Provence variety) in greenhouse for 21 years. The key soil properties were measured following standard procedures (Abou-El-Seoud and Abdel-Megeed 2012). The soil had a pH (H2O) of 8.4, and organic matter content of 18.3 g/kg. It contained 1.2 g/kg of total nitrogen, 122.3 mg/kg of alkali-hydrolyzable nitrogen, 257.4 mg/kg of available P, and 1171.6 mg/kg of available K. The climate is a typical temperate continental monsoon climate with an average annual temperature of approximately 11.5 ℃, an average annual precipitation of approximately 540 mm, an average annual sunshine of 2740 h, and a frost-free period of 183 days.
2.2 Microbial inoculants and biochar
The effective microbial inoculants of Bacillus megaterium and Paenibacillus mucilaginosus were provided by Hebei Runwo Biotechnology Company, with the bacteria number 2 × 108 cfu/mL, and the effective bacteria ratio of the two was 1:1.
The biochar was provided by Chengde Huajing Activated Carbon Company, which was manufactured from apricot shell at a final temperature of 800–900 ℃ for 0.5 h, including 73.1% of organic carbon content, 9.4 g/kg of total nitrogen content, 11.3 mg/kg of total phosphorus content, 10.6 mg/kg of total potassium content, and pH 9.6.
2.3 Experimental design
Four treatments were set up: conventional fertilization without application of biochar or microbial inoculants control (CF), conventional fertilization with 2 t/ha of biochar (B), conventional fertilization with 75 L/ha of the microbial inoculants (M), conventional fertilization with the mixture of 75 L/ha of the microbial inoculants and 2 t/ha of biochar (BM). Each treatment was repeated 3 times. A total of 12 plots were set up, each was 8.4 m × 3.6 m (length × width). One week before transplanting, the soil was rotary tilled according to the conventional practices of local vegetable growers. One day before transplanting, the experiment plots were randomly arranged. The application rates of total nutrient in the conventional fertilization were N 424 kg/ha, P2O5 332 kg/ha, and K2O 707 kg/ha.
2.4 Soil sample collection and measurement
2.4.1 Soil sample collection
Soil samples were taken at early fruiting stage (20 days after transplanting), vigorous bearing stage (60 days after transplanting), last bearing stage (100 days after transplanting), and last fruit stage (140 days after transplanting). The topsoil of 0–20 cm was taken from areas 10–15 cm away from the main root of tomato. Five soil samples were randomly taken from each plot, mixed thoroughly, a part of which was stored in a refrigerator at − 80 ℃ for the determination of soil microbial diversity. The remaining soil samples were air-dried and sieved for the determination of soil fertility.
2.4.2 Determination of soil fertility index
The available P concentration of soil was determined by sodium bicarbonate extraction and molybdenum-antimony resistance colorimetry, and the available K concentration of soil was determined by ammonium acetate extraction and flame photometry (Abou-El-Seoud and Abdel-Megeed 2012).
2.4.3 Determination of soil microbial community
DNA was extracted from the 0.5 g frozen soil samples following manufacturer’s procedures (E.Z.N.A.® soil DNA Kit) and were subsequently sequenced by Miseq sequencing to study the microbial diversity (Shanghai Majorbio Bio-pharm Technology Company). The amplification region was V3–V4 region of bacterial 16S rRNA, and the primers used were:
-
F: 515F (GTGCCAGCMGCCGCGG) and
-
R: 907R (CCGTCAATTCMTTTRAGTTT) (Yusoff et al. 2013)
The PCR thermoprofile is as follows: denaturing at 95 ℃ for 3 min, 95 ℃ for 3 s, annealing at 55 ℃ for 30 s, extending at 72 ℃ for 45 s, 27 cycles, and finally extending at 72 ℃ for 10 min. The PCR reaction was carried out in 20 μL mixture containing 4 μL of 5 × FastPfu buffer, 2 μL of 2.5 mmol/L dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL of FastPfu polymerase and 10 ng of template DNA. After amplification, purification and fluorescence quantification were performed and sequencing was performed on the Illumina MiSeq platform.
2.5 Plant sample collection and measurement
2.5.1 Plant sample collection
Plant samples were collected on days 20, 60, 100, and 140 after tomato transplanting, with 5 plants being randomly selected from each plot, and oven-dried (60 ℃) to a constant weight for nutrient analysis in plant tissues. Ripe fruits with similar appearance and size were collected at vigorous bearing stage, and five fruits from each plot were randomly collected as samples, which were mainly used to determine nitrate, vitamin C (Vc), soluble protein, and soluble sugar contents. In addition, the yields of tomato in each plot were also recorded.
2.5.2 The determination of plant samples
For the quality of tomato fruit, the nitrate content was determined by ultraviolet spectrophotometry, Vc content was determined by molybdenum blue colorimetry, soluble protein content was determined by Coomassie bright blue G-250 staining method, and soluble sugar content was determined by concentrated sulfuric acid-anthrone colorimetry. The determination of tomato tissue nutrients and calculation of P and K accumulation in tomato plants were carried out following the described previously (Zhao et al. 2021).
2.6 Statistical analysis
The diversity indices of Shannon, Sobs, Chao1, and coverage rate were calculated in Mothur and used to compare soil bacterial alpha diversity between different treatments. The significant differences in yields and quality of tomato, dry weights of plant tissues, P and K accumulation in plant tissues, bacterial alpha diversity, and the relative abundances of different taxonomic levels of bacteria between treatments were analyzed using one-way ANOVA based on Tukey’s post-hoc test using SPSS software (Version 22.0).
3 Results
3.1 Effects of biochar and microbial inoculants on the yields and quality of tomato
Compared with CF, M and BM treatments significantly increased tomato yields by 28.27% and 23.41%, respectively (p < 0.05) (Fig. 1). The BM treatment also increased tomato yields by 3.94% compared with M treatment (p < 0.05). However, there was no significant difference between the B treatment and CF in tomato yields. These results indicated that the application of 75 L/ha the microbial inoculants in greenhouse significantly increased the yields of tomato, and the application of 2 t/ha the biochar enhanced the effects of the microbial inoculants on increasing the yields of tomato.
Effects of different treatments on tomato yields. CF, conventional fertilization as control; B, conventional fertilization combined with 2 t/ha of biochar; M, conventional fertilization combined with 75 L/ha of the microbial inoculants; BM, conventional fertilization combined with both of 75 L/ha of the microbial inoculants and 2 t/ha of biochar. Different letters indicate significant differences at p < 0.05 according to Tukey’s post-hoc test
Compared with CF, the M treatment significantly increased Vc concentrations by 14.41%, and the BM and M treatments significantly increased the soluble sugar concentrations by 13.27% and 13.62%, respectively (p < 0.05) (Table 1). There was no difference on the nitrate and soluble protein in tomato fruit among different treatments. The results showed that the application of microbial inoculants could improve the quality of tomato fruit.
3.2 Effects of biochar and microbial inoculants on the dry weights of tomato tissues
The plant biomass was an important indicator for the growth of tomato. At seedling stage, the M and BM treatments significantly increased the dry weights of tomato root by 29.79% and 65.95% compared with CF (Table 2). At early fruiting stage, the M and BM treatments significantly increased the dry weights of tomato shoots by 14.66% and17.86% and roots by 21.77% and 33.04%, respectively. Similarly, the M and BM treatments also increased the dry weights of tomato shoots and roots at vigorous bearing and last bearing stages, respectively. There was no significant difference on the dry weights of tomato shoots and roots between the B treatment and CF at the last three-growth stage. Compared with the M treatment, the BM treatment also increased the dry weights of tomato shoots and roots at early fruiting and vigorous bearing stage, accounting for 4.42–6.62% and 8.73–12.88%, respectively (p < 0.05). These results showed that the application of 75 L/ha the microbial inoculants could promote the growth and development of tomato plants at different growth stages, and the application of 2 t/ha of the biochar could enhance the growth-promoting effects of microbial inoculants on tomato.
3.3 Effects of biochar and microbial inoculants on the bioavailability of phosphorus and potassium
Compared with CF, the BM treatment significantly increased the available P concentrations in soil by 24.10% and 30.02%, and available K by 37.22% and 4.33% at vigorous bearing and last bearing stage, respectively (p < 0.05) (Table 3). However, there was no significant difference in soil available P and K between BM and CF at seedling and early fruiting stage. These results showed that the combined application of microbial inoculants with biochar had “after effect” on the activation of phosphorus and potassium in soil of greenhouse tomato.
Moreover, the amount of P and K accumulation in tomato plant tissues was also different among different treatments (p < 0.05) (Table 4). Compared with CF, M, and BM treatments significantly increased the P amount in tomato plant by 17.47–32.52% and 28.72–57.14% during the whole growing stage. B treatment only increased P amounts at vigorous bearing stage. Compared with M treatment, BM treatment also increased P amounts in tomato plant by 9.03–22.22%. On the other hand, M and BM treatments significantly increased the K amounts in tomato plant by 12.60–16.89% and 19.53–29.03% during the whole growing stage compared with CF. Compared with CF, B treatment increased K amounts in tomato plant by 1.63–4.21% at the last three growth stages, respectively. Compared with M, BM treatment also increased K amounts in tomato plant at the last three growth stages. These results indicated that the microbial inoculants significantly promoted the absorption of P and K nutrients in tomato plants, and the combination of microbial inoculants and biochar was conducive to enhancing the effects of microbial inoculants.
3.4 Effects of biochar and microbial inoculants on soil bacterial community diversity
The V3 and V4 regions of 16S rRNA were sequenced in 48 soil samples from 4 treatments at different growth stages of tomato. The sequenced data included 2,090,771 valid sequences, 828,908,015 bases, and the average length of the sequences was 396.46 bp with 40,921 OTUs at a similar level of 97% (Table 5). It was found that there was a significant difference between the M treatment and the BM treatment in Sobs index at early fruiting stage (p < 0.05), but there was no significant difference between other treatments, indicating that the application of the microbial inoculants would not adversely the bacterial community diversity of the soil.
3.5 Effects of biochar and microbial inoculants on the composition of bacterial community in greenhouse soil
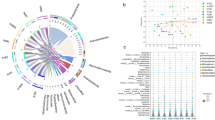
Figure 2 shows the phyla composition and distribution of soil bacterial communities under different treatments of greenhouse tomato at different stages. The dominant phyla (relative abundance > 1%) in different treatments were Proteobacteria, Actinobacteria, Acidobacteria, Bacteroidetes, Chloroflexi, Firmicutes, Planctomycetes, Gemmatimonadetes, and Nitrospirae, accounting for 89.76–95.88% of the total sequence and with relative abundances from 26.03 to 35.07%, 12.50 to 19.32%, 11.01 to 17.17%, 5.77 to 19.10%, 6.50 to 10.39%, 4.32 to 11.60%, 6.28 to 8.26%, 2.79 to 4.59%, and 1.20 to 2.13%, respectively. It was found that the species composition of bacteria in the greenhouse soil in different treatments or different tomato growth stages was the same, but the relative abundance of Acidobacteria in the soil was significantly different at seedling and vigorous bearing stages (p < 0.05). Compared with CF, the relative abundance of Acidobacteria in the M treatment decreased by 25.52% and 42.61% at seedling stage and vigorous bearing stage, respectively. At the same time, the relative abundance of Acidobacteria in the M treatment decreased by 42.19% compared with B treatment at vigorous bearing stage. Acidobacteria is an oligotrophic bacterium, of which the relative abundance will decrease with the increase of available nutrient concentration. The results indicated that the application of 75 L/ha the microbial inoculants during transplanting of tomato could improve the composition of bacterial community and promote the activation of P and K in soil, causing the decrease of the relative abundance of Acidobacteria in soil.
Composition of soil bacterial community at phyla level under different treatments of greenhouse tomato at different stages. CF, conventional fertilization as control; B, conventional fertilization combined with 2 t/ha of biochar; M, conventional fertilization combined with 75 L/ha of the microbial inoculants; BM, conventional fertilization combined with both of 75 L/ha of the microbial inoculants and 2 t/ha of biochar. Different letters at each sampling time indicate significant differences at p < 0.05 according to Tukey’s post-hoc test
The relative abundances of the predominant bacterial genera showed similar patterns to the corresponding bacterial phyla (Fig. 3). At seedling stage, compared with CF, the relative abundance of Bacillus in BM treatment increased by 63.23%; the relative abundance of Paenibacillus in M and BM treatments increased by 268.42% and 194.74%, respectively; the relative abundance of Flavobacterium in B, M, and BM treatments increased by 268.42%, 194.74%, and 94.74%, respectively. Interestingly, the relative abundance of Acidobacterium in BM treatment decreased by 41.12% compared with CF. At early fruiting stage, compared with CF, the relative abundance of Bacillus in M treatment and BM treatment increased by 77.20% and 87.09%; the relative abundance of Paenibacillus in BM treatment increased by 161.54%; but the relative abundance of Acidobacterium in BM treatment decreased by 33.58%. At vigorous bearing stage, compared with CF, the relative abundance of Bacillus in BM treatment increased by 178.45%; the relative abundance of Paenibacillus in B, M, and BM treatments increased by 41.67%, 17.65%, and 17.65%; but the relative abundance of Acidobacterium in BM treatment decreased by 55.99%. At last bearing stage, compared with CF, the relative abundance of Bacillus in M and BM treatments increased by 36.26% and 27.25%; the relative abundance of Paenibacillus in M and BM treatments increased by 106.67% and 93.33%; but the relative abundance of Acidobacterium in BM treatment decreased by 29.26%. The relative abundances of Bacillus, Paenibacillus, and Flavobacterium were significantly increased when microbial inoculants of 75L/ha was applied in greenhouse soil at seedling stage, but the relative abundance of Acidobacterium was significantly decreased (p < 0.05). The relative abundances of Bacillus and Paenibacillus were significantly increased and the relative abundance of Acidobacterium was significantly decreased from early fruiting stage to vigorous bearing stage. The results showed that the application of the microbial inoculants could not only increase the relative abundance of phosphorus-solubilizing and potassium-solubilizing functional bacteria in soil but also increase the relative abundance of plant auxin-secreting functional bacteria in soil to promote growth of tomato.
Effects of different treatments on the relative abundance of the dominant bacteria genus in the soil samples at different tomato growth stages. CF, conventional fertilization as control; B, conventional fertilization combined with 2 t/ha of biochar; M, conventional fertilization combined with 75 L/ha of the microbial inoculants; BM, conventional fertilization combined with both of 75 L/ha of the microbial inoculants and 2 t/ha of biochar. Different letters at each sampling time indicate significant differences at p < 0.05 according to Tukey’s post-hoc test
4 Discussion
Results from this study showed that the application of 75 L/ha microbial inoculants could significantly increase dry weights of tomato roots and shoots and increase yields of tomato, Vc, and soluble sugar concentrations in tomato. These results indicated that the application of microbial inoculants containing Bacillus megaterium and Paenibacillus mucilaginosus could promote the growth of tomato and improve the quality. Studies have shown that seeds of tomato treated with Paenibacillus mucilaginosus have fast germination and high germination rate and can promote the growth of root length of tomato seedlings, increase fresh weight, dry weight, and root-shoot ratio of plants (Li et al. 2017, Nuzzo et al. 2020). Our previous study also demonstrated that the application of Bacillus megaterium significantly increased the growth of chili pepper and cucumber under different greenhouse conditions (Zhao et al. 2021, 2019b). Moreover, the combined application of microbial inoculants and biochar significantly improved the growth and quality of tomato than that of only microbial inoculants treatment. Probably due to biochar’s high surface area and its ability to adsorb nutrients, it provides a highly favorable habitat to microorganisms to colonize, grow, and reproduce (Semida et al. 2019). Microbes living inside may get better protected from external factors such as desiccation, adverse pH, or toxic substances in soil. It has been reported that the combined application of microbial inoculants and biochar could increase the total K concentrations in tomato plants and fruits, increase yields and Vc concentrations of tomato, and reduce nitrate concentrations (Wang et al. 2016b). These results indicated that combined application of biochar with the microbial inoculants could enhance the growth-promoting effects of microbial inoculants.
Furthermore, the results showed that combined application of biochar with the microbial inoculants could significantly increase the soil available P and K concentrations, as well as the P and K nutrient absorption of tomato plants, and thus promote tomato growth. Biochar is characterized by high porosity on the surface area which may provide additional pore space for water and microbes for proliferation (Glodowska et al. 2017). Microbes living inside pores may get better protected from external factors such as desiccation, adverse pH, or toxic substances in soil (Chen et al. 2013). It was found that Paenibacillus mucilaginosus could decompose silicate minerals, releasing P and K (Lv et al. 2020). The exopolysaccharide produced by Paenibacillus mucilaginosus could also enhance the nonspecific immunity of plants (Chang et al. 2014). Potassium dissolution of Paenibacillus mucilaginosus is related to its secretion of polysaccharides, amino acid, and organic acid (Xi et al. 2009). When Bacillus megaterium was inoculated on egg yolk culture medium, it was found that its phosphorus dissolving circle was obvious, indicating that it had the function of dissolving organophosphorus (Korir et al. 2017; Zhou et al. 2016). Bacillus megaterium also has strong ability to degrade organophosphorus and inorganic phosphorus; the mechanism of phosphorus dissolution is determined by the metabolites during the growth of the strain. Metabolites include organic acids, protons, and polysaccharides, where organic acids can chelate insoluble phosphates to dissolve them; protons can dissolve insoluble phosphates by lowering the pH value of the surrounding environment; and polysaccharides can accelerate the dissolution of insoluble phosphates through the synergy of hydroxyl and carboxyl groups with organic acids (Munjal et al. 2016; Rocha et al. 2017; Wu et al. 2012). In general, the biochar can be considered a suitable carrier or formulation of microbial inoculants.
The diversity and composition of bacterial community in soil are an important index reflecting biological fertility of soil. Through high-throughput sequencing, it was found that Proteobacteria, Acidobacteria, Bacteroidetes, Actinobacteria, Gemmatimonadetes, Chloroflexi, and Firmicutes were the predominant bacterial phyla in all of the soil samples. Proteobacteria are eutrophic bacteria, which usually appear in soil with rich nutrition and high carbon content (Zhang et al. 2019). The combined application of the microbial inoculants with biochar did not affect the diversity of soil bacterial community but significantly increased the relative abundance of beneficial bacteria genus Bacillus, Paenibacillus, and Flavobacterium (Fig. 3). It was demonstrated that Bacillus, Paenibacillus, and Flavobacterium can not only solubilize the P and K for plant absorption but also produce auxin which can stimulate plant growth developmental (Rocha et al. 2017; Tsukanova et al. 2017; Wang et al. 2016a). These indicated that the improvement of tomato growth by co-application of biochar and microbial inoculants was probably due to the stimulated growth of autochthonous beneficial bacteria in the soil. Furthermore, co-application of biochar and microbial inoculants may promote the healthy development of soil through solubilizing the soil P and K and promoting the growth of beneficial microbial taxa.
5 Conclusions
The application of microbial inoculants at 75 L/ha promoted the growth of tomato plants and increased bioavailability of soil phosphorus and potassium and thus increased the yields of tomato, and the application of the microbial inoculants combined with 2 t/ha biochar further enhanced the growth-promoting effects of the microbial inoculants, creating a synergetic effect. The application of microbial inoculants did not affect the diversity of soil bacterial community but increased the relative abundance of bacterial genera Bacillus, Paenibacillus, and Flavobacterium and decreased the relative abundance of Acidobacterium. The combined application of the microbial inoculants together with biochar has the potential to be an effective management tool to enhance soil fertility and health and sustain longer-term production of tomatoes in greenhouse conditions.
References
Abou-El-Seoud II, Abdel-Megeed A (2012) Impact of rock materials and biofertilizations on P and K availability for maize (Zea Maize) under calcareous soil conditions. Saudi J Biol Sci 19:55–63
Chang W, Ma M, chen H, Liu L, Du B, Li J, (2014) Effects of Paenibacillus mucilaginosus on peanut growth and soil microbiological characteristics. Chin J Appl Environ Biol 20:185–191 (in chinese)
Chen J, Liu X, Zheng J, Zhang B, Lu H, Chi Z, Pan G, Li L, Zheng J, Zhang X, Wang J, Yu X (2013) Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl Soil Ecol 71:33–44
Cochard B, Giroud B, Crovadore J, Chablais R, Arminjon L, Lefort F (2022) Endophytic PGPR from tomato roots: isolation, in vitro characterization and in vivo evaluation of treated tomatoes (Solanum lycopersicum L.). Microorganisms 10:1–17
Glodowska M, Schwinghamer T, Husk B, Smith D (2017) Biochar based inoculants improve soybean growth and nodulation. Agric Sci 08:1048–1064
Gupta P, Kumar V (2017) Value added phytoremediation of metal stressed soils using phosphate solubilizing microbial consortium. World J Microbiol Biotechnol 33:1–15
Hongdan F, Zhang G, Zhang F, Sun Z, Geng G, Li T (2017) Effects of continuous tomato monoculture on soil microbial properties and enzyme activities in a solar greenhouse. Sustainability 9:317
Huang FL, Zhang Y, Zhang LP, Wang S, Feng Y, Rong NH (2019) Complete genome sequence of Bacillus megaterium JX285 isolated from Camellia oleifera rhizosphere. Comput Biol Chem 79:1–5
Korir H, Mungai NW, Thuita M, Hamba Y, Masso C (2017) Co-inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Front Plant Sci 8:141
Li Q, Lu X, Ma L, Li B, Liu W (2017) Effect of Bacillus mucilaginosus agents on four species vegetable seeds germination and seedling growth. North Hortic 10–13 (in chinese)
Lv Y, Li J, Ye H, Du D, Sun P, Ma M, Zhang TC (2020) Bioleaching of silicon in electrolytic manganese residue (EMR) by Paenibacillus mucilaginosus: impact of silicate mineral structures. Chemosphere 256:127043
Mukherjee A, Lal R (2014) The Biochar Dilemma Soil Res 52:217–230
Munjal V, Nadakkakath AV, Sheoran N, Kundu A, Venugopal V, Subaharan K, Rajamma S, Eapen SJ, Kumar A (2016) Genotyping and identification of broad spectrum antimicrobial volatiles in black pepper root endophytic biocontrol agent, Bacillus megaterium BP17. Biol Control 92:66–76
Nuzzo A, Satpute A, Albrecht U, Strauss SL (2020) Impact of soil microbial amendments on tomato rhizosphere microbiome and plant growth in field soil. Microb Ecol 80:398–409
Paz-Ferreiro J, Liang C, Fu S, Mendez A, Gasco G (2015) The effect of biochar and its interaction with the earthworm Pontoscolex corethrurus on soil microbial community structure in tropical soils. PLoS ONE 10:e0124891
Raliya R, Tarafdar J, Biswas P (2016) Enhancing the mobilization of native phosphorous in mung bean rhizosphere using ZnO nanoparticles synthesized by soil fungi. J Agric Food Chem 64:3111–3118
Rocha FYO, Oliveira CMd, da Silva PRA, Melo LHVd, Carmo MGFd, Baldani JI (2017) Taxonomical and functional characterization of Bacillus strains isolated from tomato plants and their biocontrol activity against races 1, 2 and 3 of Fusarium oxysporum f. sp. Lycopersici Appl Soil Ecol 120:8–19
Semida W, Beheiry H, Setamou M, Simpson C, Ali T, Rady M, Nelson S (2019) Biochar implications for sustainable agriculture and environment: a review. S Afr J Bot 127:333–347
Sindhu SS, Parmar P, Phour M (2014) Nutrient cycling: potassium solubilization by microorganisms and improvement of crop growth, geomicrobiology and biogeochemistry. Soil Biol 175–198
Singh H, Northup BK, Rice CW, Prasad PVV (2022) Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: a meta-analysis. Biochar 4:1–17
Singh VK, Rai S, Singh D, Upadhyay RS (2021) Application of soil microorganisms for agricultural and environmental sustainability: a review. In: Dubey SK , Verma SK (Editors), Plant, Soil and Microbes in Tropical Ecosystems. Springer Singapore, Singapore 151–175
Tripti KA, Usmani Z, Kumar V, Anshumali, (2017) Biochar and flyash inoculated with plant growth promoting rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant. J Environ Manag 190:20–27
Tsukanova KA, Chebotar VК, Meyer JJM, Bibikova TN (2017) Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S Afr J Bot 113:91–102
Wang P, Wu S-H, Wen M-X, Wang Y, Wu Q-S (2016a) Effects of combined inoculation with Rhizophagus intraradices and Paenibacillus mucilaginosus on plant growth, root morphology, and physiological status of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under different levels of phosphorus. Sci Hortic 205:97–105
Wang X, Li L, Pan G, Zhang X, Zheng J, Zheng J, Liu X, Cheng K, Wang J, Yu X (2016b) Compound of Bacillus mucilaginosus and biochar and its effects on tomato yield and quality. Siols 48:479–485 (in chinese)
Wu F, Wan Judy Hon C, Wu S, Wong M (2012) Effects of earthworms and plant growth–promoting rhizobacteria (PGPR) on availability of nitrogen, phosphorus, and potassium in soil. Soil Sci Plant Nutr 175:423–433
Xi L, Song A, Gong M, Zhang L (2009) Preliminary study on the potassium-dissolving mechanism of silicate bacteria from cotton rhizosphere. Acta Agric Boreali-Occident Sin 18:309–314 (in chinese)
Yagmur B, Gunes A (2021) Evaluation of the effects of plant growth promoting rhizobacteria (PGPR) on yield and quality parameters of tomato plants in organic agriculture by principal component analysis (PCA). Gesunde Pflanzen 73:219–228
Yusoff MZ, Hu A, Feng C, Maeda T, Shirai Y, Hassan MA, Yu CP (2013) Influence of pretreated activated sludge for electricity generation in microbial fuel cell application. Bioresour Technol 145:90–96
Zhang J, Wang P, Tian H, Xiao Q, Jiang H (2019) Pyrosequencing-based assessment of soil microbial community structure and analysis of soil properties with vegetable planted at different years under greenhouse conditions. Soil Tillage Res 187:1–10
Zhao F, Zhang Y, Li Z, Shi J, Zhang G, Zhang H, Yang L (2019a) Vermicompost improves microbial functions of soil with continuous tomato cropping in a greenhouse. J Soils Sediments 20:380–391
Zhao Y, Zhang M, Yang W, Di HJ, Ma L, Liu W, Li B (2019b) Effects of microbial inoculants on phosphorus and potassium availability, bacterial community composition, and chili pepper growth in a calcareous soil: a greenhouse study. J Soils Sediments 19:3597–3607
Zhao Y, Mao X, Zhang M, Yang W, Di HJ, Ma L, Liu W, Li B (2021) The application of Bacillus Megaterium alters soil microbial community composition, bioavailability of soil phosphorus and potassium, and cucumber growth in the plastic shed system of North China. Agr Ecosyst Environ 307:107236
Zheng X, Wang Z, Zhu Y, Wang J, Liu B (2020) Effects of a microbial restoration substrate on plant growth and rhizosphere bacterial community in a continuous tomato cropping greenhouse. Sci Rep 10:13729
Zhou C, Ma Z, Zhu L, Xiao X, Xie Y, Zhu J, Wang J (2016) Rhizobacterial strain Bacillus megaterium BOFC15 induces cellular polyamine changes that improve plant growth and drought Resistance. Int J Mol Sci 17:976
Zhu X, Chen B, Zhu L, Xing B (2017) Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ Pollut 227:98–115
Funding
This work was financially supported by the Hebei Province Agricultural Industry System Project (HBCT2018030206); the Innovation Team Project of State Key Laboratory of North China Crop Improvement and Regulation (NCCIR2020CX6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Yuan Ge
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei Yang and Yingnan Zhao contributed equally to this work.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, W., Zhao, Y., Yang, Y. et al. Co-application of biochar and microbial inoculants increases soil phosphorus and potassium fertility and improves soil health and tomato growth. J Soils Sediments 23, 947–957 (2023). https://doi.org/10.1007/s11368-022-03347-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-022-03347-0