Abstract
Purpose
To better control and manage phosphorus (P) in the aquatic environment of the Qiuxi River, a typical river severely influenced by anthropogenic activities in southwest China, the morphological characteristics and the release potential of P in sediments during different water seasons were determined.
Materials and methods
The overlying water and sediment samples were collected from the Qiuxi River in the normal-water season (NWS), low-water season (LWS), and high-water season (HWS), and physicochemical properties were quantified. Total phosphorus (TP) in sediment and the inorganic phosphorus (Pi) fraction were analyzed using the standard measures technology (SMT) procedure, and organic phosphorus (Po) was further determined to be labile Po (LOP), moderately labile Po (MLOP), or non-labile Po (NLOP). The release potential of P from the sediments was studied by experiments under simulated conditions.
Results and discussion
The results showed that P pollution in the Qiuxi River was the most serious during the LWS, with mean TP concentrations in the sediment of 854.6 mg kg−1, and the release risk of the sediment P was also highest during this water season. The variation in P concentration was significantly influenced by the discharge of domestic sewage, agricultural diffusion, flow rate, and OM. The concentrations of P fractions varied with water season, but Pi was constantly higher than Po. HCl-extractable Pi (HCl-Pi) accounted for most of the P in the sediments, which was related to its characteristics and the geographical location of the Qiuxi River. The maximum P release (Rmax) was 25.9 mg kg−1 in the LWS and 15.3 mg kg−1 in the HWS, which were positively correlated with the TP (r2 = 0.848, p < 0.01) and OM (r2 = 0.847, p < 0.01) concentrations in the sediments and explained why the release risk of the sediment P in the LWS was higher than that in the HWS.
Conclusions
Phosphorus pollution and the P release potential in the sediments of the Qiuxi River were most severe in the LWS, so more effective measures to restrain the release of P should be taken during the LWS. Moreover, OM in sediments should be given more attention owing to its significantly positive correlation with the risk of P release.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phosphorus (P) is an essential element in the biogeochemical cycle and has important nutritional value in surface water (Wang et al. 2006b; Zhang and Shan 2008; Yang et al. 2019). However, anthropogenic activities, including over fertilization, pesticide application, fossil fuel consumption, and wastewater discharge, in catchments input a large amount of P into surface water and are responsible for eutrophication (Withers and Jarvie 2008; Zafar et al. 2016; Ye et al. 2017; Yuan et al. 2018; Yang et al. 2019). Currently, agricultural diffusion P accounts for approximately 50% of the total amount of P emissions in Germany and many other European countries (Umweltbundesamt 2014). Since the 2000s, the intensive development of agriculture has increased the P load of fertilizer and livestock breeding in agricultural areas. Nevertheless, only 10–15% of this P can be absorbed by the soil, and the rest is lost to surface or underground runoff (McDowell et al. 2001; Zhang and Shan 2008; Bortoluzzi et al. 2013; Zafar et al. 2016), demonstrating that agricultural pollution is an environmental problem that cannot be ignored. Sediment is a reservoir for pollutants from overlying water, and the continual release of sediment P under certain environmental conditions is one of the major sources of P in overlying water (Søndergaard et al. 1999; Wang et al. 2006b, 2009 Shilla et al. 2009).
The release of P from sediment to overlying water is considerably influenced by sediment and overlying water properties such as P concentration, temperature, pH, oxidation-reduction potential (ORP), organic matter (OM), and hydraulic conditions (Kim et al. 2003; Wang et al. 2006a; McDowell and Hill 2015; Zafar et al. 2016). The previous study demonstrated that the physicochemical properties of overlying water and sediment, stream discharge, and coverage of hydrophytes change with water season (Bryan et al. 2007; Zafar et al. 2016; Yang et al. 2019). Correspondingly, the fraction and release of P in sediment will vary with water season due to the influence of river properties, which causes different levels of P pollution in rivers during different water seasons (Zafar et al. 2016). Therefore, it is necessary to research the release characteristics of P in sediment according to different water seasons. The maximum P release (Rmax) is an effective parameter used to evaluate the release risk and characteristics of P from sediment to overlying water. The release kinetics experiment simulates the release process of sediment, and Rmax is obtained by fitting the first-order kinetics equation (Pettersson 2001; Wang et al. 2009).
However, not all of the P fractions are released from sediment (Ruban et al. 2001; Wang et al. 2006b; Ni et al. 2019a). The concentration of various P factions can indicate internal pollution status as well as the risk of sediment in a more effective way than total phosphorus (TP) concentration in sediment (Wang et al. 2006b; Ni et al. 2019a). Understanding the concentrations of different P fractions will help to identify the sources of P and explain the release characteristics of P in sediment (Ni et al. 2019a, b). Phosphorus exists in the form of complex salts with calcium, iron, or aluminum and OM in sediment and can also be adsorbed onto the mineral surface (Ruttenberg 1992; Ruban et al. 2001). The inorganic phosphorus (Pi) fraction is usually loosely sorbed, potentially labile P (reductant-soluble P and ferrous-bound phosphorus), non-labile P (mostly calcium-bound phosphorus (Ca-P)), and occluded P (Ruttenberg 1992; Ruban et al. 2001; Ni et al. 2019a). Organic phosphorus (Po) compounds such as saccharide phosphates, nucleotides, hemic acid, and fulvic acid are usually treated as residues or refractory parts of the P cycle (Ruttenberg 1992; Ni et al. 2019b). However, Po can be converted into Pi by phosphate hydrolysis, bacterial degradation, non-biological hydrolysis, and photolysis (Ahlgren et al. 2006; Ni et al. 2019b). Thus, the role of Po in the sediment-overlying water P cycle should not be ignored. In recent years, the Po sequential fractionation scheme has been widely used, with the fractions determined to be labile Po (LOP) (NaHCO3-Po), moderately labile Po (MLOP) (HCl-Po and fulvic acid-Po), and non-labile Po (NLOP) (hemic acid-Po and residual-Po) (Ivanoff et al. 1998; Ni et al. 2019a). Different fractions of Po have different chemical characteristics and release risks (Wang et al. 2006b; He et al. 2015; Ni et al. 2019a). It is rare for NLOP to be absorbed biologically, while LOP can be easily converted into Pi by microorganisms and released into water (Hund et al. 2013).
There are a large number of catchments severely polluted by agriculture and urban sewage in southwestern China due to the lack of domestic sewage collection systems and agricultural diffusion source pollution management, and the Qiuxi River is one of these catchments. To date, only a few studies have been conducted on P in sediments in typical diffusion-polluted regions. Our study is one of the first to research the variation in P pollution and release risk in different water seasons of these types of rivers. The main objectives of our research were to (1) evaluate seasonal characteristics of P pollution in sediments and overlying waters of the Qiuxi River; (2) determine the morphological characteristics of P in sediments and different fractions of P influenced by factors such as OM, ORP, particle size, temperature, pH, and hydraulic conditions in different water seasons; and (3) conduct simulation experiments to study the release characteristics of P in different water seasons.
2 Materials and methods
2.1 Study area
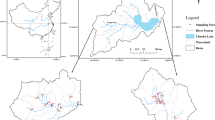
The study area is located in the Qiuxi River, Sichuan Province, southwest China. As the first-level tributary of the Tuojiang River, the Qiuxi River is the most important source of drinking water for the surrounding area, with a population of nearly 1.8 million. This river is a typical watershed in southwest China, most of which are secondary tributaries of the upper reaches of the Yangtze River, which is the largest river in China. There are 63 towns around the Qiuxi River, where the population is approximately 3.7 million. Phosphorus pollution in the Qiuxi River was most affected by domestic, farm, and agricultural factors, and the pollutants came mainly from three tributaries, including the Longshui River, Tongjiang River, and Qingshui River (Figs. 1 and 2) (Sichuan Provincial Department of Environmental Protection (SPDEP) 2017).
The proportion of P pollutants discharged into rivers from different pollution sources (a), the proportion of total P pollutants in different tributaries (b). The total amount of P emissions from the five pollution sources was 693.50 t/year (SPDEP 2017)
The concentration of TP in the overlying water was monitored in the national control section from 2011 to 2016, which is located in the confluence section of the Qiuxi River and Tuojiang River (Table 1). The TP concentration in the overlying water of the Qiuxi River was higher than the standard of grade V of 0.2 mg L−1 and even reached 0.42 mg L−1 in 2016 (Standard of Surface Water Environment Quality of China (GB 3838–2002) 2002). After 2016, the growth rate of industrial development in the Qiuxi River basin was approximately 8.5%, and the population growth rate was approximately 2.6%, which increased P emissions.
The rainfall is unevenly distributed during different seasons, and precipitation is highest in summer, followed by autumn, and lowest in spring and winter (SPDEP 2017). According to the water level and flow rate, the Qiuxi River is divided into three water seasons: the normal-water season (NWS), low-water reason (LWS), and high-water season (HWS) (Sichuan Provincial Government 2017; Table 2).
2.2 Sample collection
According to the hydrogeological characteristics of the Qiuxi River and the amount of pollutant input, we selected six representative sampling sites (Table 3, Fig. 1). They are distributed on the main stream and in three tributaries of the Qiuxi River (SPDEP 2017). A total of eighteen surface sediment (0–10 cm) samples and eighteen water samples were collected during the NWS (November 2017), LWS (March 2018), and HWS (September 2018). Each site was located with a global position system (GPS) unit, and the coordinates are provided in Fig. 1. In order to ensure that the sediment sample is representative, 500 g sediment sample and 800 mL water sample are composed of a mixture of samples collected at three sites. Prior to analysis, the sediment samples were immediately stored in sealable plastic bags at a temperature of 2–5 °C. The sediment samples were dried at 105 °C and then sieved with a 2-mm sieve before the experiment to ensure homogeneity prior to analysis. The water samples were stored in polyethylene bottles and placed at 4 °C. The concentration of TP in the water samples was determined within 3 days.
2.3 Analytical methods
2.3.1 Physicochemical properties of sediment and TP in overlying water
The temperature, pH, and ORP of the sediment at every sampling site were monitored in situ by portable parameter water quality meter. The organic matter was measured as loss on ignition (550 °C for 2 h) (Jensen et al. 1992). The sediment grain size distribution was measured with a laser particle size analyzer (Microtrac S2000) and was classified into three categories: clay (< 0.002 mm), silt (0.002–0.02 mm), and sand (0.02–2 mm) (Day 1965).
2.3.2 TP determination and Pi and Po fractionation scheme in sediment
The TP concentration and P fractions of sediments were analyzed using the standard measures technology (SMT) procedure (Ruban et al. 2001) (Fig. 3). Sediment P was separated into Po, Pi, NaOH-Pi, and HCl-Pi. NaOH-Pi is NaOH-extractable P including P bound to Al, Fe, Mn oxides, or hydroxides. HCl-Pi is HCl-extractable P including Ca-bound P. Organic P extraction method used in this study was from Ivanoff et al. (1998) (Fig. 3). Three Po fractions, including LOP (NaHCO3-Po), MLOP (HCl-Po and fulvic acid-Po), and NLOP (hemic acid-Po and residual-Po), were obtained using the sequential extraction procedure. Due to the difficulty of sampling S1, S3, and S4 in the NWS, a small number of sediment samples were collected, and only Po fractions in the NWS were determined.
TP determination method and sequential extraction scheme of the Pi (Ruban et al. 2001) and Po (Ivanoff et al. 1998) fractions in sediments. SRP soluble reactive phosphorus, TP total phosphorus, Pi inorganic phosphorus, Po organic phosphorus, HCl-Pi HCl-extractable inorganic phosphorus, NaOH-Pi NaOH-extractable inorganic phosphorus, LOP labile organic phosphorus, MLOP moderately labile organic phosphorus, NLOP non-labile organic phosphorus
2.3.3 Sediment P release kinetics experiment
The dry sediments (0.25 g) were put into centrifuge tubes and mixed with 25 mL of KCl solution (0.02 mol L−1). These centrifuge tubes were shaken at 200 r min−1 at 25 °C in a shaker. Samples were collected after 0.5, 1, 2, 4, 8, 12, 24, and 36 h and centrifuged at 5000 r min−1 for 10 min. The soluble reactive P concentration of the supernatant was measured, and sediment P release was calculated using the following Formula (1) (Tang et al. 2014):
where R represents the amount of P (mg kg−1) released from sediment under experimental conditions, Ce is the concentration (mg L−1) of P in the solution and C0 is the initial concentrations of P in the solution, which is 0 mg L−1, V is the volume (mL) of added water, and W is the quality (g) of the dried sediment.
Phosphorus release kinetics is fitted with a first-order kinetic equation (Hansen and Strawn 2003) (Eq. (2)).
where Rt is the amount of P released at time t (mg kg−1), Rmax is the maximum release of P (mg kg−1), k is the release rate constant of P, and t is the release time (h).
2.4 Data analysis and quality control
Pearson correlation analysis was carried out to identify the relationships among the various forms of P in the studied sediments, physicochemical characteristics of sediment, and Rmax. Data were subjected to ANOVA tests to evaluate the data of different water seasons and sampling site. Significant differences were reported at the p < 0.05 level. All statistical analyses were conducted using the SPSS 22.0 (IBM, USA) software package. Figures were created using the Origin 8.0 (OriginLab, USA) software package. Measurements in triplicate of each sample were conducted, and their arithmetic mean values were reported.
3 Results
3.1 Physicochemical characteristics of sediment
The physicochemical characteristics of the sediments in the Qiuxi River had seasonal differences because of the significant differences in climate characteristics (Table 4). In general, OM concentration in sediments was the largest in the HWS, which was similar to that in the LWS, and the smallest concentration was observed in the NWS. This indicated that the input or sedimentation of OM in the HWS was the largest, and OM may accumulate gradually from the NWS to the HWS. In the NWS and HWS, the sediments were in a state of oxidation, while there were reduction conditions in the LWS, with an average ORP of − 211.0 mV. The sand fraction (0.02–2 mm) dominated in the studied sediments, with averages of 72.2%, 85.1%, and 84.1% in the NWS, LWS, and HWS, respectively. The results of Ye et al. (2017) showed that the contents of clay, silt, and sand in the Tiaoxi River watershed were 5.4–20.7%, 68.5–75.7%, and 6.6–26.1%, respectively. The temperature gradually increased owing to the sampling times in NWS, LWS, and HWS being in winter (November), spring (March), and summer (September), respectively. The pH in the sediment increased from the LWS to HWS.
3.2 Seasonal variation of TP in overlying water and sediment
In general, P pollution in sediments and overlying water was the most severe in the LWS, followed by the NWS and HWS, and it was correspondingly suggested that P pollution is addressed during the LWS. The average TP concentrations of the overlying water was higher in the LWS (1.2 mg L−1) than that in the HWS (0.3 mg L−1) and NWS (0.2 mg L−1) (Fig. 4a), which was attributed to the low water volume in the LWS. The overlying water TP concentrations had no significant trend in the upper and lower reaches of the river in the NWS and HWS, while the mainstream TP concentrations of the Qiuxi River were higher than those of the other reaches in the LWS. The seasonal distribution of the concentration of TP in the sediment varied considerably, and the average concentration of TP (854.6 mg kg−1) in the LWS was higher than that in the HWS (612.2 mg kg−1) (Fig. 4b). The concentration of TP in sediments at all sampling sites did not exceed sediment quality guidelines for the “severe effect level” of 2000 mg kg−1 set by the Canadian Province of Ontario (Persaud et al. 1993; Owens and Walling 2002). However, according to Chinese environmental dredging common standards, when TP > 500 mg kg−1, the sediment is considered to be polluted and should be dredged (Liu et al. 1999). The TP concentration of sediments from five sampling sites of the LWS reached this limit, and four sampling sites reached this limit in the HWS. Therefore, effective measures need to be taken to control the source of P pollution and remove contaminated sediment.
TP concentrations in the overlying water (a) and sediments (b) in different water seasons. Different uppercase letters indicate that the concentration of TP in same site has significant differences (p < 0.05) among different water seasons. Different lowercase letters indicate that the concentration of TP in same water season has significant differences (p < 0.05) among different sampling sites. Due to the difficulty of sampling and TP in NWS was not determined
3.3 P fraction seasonal variation in sediment
3.3.1 Pi fraction seasonal variation
The seasonal distribution of the P fractions varied considerably in the Qiuxi River, and Pi was always the primary constituent of P. Due to the difficulty of sampling in the NWS, a small number of sediment samples were collected, and the Pi fraction in the NWS was not determined. The average inorganic P fraction accounted for 84.6% of the TP in the sediments during the LWS and HWS (Fig. 5). The average concentrations of Pi in the LWS and HWS were 732.6 mg kg−1 and 517.0 mg kg−1, accounting for 85.7% and 84.6% of the TP, respectively. The average Po concentrations in the sediments were 122.0 mg kg−1 and 95.2 mg kg−1 , accounting for 14.28% and 15.4% of the TP in the LWS and HWS, respectively. With waster season transfer from the LWS to the HWS, the concentrations of sediment Pi and Po decreased, but the proportions of TP maintained stability, indicating that both Pi and Po were released to the overlying water. From Fig. 5, HCl-Pi was the main fraction of Pi accounting for an average of 77.6% of the Pi fraction. The average concentrations of HCl-Pi were 519.9 mg kg−1 and 419.3 mg kg−1 and accounted for 72.4% and 82.9% of Pi in the LWS and HWS, respectively. The high HCl-Pi concentration was attributed to the calcareous terrain of the recharge area and land utilization (Wang et al. 2006b; Ni et al. 2019a). Although HCl-Pi concentrations in sediments decreased from the LWS to the HWS, its proportion in Pi increased, suggesting that NaOH-Pi has a weaker potential to be released than that of other forms of P. The average concentration of NaOH-Pi was 138.9 mg kg−1, accounting for 19.0% of the Pi fraction in the LWS, and decreased to 51.7 mg kg−1 (only 8.7% of the Pi fraction) in the HWS, which indicated that NaOH-Pi was released from the LWS to the HWS.
Concentrations and percentages of different Pi fractions in the sediments in the LWS (a, b) and HWS (c, d). Pi inorganic phosphorus, Po organic phosphorus, HCl-Pi HCl-extractable inorganic phosphorus, NaOH-Pi NaOH-extractable inorganic phosphorus. Different uppercase letters indicate that the concentration of same P fraction has significant differences (p < 0.05) among different water seasons. Different lowercase letters indicate that the concentration of same P fraction has significant differences (p < 0.05) among different sampling sites. Due to the difficulty of sampling in NWS, a small amount of sediment samples were collected, and Pi fractions in NWS was not determined
3.3.2 Po fraction seasonal variation
For the Po fractions, the ratios of LOP to MLOP to NLOP were 34.1:62.2:3.7, 30.8:61.7:7.5, and 9.2:50.9:40.6 in the NWS, LWS, and HWS, respectively (Fig. 6). The main component of Po fractions of sediments was MLOP and LOP in the NWS and LWS and NLOP in the HWS. These suggested that the MLOP and LOP of the main component of sediment Po in the Qiuxi River was consistent with the results in Lakes Meiliang (47.8%), Yuantouzhu (47.3%), and Gonghu (47.3%) (Zhang et al. 2008). Furthermore, LOP was high in the NWS and LWS, with an average value of 34.1% and 30.8%, respectively. Overall, from the NWS to the HWS, the proportions of LOP (30.8 to 9.2%) and MLOP (61.7 to 50.9%) of the Po fraction decreased, and that of NLOP (3.7 to 40.6%) increased. Compared with LOP and MLOP, the main source of plant-available P, NLOP had weaker bioavailability (Turner et al. 2005).
Seasonal distribution of the Po fractions, including labile organic phosphorus (LOP) (a), moderately labile organic phosphorus (MLOP) (b), and non-labile organic phosphorus (NLOP) (c). Values represent mean ± SD; different letters indicate significant differences among different water seasons (p < 0.05)
According to the fraction method of Ivanoff et al. (1998), LOP represented NaHCO3-Po, MLOP included HCl-Po and fulvic acid-Po, and NLOP denoted hemic acid-Po and residual-Po. We observed the following results in the NWS and LWS: HCl-Po > NaHCO3-Po > fulvic acid-Po > hemic acid-Po > residual-Po; the average HCl-Po to NaHCO3-Po to fulvic acid-Po to hemic acid-Po to residual-Po ratio was 35.8:22.3:4.9:1.4:0.9 in the NWS and 47.6:30.8:14.0:4.6:3.0 in the LWS (Fig. 7). However, the pattern was HCl-Po > residual-Po > fulvic acid-Po > hemic acid-Po > NaHCO3-Po in the HWS, and the average HCl-Po to NaHCO3-Po to fulvic acid-Po to hemic acid-Po to residual-Po ratio was 38.9:32.0:12.0:8.8:8.5 (Fig. 7). The concentration of HCl-Po in the sediments of the Qiuxi River was the highest compared to that of other fractions of Po in general and ranged from 12.9 to 84.7%, with the highest average value of 47.6% in the LWS. The HCl-Po is in the fraction of biomacromolecules and is labile to be decomposed, hydrolyzed, and mineralized and has potential biological effectiveness (Zhang et al. 2008). This suggested that there was a high possibility of release of Po from the sediments of the Qiuxi River. The dissolved Po compounds in overlying water are bioavailable, which could lead to a high risk of eutrophication, especially during the LWS (Wetzel 2001). The proportion of NaHCO3-Po (LOP) was second only to HCl-Po in the NWS (accounting for 22.3% of Po) and LWS (30.8%) and gradually decreased to the least prevalent component in the HWS (8.5%) because NaHCO3-Po mainly consisted of phospholipids, nucleic acids, and phosphosaccharides and was easy to mineralize and decompose (Zhu et al. 2013). The NaHCO3-Po (LOP) concentration was positively correlated with the nutritional loading of sediments, which suggests that the proportion of LOP is high in sediments with high nutrient loading (Zhang et al. 2008). Fulvic acid-Po belongs to the MLOP fraction, which accounted for 3.1–11.6% of Po in the NWS, 4.5–22.9% in the LWS, and 4.9–16.2% in the HWS. The proportion of hemic acid-Po, which consists of inositol five-phosphate and inositol hexa-phosphate (phytic acid), was 0.2–4.0% in the NWS, 0.02–7.1% in the LWS, and 4.1–13.8% in the HWS (Ni et al. 2019a). The concentration of residual-Po, which consists of phytic acid–bound P and provides resistance to biodegradation, was 0.4–3.7% in the NWS, 1.4–18.0% in the LWS, and 19.9–44.4% in the HWS (Bowman and Cole 1978).
The relationship between geochemical fractions of P and physicochemical properties was analyzed using the Pearson correlation (Table 5). The TP in sediment was significantly positively correlated with Pi during the HWS (r2 = 0.990, p < 0.01) and LWS (r2 = 0.998, p < 0.01) since Pi was always the primary fraction of P in the Qiuxi River sediments. HCl-Pi was the main fraction of Pi; therefore, the correlation between HCl-Pi and TP (r2 = 0.982 and 0.951, p < 0.01) was also the most significant. Comparatively, Po and TP showed a certain correlation only in the HWS, in which MLOP (r2 = − 0.865, p < 0.05) and NLOP (r2 = 0.881, p < 0.05) were correlated with TP. There was also a correlation between Pi and Po; in the LWS, only NaOH-Pi and NLOP were positively correlated (r2 = 0.833, p < 0.05). In addition, HCl-Pi was positively correlated with NLOP (r2 = 0.879, p < 0.05) in the HWS, and NaOH-Pi was significantly positively correlated with LOP (r2 = 0.934, p < 0.01). This may be because both HCl-Pi and NLOP were stable P forms, and NaOH-Pi and LOP were easily released P forms.
3.4 P release characteristics in different water seasons
Phosphorus release from sediments to overlying water is a complex kinetic process, including fast and slow-reaction stages (Fig. 8) (Hansen and Strawn 2003; Wang et al. 2009). In our study, P release was a fast-reaction stage for the first 8 h, with an average release rate of 2.0 mg (kg h)−1 in LWS and 1.7 mg (kg h)−1 in HWS. After that, P release stabilized in a slow-reaction stage and tended to reach a dynamic balance, with an average release rate of 0.08 mg (kg h)−1 in the LWS and 0.04 mg (kg h)−1 in the HWS. The results revealed that the release rate in the fast-reaction stage was much higher than that in the slow-reaction stage. To further characterize the risk of P release in sediment, the initial release rate v0 of sediment was introduced and defined as the P release amount per unit mass of sediment within 0.5 h from the start of the reaction (Wang et al. 2006b). The v0 was 10.1–25.9 mg (kg h)−1, with an average of 18.0 mg (kg h)−1 in the LWS and 0.8–32.5 mg (kg h)−1, with an average of 9.4 mg (kg h)−1 in the HWS (Table 6). The whole P release process in the sediments of the Qiuxi River was fitted with the first-order kinetic equation (p < 0.05). The Rmax evaluates the release potential and risk of sediment in this study condition (He et al. 2015). The Rmax of sediments in our study was 9.4–44.0 mg kg−1, with an average value of 25.9 mg kg−1 in the LWS, and 6.0–36.7 mg kg−1, with an average value of 15.3 mg kg−1 in the HWS (Table 6). Comparing the Rmax and v0 of the Qiuxi River in the LWS and HWS, the results show that the release risk of P in the LWS was greater than that in the HWS, except for S3, which might be attributed to the TP concentration in the LWS sediments being higher than that in the HWS sediments.
4 Discussion
4.1 Correlation between P fractions and sediment properties
Generally, P in sediments is transported to the overlying water and organisms through interstitial water under the influence of environmental factors such as temperature, pH, OM, ORP, sediment particle size, and aquatic plants (Zhang et al. 2008; Wu et al. 2014; McDowell and Hill 2015; Yang et al. 2019). Moreover, physicochemical properties and sediment compositions directly affect P fraction distributions, and their relationships can reflect the transformation of different P in sediment (Yang et al. 2019). According to the correlation analysis (Table 5), OM was the most significant correlation with the fraction distribution of P among all sediment property parameters in the LWS. The concentration of OM was positively correlated with the concentrations of TP, NaOH-Pi, MLOP, and NLOP (r2 = 0.840, 0.843, 0.824, and 0.904, p < 0.05) in the LWS; therefore, much more attention should be paid to the effect of OM on sediment P during the LWS in the Qiuxi River than during other seasons. In general, the sources of OM in sediments mainly include (1) bacteria, phytoplankton, and plants in nature (Otero et al. 2000) and (2) anthropogenic activities such as agriculture, urban runoff, and industry (Watanabe and Kuwae 2015; Ni et al. 2019a). There were a large number of farmland and residential buildings in the Qiuxi River Basin. Non-point agricultural sources and untreated domestic sewage were responsible for the increase in the concentration of OM in sediments. Organic matter can change the function of sediments as “source” and “sink” by changing the adsorption process and mode of various fractions of Po (Wang et al. 2012; Yang et al. 2019). In addition, microorganisms in sediment can consume OM combined with Po, convert Po into Pi, and then release it into overlying water (Wang et al. 2008). Moreover, OM exhibited a correlation with Pi by interfering with metal cation binding to P. Specifically, Ca2+ can form a binary complex with OM (OM-Ca2+), which can form a poorly soluble P-Ca-OM (part of HCl-Pi) complex (Lei et al. 2018). Furthermore, OM can inhibit P adsorption on iron oxides by forming negatively charged surface sites. However, in this study, there was a poor correlation between HCl-Pi and OM, which was inconsistent with other studies (Lei et al. 2018; Ma et al. 2019). The possible reason for this might be that HCl-Pi is usually closely related to inorganic apatite and carbonates from rock debris and soils (Ni et al. 2019a). In addition, we regard HCl-Pi as Ca-P, but HCl can also extract the P combined with other metals in addition to Ca-P.
The pH also affected the distribution of P, and MLOP had a positive correlation with pH in the LWS (r2 = 0.859, p < 0.05). Acid and alkaline conditions are favorable for the release of P, and the amount of P release was lowest under neutral conditions (Liang et al. 2015). The sediments (the average pH values in the NWS, LWS, and HWS were 9.1, 8.3, and 8.9, respectively) were under alkaline conditions in the Qiuxi River, thereby accelerating the release of Pi and Po. For Po, acidic conditions are favorable for HCl-Po (MLOP) release, while alkaline conditions accelerate hemic acid-Po (MLOP) and fulvic acid-Po (NLOP) release (Wu et al. 2014; Liang et al. 2015). Comparatively, Pi is mainly released by ion exchange, which is impacted by the pH affecting the combination of P and metal ions, as OH− competes with phosphate anions and weakens Fe and Al fixation of P, leading to an increase in P release (Beutel et al. 2008; Liang et al. 2015; Ma et al. 2019). The effect of pH on P release is reflected in the morphogenesis of sediments, and different sediments have different fraction conditions of P, so the effect of pH on P release in sediments is also different (Wang et al. 2006b; Liang et al. 2015).
The TP concentration of the sediments in the LWS was significantly higher than that in the HWS, which was similar to the Lower Changjiang River, China (Duan et al. 2008; Table 7). However, some previous studies had opposite results (Table 7). This result suggested that the TP concentrations of sediments in different catchments show different rules with the variation in the water season. On the one hand, in the HWS, runoff would bring shoreside pollutants into the catchment, leading to an increase in the concentration of P in the river (Duan et al. 2008; Varol 2012; Zafar et al. 2016). On the other hand, during the HWS, a large amount of river water had a dilution effect on the pollutants in the catchment (Lei and Keming 2011). The dilution effect of runoff on the pollutants was dominant. The dilution capacity of the overlying water was different due to the variation in the flow rate of the river (the average values were 10.0 m3 s−1 in the LWS and 31.6 m3 s−1 in the HWS), resulting in the concentrations of TP in the overlying water decreasing from 1.2 mg L−1 in the LWS to 0.3 mg L−1 HWS (SPDEP 2017). In addition, we analyzed the other reasons why P pollution was worst in the LWS compared to that in the HWS in the Qiuxi River and considered the following: (1) the release of P from the sediment to the overlying water increased because the activities of microorganisms and enzymes increased as the temperature increased from 21.0 to 24.5 °C (Table 4) (Søndergaard et al. 1999; Bryan et al. 2007; Liu et al. 2018), and (2) the exposed sediments were submerged, leading to the sediment-water interface changing from an oxidation to a reduction state; therefore, Fe3+ would be reduced to Fe2+, resulting in the release of Fe3+-P (Jensen et al. 1992; Wang et al. 2006b; Pellegrini et al. 2010). However, different from the overall trend, the concentration of TP in the sediment at S3 increased from the LWS to the HWS since there were a large number of submerged plants in the sediments at this sampling site in the LWS, which mitigated the resuspension of sediments and release of P, resulting in the accumulation of P in sediments, while a large number of dead tape grass in the HWS caused P fixed in the tape grass to be released to the sediment and overlying water (Wang et al. 2018). The release of P from sediments was influenced by many environmental factors and needs to be further studied through simulation experiments (Table 8).
4.2 Influence of anthropogenic activities on P fractions in sediment
The anthropogenic activities in the Qiuxi River, including diffuse pollution of agriculture and discharge of sewage, especially wastewater, also affect the P concentration (Zafar et al. 2016; SPDEP 2017; Ni et al. 2019a, b). There are approximately 0.4 million urban and 1.3 million rural residents around this area, making this area a representative catchment severely influenced by anthropogenic activities in southwest China. In the Qiuxi River, the P pollutants discharged from domestic sewage accounted for 49% of the total pollution sources, and agricultural pollution sources accounted for 24% the total pollution sources (Fig. 2). There were three sampling sites mainly polluted by domestic sewage, including S1, S2, and S5, which were located downstream of the town. For the concentration of sediment TP in these three sampling sites, the average was 1125.9 mg kg−1 in the LWS and 601.5 mg kg−1 in the HWS. In comparison, S3, S4, and S6 are located in an agricultural area mainly affected by agricultural activities (SPDEP 2017). The concentration of sediment TP in these three sampling sites (the average was 583.4 mg kg−1 in the LWS and 622.9 mg kg−1 in the HWS) was similar to the mean value of the Bronx River sediments (580 mg kg−1 in 2006 and 793 mg kg−1 in 2007) with agricultural pollution, while it was higher than the mean value of sediment TP in Illinois streams (360 mg kg−1) (McDaniel and David 2009; Wang and Hari 2011).
The previous study demonstrated that urban untreated discharges may be a single major P source if not wisely managed, proving a major threat to water quality (Zafar et al. 2016; Ni et al. 2019a). Sampling site S5 was located downstream of Beidou Town with a permanent population of approximately 50,000; therefore, it was the most severely influenced by domestic sewage among all sampling sites. A large amount of domestic sewage was discharged into the stream in this area due to the lack of a sewage pipeline system in the households along the riparian. Previous studies found that domestic sewage usually contains NaOH-Pi, and the concentration of NaOH-Pi in sediments can be used as an effective index of P load in industrial and domestic sewage (Ni et al. 2019a). It can also be observed from correlation analysis that there was a positive correlation between TP in sediment and NaOH-Pi (r2 = 0.814, p < 0.05). It is worth noting that previous studies have shown that domestic sewage contains a large amount of Fe/Al-P. However, due to the limitations of the method, the extracted P cannot be accurately classified as a certain type of metal-bound P, which may become the focus of our future research. In this study, NaOH-Pi is regarded as Fe/Al-P, but NaOH may extract P combined with other metals. NaOH-Pi was once used for the estimation of available P in the sediments and was an indicator of algal available P (Wang et al. 2006b; Ye et al. 2017). Therefore, the NaOH-Pi from domestic sewage would increase the risk of eutrophication.
Furthermore, agricultural activities also significantly increase the concentration of Pi in sediments (Ni et al. 2019a). The main reason for this increase is P emission from fertilizer and livestock farming, as the farmland soil can only absorb 10–15% of the P, and the rest is lost to the river through surface runoff or soil erosion, leading to a large increase in P such as phytate P in the river (Zhang and Shan 2008; McDaniel and David 2009). Phytate P, belonging to the NLOP, includes hemic acid-Po and residual-Po and comes mainly from the indigestible P components of crops (such as legumes, triticeae, cereals) (Waldrip et al. 2005; Ni et al. 2019a). In the HWS, the correlation between NLOP and TP in sediment was positive (r2 = 0.881, p < 0.05) and was an important component of Po, accounting for 40.6% of Po on average in the Qiuxi River. Non-labile Po is difficult to release in sediments, but it can be hydrolyzed by phytase hydrolyzation (Zhu et al. 2013). Nevertheless, agricultural development has increased the concentration of phytase hydrolyzation in soil, which induces an increase in NLOP release in sediments when P migrates to rivers (Suzumura and Kamatani 1995; Cheng and Lim 2006).
In addition, a previous study illustrated that the concentration of HCl-Pi in sediments of agricultural lakes or rivers was relatively high (accounting for 70% of TP) because P fertilizer usually contains a large amount of calcium (Dittrich et al. 2013), and the average proportion of HCl-Pi in our research was 77.6% in the Qiuxi River. Moreover, the background value of Ca concentration in the soil will also affect the concentration of HCl-Pi in sediments. In southwest China, a large amount of carbonate apatite exists in the soil, and calcareous soil accounted for the majority of carbonate apatite in the soil. The soil area with calcium carbonate concentrations ≥ 1% accounted for 64.1% of the total cultivated land area (Wang et al. 2006b; Ni et al. 2019a). HCl-Pi is stable in sediments and is generally not bioavailable (Wang et al. 2006b; Ye et al. 2017). A large amount of input and release difficulty is the reason for the increase in HCl-Pi in the sediment. All of the above results illustrated that the P pollution of agricultural pollution sources and domestic sewage pollution sources cannot be ignored.
4.3 Influence of sediment properties on P release in sediment
Sediment release is a complex kinetic process affected by many factors, such as OM, ORP, and sediment particle size; therefore, investigating the kinetic characteristics of sediment release could be useful to evaluate the P release risk (Kim et al. 2003; Wang et al. 2006a, 2009; Zafar et al. 2016). In our study, OM was positively correlated with Rmax and v0 (r2 = 0.847, p < 0.01; r2 = 0.702, p < 0.05), which implied that P release was greatly affected by OM. The results from He et al. (2015) and Wang et al. (2017) also demonstrated that the river carbon cycle had a significant impact on the migration and transformation of P in sediments. Moreover, a study revealed that there is a positive correlation between OM and alkaline phosphatase activity, thereby indicating that the higher the OM concentration is, the higher the alkaline phosphatase activity; therefore, more Po is released (Wang et al. 2006a, 2012; Yang et al. 2019). According to the results of correlation analysis, Rmax had a negative correlation with ORP (r2 = − 0.704, p < 0.05) demonstrating that the risk of P release from sediment increases when sediment is in a reduced state since iron is labile and will combine with P to increase the migration of P and, and the reduction of Fe3+ and sulfate promotes the mobilization of P under reducing conditions (Dieter et al. 2015). Furthermore, fine particles (e.g., clay, silt) usually have higher specific surface area, more P-retention components (e.g., Al/Fe oxides or hydroxides), and correspondingly stronger adsorption abilities than coarser particles (sand) (Zafar et al. 2016; Ye et al. 2017; Yang et al. 2019). However, sediment particle size had no correlation with Rmax and v0 (p > 0.05).
A significantly positive correlation between Rmax and TP (r2 = 0.848, p < 0.01) illustrated that the higher the TP concentration in sediments was, the greater the Rmax. Similarly, a previous study also showed that the magnitude of the P released is different among the different concentrations of P sediments, and a high P concentration has a high risk for P release (Gloor et al. 2005; Wang et al. 2006b, 2009). This explained why the Rmax in the Qiuxi River during the LWS was greater than that in the HWS (the average concentration of sediment TP was 854.6 mg kg−1 and 612.2 mg kg−1, and the average Rmax was 25.9 mg kg−1 and 15.3 mg kg−1 in the LWS and HWS, respectively) (Fig. 4b; Table 6). However, the Rmax of P in S3 sediment was higher in HWS (36.7 mg kg−1) than in LWS (21.8 mg kg−1) might be because the concentration of sediment TP in the HWS (916.5 mg kg−1) was higher than that in the LWS (679.0 mg kg−1).
In addition, the difference in P forms and the different bonding interactions between different P forms and sediments have an impact on the release risk of P in sediments (Wang et al. 2006b, 2009). Inorganic P was the main P fraction in the sediments of the Qiuxi River, and Rmax was significantly positively correlated with HCl-Pi and NaOH-Pi (r2 = 0.808 and 0.731, p < 0.01). The proportion of NaOH-Pi in Pi was not high (14.0%), while it was a potentially active P that was labile for release to the overlying water (Kaiserli et al. 2002; Wang et al. 2006b). The average concentration of NaOH-Pi decreased 62.8% from the LWS to HWS (Fig. 5). Wang et al. (2006b) studied P release from lake sediments in the middle and lower reaches of the Yangtze River areas and found that over 50% of P released was from NaOH-Pi, and approximately 10% was from HCl-Pi. The most NaOH-Pi is only temporarily immobilized in sediments and is very sensitive to changes in environmental factors such as pH and temperature. As sediment is suspended or other river biochemical reactions are processed, temporarily bound NaOH-Pi is readily released into the overlying water again (Jensen et al. 1992; Ruttenberg 1992). Generally, the condition of higher temperature and slight acid or alkaline is beneficial to the release of NaOH-Pi from the sediment (Table 4 and Fig. 4) (Wang et al. 2006a; Zafar et al. 2016). The result shows that pH (from 8.3 to 8.9) and temperature (from 21.0 to 24.5 °C) increased from LWS to HWS, and the NaOH-Pi decreased (from 138.9 to 51.7 mg kg−1). Comparatively, HCl-Pi was unlikely to be released or utilized in sediments, but it accounted for a high proportion of TP (77.6%), and a small amount of release would affect Rmax. The total Po concentration was also significantly positively correlated with Rmax (r2 = 0.781, p < 0.01). The Po compounds can be hydrolyzed by alkaline phosphatase into various orthophosphate, which is then released into the overlying water (Ni et al. 2019b).
5 Conclusions
This study is one of the first studies to research the seasonal variation of the pollution status, fractions, and release characteristics of P in a typical river catchment that is severely polluted by agriculture and urban sewage in southwestern China. We found that P concentration and the risk of P release in sediments had similar seasonal variation trends in the Qiuxi River, both of which were highest in the LWS owing to the influence of the discharge of domestic sewage, agricultural diffusion, flow rate, and OM. It is necessary to take appropriate measures to inhibit the release of sediment P during the LWS. In particular, OM was significantly correlated with P fractions and Rmax; therefore, the seasonal influence of OM on P migration and transformation in sediments is worthy of further investigation. Inorganic phosphorus (accounting for 84.6% of TP) was the main component of P, in which the HCl-Pi average accounted for 77.6%. Comparatively, MLOP was the main component of Po, and HCl-Po constituted most of the MLOP. Moreover, as the water season changed from the LWS to HWS, the average Po concentrations in sediments decreased by 26.8 mg kg−1, indicating that the contribution of the release of sediment Po to P pollution in the aquatic environment cannot be ignored. We also observed that there was a significantly positive correlation between Rmax and TP in sediments (r2 = 0.848, p < 0.01), which demonstrated the necessity of decreasing the P concentration in sediment to control P pollution in the overlying water. This paper explores the migration and transformation of P in sediments of the Qiuxi River, which provides information for sustainable development of local agriculture and data support to the study of sediments from typical agricultural polluted rivers. Moreover, the research results show that the P concentration and release risk in the sediment during the LWS was higher than that during the HWS. Therefore, taking measures to control river P pollution during the LWS should be recommended.
References
Ahlgren J, Reitzel K, Danielsson R, Gogoll A, Rydin E (2006) Biogenic phosphorus in oligotrophic mountain lake sediment: differences in composition measured with NMR spectroscopy. Water Res 40:3705–3712
Beutel MW, Horne AJ, Taylor WD, Losee RF, Whitney RD (2008) Effects of oxygen and nitrate on nutrient release from profundal sediments of a large, oligo-mesotrophic reservoir, Lake Mathews, California. Lake Reserv Manage 24:18–29
Bortoluzzi EC, dos Santos DR, Santanna MA, Caner L (2013) Mineralogy and nutrient desorption of suspended sediments during a storm event. J Soils Sediments 13:1093–1105
Bowman RA, Cole CV (1978) An exploratory method for fractionation of organic phosphorus from grassland soils. Soil Sci 12:95–101
Bryan MS, Laurence C, Rupert P, Alex K, David MP (2007) Sediment phosphorus cycling in a large shallow lake: spatio-temporal variation in phosphorus pools and release. Hydrobiologia 584:37–48
Cheng C, Lim BL (2006) Beta-propeller phytases in the aquatic environment. Arch Microbiol 185:1–13
Day PR (1965) Particle fractionation and particle-size analysis. In: Black CA et al (eds) Methods of soil analysis, 1 part. American Society of Agronomy, pp 545–567
Dieter D, Herzog C, Hupfer M (2015) Effects of drying on phosphorus uptake in re-flooded lake sediments. Environ Sci Pollut Res 22:17065–17081
Dittrich M, Chesnyuk A, Gudimov A (2013) Phosphorus retention in a mesotrophic lake under renascent loading conditions: insights from a sediment phosphorus binding form study. Water Res 47:1433–1447
Duan SW, Liang T, Zhang S, Wang LJ, Zhang XM, Chen XB (2008) Seasonal changes in nitrogen and phosphorus transport in the lower Changjiang River before the construction of the Three Gorges Dam. Estuar Coast and Shelf Sci 79:235–240
Ecological Environment Protection Department of Meishan City (2018) Monthly rainfall. http://ssthjj.ms.gov.cn/hbyw1/hjzlgg.htm (in Chinese)
Gloor M, Wuest A, Munnich M (2005) Benthic boundary mixing and resuspension induced by internal seiches. Hydrobiologia 284:59–68
Hansen JC, Strawn DG (2003) Kinetics of phosphorus release from manure-amended alkaline soil. Soil Sci 168:869–879
He J, Chen CY, Deng WM, Xu XM et al (2015) Phosphorus forms distribution and potential release characteristics at water-sediment interface in Dianchi Lake. Lake Sci 27:799–810 (in Chinese)
Hund SV, Brown S, Lavkulich LM, Oswald SE (2013) Relating P lability in stream sediments to watershed land use via an effective sequential extraction scheme. Water Air Soil Pollut 224:1643
Ivanoff DB, Reddy KR, Robinson S (1998) Chemical fractionation of organic phosphorus in selected histosols. Soil Sci 163:36–45
Jensen HS, Kristensen P, Jeppesen E, Skytthe A (1992) Iron: phosphorus ratio in surface sediment as an indicator of phosphate release from aerobic sediments in shallow lakes. Hydrobiologia 1:731–743
Kaiserli A, Voutsa D, Samaran C (2002) Phosphorus fractionation in lake sediments-Lakes Volvi and Koronia, N. Greece. Chemosphere 46:1147–1155
Kim LH, Choi E, Stenstrom MK (2003) Sediment characteristics, phosphorus types and phosphorus release rates between river and lake sediments. Chemosphere 50:53–61
Lei G, Keming MA (2011) Seasonal dynamics of nitrogen and phosphorus in water and sediment of a multi-level ditch system in Sanjiang Plain, Northeast China. Chin Geogr Sci (English) 21:437–445
Lei Y, Song B, Saakes M, van der Weijden RD, Buisman CJN (2018) Interaction of calcium, phosphorus and natural organic matter in electrochemical recovery of phosphate. Water Res 142:10–17
Liang Z, Liu ZM, Zhen SM, He R (2015) Phosphorus speciation and effects of environmental factors on release of phosphorus from sediments obtained from Taihu Lake, Tien Lake, and East Lake. Environ Toxicol Chem 97:335–348
Liu HL, Jin XC, Jing YF (1999) Environmental dredging technology of lake sediment. Chin Eng Sci 1:81–84 (in Chinese)
Liu Q, Ding S, Chen X, Sun Q, Chen M, Zhang C (2018) Effects of temperature on phosphorus mobilization in sediments in microcosm experiment and in the field. Appl Geochem 88:158–166
Ma YL, Ma J, Peng H, Chen YL, Li YT (2019) Effects of iron, calcium, and organic matter on phosphorus behavior in fluvo-aquic soil: farmland investigation and aging experiments. J Soils Sediments 19:3994–4004
McDaniel MD, David MB (2009) Relationships between benthic sediments and water column phosphorus in Illinois streams. J Environ Qual 38:607–617
McDowell RW, Hill SJ (2015) Speciation and distribution of organic phosphorus in river sediments: a national survey. J Soils Sediments 15:2369–2379
McDowell RW, Sharpley AN, Condron LM, Haygarth PM, Brookes PC (2001) Processes controlling soil phosphorus release to runoff and implications for agricultural management. Nutr Cycl Agroecosys 59:269–284
Ni ZK, Wang SR, Wu Y, Jia P (2019a) Response of phosphorus fractionation in lake sediments to anthropogenic activities in China. Sci Total Environ 134242
Ni ZK, Wang SR, Zhang BT, Wang Y, Li H (2019b) Response of sediment organic phosphorus composition to lake trophic status in China. Sci Total Environ 652:495–504
Otero E, Culp R, Noakes JE, Hodson RE (2000) Allocation of particulate organic carbon from different sources in two contrasting estuaries of southeastern USA. Limnol Oceanogr 45:1753–1763
Owens PN, Walling DE (2002) The phosphorus content of fluvial sediment in rural and industrialised river basins. Water Res 36:685–701
Pellegrini JBR, Santos DR, Goncalves CS et al (2010) Impacts of anthropic pressures on soil phosphorus availability, concentration, and phosphorus forms in sediments in a Southern Brazilian watershed. J Soils Sediments 10:451–460
People’s Republic of China (2002) Standard Surface Water Environment Quality (GB 3838-2002 (National Standard)) (in Chinese)
Persaud D, Jaagumagi R, Hayton A (1993) Guidelines for the protection and management of aquatic sediment quality in Ontario. Ontario Ministry of Environment and Energy. Queens Printer for Ontario, Ontario
Pettersson K (2001) Phosphorus characteristics of settling and suspended particles in Lake Erken. Sci Total Environ 266:79–86
Renjith KR, Chandramohanakumar N, Joseph MM (2011) Fractionation and bioavailability of phosphorus in a tropical estuary, Southwest India. Environ Monit Assess 174:299–312
Ribeiro DC, Martins G, Nogueira R, Cruz JV, Brito AG (2008) Phosphorus fractionation in volcanic lake sediments (Azores-Portugal). Chemosphere 70:1256–1263
Ruban V, Lopez-Sanchez JF, Pardo P, Rauret G, Muntau H, Quevauviller P (2001) Development of a harmonised phosphorus extraction procedure and certification of a sediment reference material. J Environ Monit 3:121–125
Ruttenberg KC (1992) Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol Oceanogr 37:1460–1482
Shilla DA, Asaeda T, Kalibbala M (2009) Phosphorus speciation in Myall Lake sediment, NSW, Australia. Wetl Ecol Manag 17:85–91
Sichuan Provincial Government (2017) Notice of Sichuan provincial development and reform commission on matters related to the adjustment of the pricing policy of Sichuan power grid (Sichuan development and reform commission [2017] no. 582). http://www.sc.gov.cn/10462/11857/13305/13312/2017/12/1/10440883.shtml (in Chinese)
Sichuan Provincial Department of Environmental Protection (SPDEP) (2017) Qiuxi River basin water quality compliance plan. http://sthjt.sc.gov.cn (in Chinese)
Søndergaard M, Jensen JP, Jeppesen E (1999) Internal phosphorus loading in shallow Danish lakes. Hydrobiologia 408:145–152
Suzumura M, Kamatani A (1995) Origin and distribution of inositol hexaphosphate in estuarine and coastal sediments. Limnol Oceanogr 40:1254–1261
Tang XQ, Wu M, Li QY, Lin L, Zhao WH (2014) Impacts of water level regulation on sediment physic-chemical properties and phosphorus adsorption–desorption behaviors. Ecol Eng 70:450–458
Turner BL, Cade-Menun BJ, Condron LM, Newman S (2005) Extraction of soil organic phosphorus. Talanta 66:294–306
Umweltbundesamt (2014) Stickstoff-und Phosphoreinträge aus Punktquellen und diffusen Quellen in die Oberflächengewässer in Deutschland. www.umweltbundesamt.de/sites/default/files/medien/384/bilder/dateien/2_abb_n-p-eintraege_2014-09-23_1.pdf (accessed 16.11.2016)
Varol M (2012) Temporal and spatial dynamics of nitrogen and phosphorus in surface water and sediments of a transboundary river located in the semi-arid region of Turkey. Catena 100:1–9
Waldrip HM, Pagliari PH, He Z, Harmel RD, Cole NA, Zhang M (2005) Legacy phosphorus in calcareous soils: effects of long-term poultry litter application. Soil Sci Soc Am J 79:1601–1614
Wang C, Liu ZS, Zhang Y, Liu BY, Zhou Q, Zeng L, He F, Wu Z (2018) Synergistic removal effect of P in sediment of all fractions by combining the modified bentonite granules and submerged macrophyte. Sci Total Environ 626:458–467
Wang JY, Hari K (2011) Assessments of potential spatial-temporal variations in phosphorus distribution and fractionation in river bed sediments. Clean-Soil Air Water 39:148–156
Wang SR, Jiao LX, Yang SW, Jin XC, Yi WL (2012) Effects of organic matter and submerged macrophytes on variations of alkaline phosphatase activity and phosphorus fractions in lake sediment. J Environ Manag 113:355–360
Wang SR, Jin XC, Bu QY, Zhou XN, Wu FC (2006a) Effects of particle size, organic matter and ionic strength on the phosphate sorption in different trophic lake sediments. J Hazard Mater 128:95–105
Wang SR, Jin XC, Zhao HC, Wu FC (2006b) Phosphorus fractions and its release in the sediments from the shallow lakes in the middle and lower reaches of Yangtze River area in China. Colloids Surf A Physicochem Eng Asp 273:109–116
Wang SR, Jin XC, Zhao HC, Zhou XN, Wu FC (2008) Effects of organic matter on phosphorus release kinetics in different trophic lake sediments and application of transition state theory. J Environ Manag 88:845–852
Wang SR, Jin XC, Zhao HC, Wu FC (2009) Phosphorus release characteristics of different trophic lake sediments under simulative disturbing conditions. J Hazard Mater 161:1551–1559
Watanabe K, Kuwae T (2015) How organic carbon derived from multiple sources contributes to carbon sequestration processes in a shallow coastal system? Glob Chang Biol 21:2612–2623
Wetzel RG (2001) Limnology: lake and river ecosystems, San Diego
Withers PJA, Jarvie HP (2008) Delivery and cycling of phosphorus in rivers: a review. Sci Total Environ 400:379–395
Wu YH, Wen YJ, Zhou JX, Wu YY (2014) Phosphorus release from lake sediments: effects of pH, temperature and dissolved oxygen. KSCE J of Civ Eng 18:323–329
Yang B, Zhou JB, Lu DL, Dan SF, Zhang D, Lan WL, Kang ZJ, Ning ZM, Cui DY (2019) Phosphorus chemical speciation and seasonal variations in surface sediments of the Maowei Sea, northern Beibu Gulf. Mar Pollut Bull 141:61–69
Ye H, Yuan X, Han L, Yin H, Jin J (2017) Comparison of phosphorus fraction distribution and influencing factors of suspended and surface sediments in the Tiaoxi watershed, China. Water Sci Technol 75:2108–2118
Yuan ZW, Jiang SY, Sheng H, Liu X, Hua H, Liu XW, Zhang Y (2018) Human perturbation of the global phosphorus cycle: changes and consequences. Environ Sci Technol 52:2438–2450
Zafar M, Tiecher T, de Castro Lima JAM, Schaefer GL, Santanna MA, dos Santos DR (2016) Phosphorus seasonal sorption-release kinetics in suspended sediment in response to land use and management in the Guaporé catchment, Southern Brazil. Environ Monit Assess 188:643
Zhang H, Shan BQ (2008) Historical distribution and partitioning of phosphorus in sediment in an agricultural watershed in the Yangtze-Huaihe region, China. Environ Sci Technol 42:2328–2333
Zhang RY, Wand FC, Li CQ (2008) Characteristics of organic phosphorus fractions in different trophic sediment of lakes from the middle and lower reaches of Yangtze River region and Southwestern Plateau, China. Environ Pollu 27:366–372
Zhu YR, Wu FC, He ZQ, Guo J, Qu X, Xie F, Giesy JP, Liao H, Guo F (2013) Characterization of organic phosphorus in lake sediments by sequential fractionation and enzymatic hydrolysis. Environ Sci Technol 47:7679–7687
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Patrick Laceby
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Chen, X., Zhang, W., Yin, Y. et al. Seasonal variation characteristics and release potential of phosphorus in sediments: a case study of the Qiuxi River, a typical diffuse source pollution river in southwestern China. J Soils Sediments 21, 575–591 (2021). https://doi.org/10.1007/s11368-020-02805-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02805-x