Abstract
Purpose
For the sake of risk assessment for arsenic-contaminated sites, the purpose of this study is to estimate the bioavailable arsenic fractions in soil by using the three arsenic-specific sequential extraction procedures (SEPs) and distinguish which SEP can reliably identify and estimate the bioavailable arsenic, so as to screen the most suitable SEP for the risk assessment of arsenic-contaminated sites.
Materials and methods
The arsenic uptake by spinach and amaranth was used to evaluate bioavailability of arsenic fractions defined by the SEPs proposed by Shiowatana, Larios, and Wenzel, respectively, as well as the ability of these three SEPs to identify and estimate bioavailable arsenic.
Results and discussion
The results showed that besides the highly mobile arsenic fractions defined by each SEP, the less mobile HCl-extractable arsenic (mainly carbonate-bound arsenic) in Shiowatana SEP was also the source of bioavailable arsenic, and their contribution to bioavailable arsenic depended not only on their mobility but also on their content, suggesting that the independent extraction of carbonate-bound arsenic should be considered in the design of arsenic-specific SEPs.
Conclusions
All three SEPs could provide approximate estimation of bioavailable arsenic fractions. Although Wenzel SEP performed slightly worse than Larios SEP and Shiowatana SEP, all three SEPs had acceptable accuracy and reproducibility in arsenic fractionation. However, the Shiowatana SEP performed more comprehensive in extracting potential bioavailable arsenic fractions and identifying the source of bioavailable arsenic, indicating that it might be more suitable for the risk assessment of arsenic-polluted sites based on arsenic fractionation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arsenic is a toxic metalloid element that may cause skin diseases and cardiovascular diseases and has potential carcinogenic effect (Kapaj et al. 2006; Jomova et al. 2011). The extensive use of arsenic in industry and agriculture (Wang and Mulligan 2006), such as metal mining and smelting, and the use of fertilizers and pesticides (Mandal and Suzuki 2002), has resulted in a large number of arsenic-contaminated sites, which directly or indirectly lead to the treat of arsenic pollution to tens of millions of people (Zhang and Wang 2018). Soil arsenic pollution has become a noteworthy problem not only in the developing countries, like Bangladesh, India, Vietnam, Argentina, and China, but also in the developed countries such as Germany, Australia, and the USA (Mandal and Suzuki 2002; Singh et al. 2015; Keshavarzifard et al. 2019); risk assessment for arsenic-contaminated sites will be an important and practical issue.
The chemical fractions of metal(loid)s in site soils and their bioavailability are of great significance to site risk assessment. On the one hand, it has been widely recognized that the total concentration–based risk assessment methods often overestimated the potential risk (Whitacre et al. 2017; Zhang et al. 2018; Srithongkul et al. 2019), and the chemical fractions of metal(loid)s rather than their total concentration usually provide more accurate risk assessment information (Islam et al. 2017). On the other hand, bioavailability has become a key factor in site risk assessment. In recent years, some risk assessment methods taking bioavailability into account have already been proposed and indeed in many countries, like USA, Canada, and some European countries, have incorporated such risk assessment methods into their legislation on risk assessment and management (CCME 2007; U.S.EPA 2007; EuropeanCommission 2009). In view of this, identification and quantification of various fractions of metal(loid)s and their bioavailability are the premise and basis for site risk assessment.
Up to now, although there are various understandings about the bioavailability of metal(loid)s in soils (Ehlers and Luthy 2003; Semple et al. 2004; Ehlers and Loibner 2006; Berthelot et al. 2008; Rosado et al. 2016), it can be generalized that bioavailability refers to the fractions of metal(loid)s that can play a role in organisms. In this context, some bioassay methods have been proposed to measure the bioavailability of metal(loid)s in soils. Among them, it is a common and reliable way to assess the bioavailability of metal(loid)s in soils by directly measuring the amount of metal(loid)s absorbed by organisms, including oligochaetes (e.g., earthworm), insects, and various terrestrial plants (Rosado et al. 2016). Nevertheless, due to the time-consuming, cumbersome, difficult to reproduce, and high cost, the application of such methods is limited, especially in the case of multi-sample analysis requirements for site risk assessment (Turner and Olsen 2000). In addition, it should be emphasized that the amount of bioavailable pollutants does not necessarily correspond to the amount absorbed by an organism, although it represents the maximum amount of pollutants that can be absorbed (Ianni et al. 2010). Therefore, for the sake of risk assessment, the exploration of chemical testing methods for the bioavailability of metal(loid)s has never stopped.
Sequential extraction procedure (SEP) is an important technique for fractionation of metal(loid)s in soils, which uses chemical reagents with different extraction abilities to extract metal(loid) fractions from soil in turn (Bacon and Davidson 2008). That is to say, the SEP can provide detailed information on the binding states and distribution of metal(loid)s in soils and then can effectively evaluate the mobility of metal(loid)s (Adamo and Zampella 2008), which is closely related to the bioavailability of metal(loid)s. Based on this fact, the SEP has been widely used to evaluate the bioavailability of metal(loid)s in soils (Remon et al. 2013; Adamo et al. 2014). Indeed, many studies have demonstrated that there is a high correlation between the mobile fractions of metal(loid)s extracted by the SEP and the bioavailable fractions of metal(loid)s quantified by the bioassays (Lee et al. 2011; Chakraborty et al. 2014; Kumpiene et al. 2017). In addition, the use of SEP to evaluate the bioavailability of metal(loid)s has incomparable advantages in site risk assessment, for example, it is simple in operation, low in cost, suitable for various soils, and with easily understandable and comparable results (Rosado et al. 2016).
Among many SEPs, both the BCR (Ure et al. 1993) SEP developed by the European Community Bureau of Reference and the one proposed by Tessier et al. (1979) are widely used currently, but they are not fully applicable for the extraction of arsenic in soils. The reason is that they are mainly designed for the metals in the form of cations in soils. Many extractants for cationic metals are not suitable for arsenic, such as hydrogen peroxide and hydroxylamine hydrochloride, which are not selective to the extraction of arsenic, because arsenic exists in soil as anions (Gleyzes et al. 2002). To cope with this shortage, some arsenic-specific SEPs have been proposed. Among them, three SEPs, i.e., the SEP proposed by Wenzel et al. (2001), the SEP developed by Shiowatana et al. (2001), and the SEP designed by Larios et al. (2013), have been mentioned more. Although the arsenic fractions defined by these three SEPs are different from each other, they can be classified into four categories: acid-extractable arsenic, reducible arsenic, oxidizable arsenic, and residual arsenic. Many studies confirm the accuracy, precision, and reproducibility of these SEPs in the fractionation of soil arsenic (Mester et al. 1998; Alborés et al. 2000; Larios et al. 2012), while few studies pay attention to their ability of identifying and estimating the bioavailability of soil arsenic (Rosado et al. 2016; Wan et al. 2017). Therefore, their use to quantitate the bioavailable arsenic fractions in soil remains to be questioned.
The purpose of this study is to estimate the bioavailable arsenic fractions in soil by using the above three arsenic specific SEPs, compare the obtained results with the arsenic enriched by two kinds of leaf plants (spinach and amaranth), and distinguish which SEP can reliably identify and estimate the bioavailable arsenic, so as to screen the most suitable SEP for the risk assessment of arsenic-contaminated sites.
2 Materials and methods
2.1 Soil samples and their source
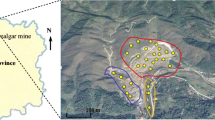
With two topsoil samples (30 cm in depth, each weighing about 5 kg) at each site, eight contaminated soil samples that were labelled with S1-S8 were randomly collected from four arsenic-contaminated sites in Hunan and Jilin Provinces of China. These sampling points were respectively located in a realgar mining area (sample S1 and S2), in a tin mining area (sample S3 and S4), near an abandoned pesticide factory (sample S5 and S6), and in a site contaminated by chemical weapons left over from Japan (sample S7 and S8).
In addition, two arsenic-free topsoil samples were collected in a mountainous area in Beijing suburb, added with the mixed solution of sodium arsenate and sodium arsenite, and then aged for 60 days under normal temperature and cool environment to prepare the spiked arsenic soil samples. They were labelled with S9 and S10.
After natural air-drying, all 10 soil samples were crushed and sifted through 2-mm sieve and then stored in seal environment at 4 °C for later use.
Some physicochemical properties of these soil samples were measured, including pH, oxidation-reduction potential (ORP), organic matter (OM), cation exchange capacity (CEC), carbonate content, and the contents of Fe, Al, and Mn. Among them, pH, ORP, OM, and CEC were measured by the test methods used in our previous study (Dong et al. 2017), the carbonate content was measured by the “Karbonat-Bombe” method of Müller and Gastner (1971), and the contents of Fe, Al, and Mn were measured using a portable XRF spectrometer (Explorer 9000) (Kalnicky and Singhvi 2001).
2.2 Pot experiment
Carried by pot experiment, and through their uptake of arsenic, amaranth and spinach were selected as indicator plants to quantity the bioavailable arsenic in the 10 soil samples.
After completely mixed and homogenized, 500 g of each soil sample was transferred to a ϕ14.5 cm × 12 cm plastic pot. The seeds of amaranth (0.15 g) or spinach (0.30 g) were sowed evenly on the soil surface at each pot and covered with about 60 g of the same soil. With triplicates set for each soil sample and indicator plant, 60 pots were prepared in total. All these pots were placed in an artificial climate incubator for plant growing away from the impact of ambient environment. During plant growth period, the growth conditions were set as follows: 16/8 h of day-night cycle, 12,000 Lux of daytime light intensity, 25 ± 1 °C temperature, and 22 ± 1 °C of night temperature. We observe plant growth regularly and add deionized water to the soil in the pot to ensure soil moisture constant at 50% saturation.
Plant samples were harvested in a manner of whole pot after 45 days of cultivation. The amaranth or spinach plants in each pot were uprooted and rinsed with deionized water trice to remove adherent soil particles and then sealed and stored below 0 °C for later biomass testing and arsenic content analysis.
2.3 Extraction of arsenic in soil samples
2.3.1 Extraction of total arsenic
For each soil sample, after completely mixed and homogenized, 0.2 g of test samples was taken, ground to below 0.149 mm, and then digested with aqua regia/HF (1:1 in v/v) at 96 °C in water bath for 2 h (Wan et al. 2017). The digestion solution was collected after centrifugal separation for later arsenic analysis.
2.3.2 Extraction of arsenic fractions by three SEPs
Inclusive of the defined arsenic fractions, the specific extraction procedures of three SEPs are listed in Table 1.
Like the extraction of total arsenic, 2 g of test samples of each soil was taken for each SEP and ground to below 0.149 mm.
After each extraction step of the selected SEP, the extract was centrifuged at 4000 rpm for 15 min and filtered through a 0.1-mm cellulose vacuum filter. The extracted residue of soil was suspended in 20 mL deionized water, shaken fully for 15 min, centrifuged at 4000 rpm for 10 min, and filtered through the same filter. The filtrates from both filtrations were collected and added to the extract for later arsenic analysis. The filter was rinsed with deionized water for the use in the next extraction step.
2.4 Extraction of arsenic in plant samples
Each plant sample was dried to constant weight at 60 °C to weigh and record its dry weight.
When extracting arsenic in plant samples, 0.5 g of test samples was taken from each homogenized dry plant sample and then digested with 10 mL concentrated HNO3 and 5 mL 30% H2O2 at 120 °C for 1 h in the presence of 800 W microwave (Wan et al. 2017).
2.5 Arsenic analysis
The arsenic concentration in the digestion solutions/extracts of soil and plant samples was measured by a double channel atomic fluorescence spectrometer (AFS3100, BJHG, China) with the following operating parameters: mixed solution of KHB4 (1%) and KOH (0.2%) used as reducing agents at a flow rate of 4.80 mL min−1, 10% HCl solution used as sample carrier liquid at a flow rate of 2.50 mL min−1, and high-purity argon used as carrier gas at a flow rate of 300 mL min−1.
All analytical tests were performed in triplicates; meanwhile, the analytical quality was validated by certified standard samples. Among them, the analytical quality of soil samples was evaluated by the certified soil samples of limestone soil (GBW07404, 58 ± 6 mg kg−1) and yellow-red soil (GBW07405, 412 ± 16 mg kg−1) provided by the Institute of Geophysical and Geochemical Exploration, China, with a percentage recovery of 98.02% and 97.51%, respectively, and the detection limit (LOD) of 35 μg kg−1, while the analytical quality of plant samples was evaluated by the certified plant samples of citrus leaf (GBW10020, 1.1 ± 0.2 mg kg−1) and beans (GBW10021, 0.15 ± 0.02 mg kg−1) from the same Institute, with a percentage recovery of 97.36% and 95.82%, respectively, and the LOD of 20 μg kg−1.
2.6 Data analysis
The test results of each sample (inclusive of soil and plant samples) were expressed as the mean ± standard deviation of three measurements. SPSS 22.0 was used for statistical analysis (inclusive of variance analysis, Pearson correlation analysis, and partial correlation analysis), and Excel 2010 was used for graph drawing.
3 Results and discussion
3.1 Characterization of the soil samples
With greatly varied arsenic content, these soil samples involved different soil types and different pollution history, had different pH value and OM content, higher ORP and moderate CEC, and contained relatively large quantity of potential arsenic scavengers such as Fe, Al, Mn, and carbonate (Table 2) (Filgueiras et al. 2002), covering almost various potential factors and conditions that affect the distribution of arsenic fractions in soils. They were relatively ideal test samples for comparing different SEPs in identifying and estimating bioavailable arsenic in soils.
3.2 Arsenic fractions in soil samples determined by three SEPs
The arsenic fractions in the 10 soil samples determined by each SEP are listed in Table 3, together with the recovery rate and the coefficient of variation (CV), related to the accuracy and reproducibility of each SEP. Among them, the recovery rate is defined as the percentage ratio of the sum of various arsenic fractions measured by each SEP to the total arsenic directly measured, while the CV is defined as the percentage ratio of standard deviation to mean tested in triplicates.
The accuracy of the three SEPs was satisfactory and acceptable in terms of the recovery rates and their fluctuation ranges of each SEP (Silva et al. 2014; Wan et al. 2017; Xie et al. 2019). However, the recovery rates of the Larios and Shiowatana SEPs were not significantly different from each other at a confidence limit of 95%, and they were statistically significantly higher than that of the Wenzel SEP (Table 3, recovery rate [%]), implying that the two SEPs performed equally well and both performed better than the Wenzel SEP in arsenic fractionation with respect to accuracy. The reason might be that the Wenzel SEP did not extract independently such key arsenic fractions as the bound to carbonate and the bound to organic matter.
In addition, except for higher CVs (exceeding 10%) found mainly in the first arsenic fraction in each SEP, the CVs for different arsenic fractions tested by each SEP in triplicates were lower on the whole (mostly less than 5.0%) without significant differences among them at the confidence limit of 95% (Table 3; CV [%]), meaning that the three SEPs had comparable and acceptable reproducibility in soil arsenic fractionation analysis. Besides the heterogeneity of soil samples, the main reason for these higher CVs might be the low concentration of arsenic in the extracted arsenic fractions.
As discussed above, bioavailable arsenic in soil refers to the part that can play a role in organisms (Caussy 2003; Bagherifam et al. 2019). Arsenic in soil can be roughly classified into mobile and stable fractions in accordance with its mobility. The mobile arsenic fraction in soil refers to that exists freely or can be directly converted to a free state under particular environmental conditions, which generally has high mobility and can be regarded as the potential bioavailable arsenic. Due to the differences in definitions of arsenic fractions and their operation conditions, there were significant differences in the extraction of some arsenic fractions by the three SEPs (Fig. S1), especially the iron-/aluminum-bound arsenic and carbonate-bound arsenic. Considering that bioavailable arsenics are highly dependent of mobile arsenic fractions in soil, only the mobile arsenics extracted by three SEPs were compared in this study. The acid-extractable fractions (including the water-soluble fraction, exchangeable fraction and the fraction bound to carbonate) are generally regarded as the mobile ones in metal(loid)s with high bioavailability (Filgueiras et al. 2002; Wan et al. 2017). Some risk assessment methods often evaluated the ecological risk of metal(loid)s polluted sites based on the content of these fractions (Perin et al. 1997; Yang et al. 2013; Wang et al. 2016). Therefore, these arsenic fractions were compared as potential bioavailable ones (Fig. 1); it could be found that total content of bioavailable arsenic extracted by the other two SEPs were close to each other except for a few samples with statistically significant differences and were statistically significantly lower than that extracted by the Shiowatana SEP. The main reason might be that besides the use of similar weak alkaline extractants to extract water-soluble and adsorbed arsenic, the hydrochloric acid extraction step was specially designed, which made it possible to extract carbonate-bound arsenic independently and greatly improved the extraction of carbonate-bound arsenic from soil. On the other hand, as indicated by Srithongkul et al. (2020), the operation conditions such as extraction time, the dosage of extractants, and soil/solvent ratio had an effect on the extraction of arsenic fractions in soil; for the other two SEPs, the designed extraction steps and their operation conditions could not be able to distinguish the carbonate-bound arsenic from the Fe/Al-associated arsenic.
Mobile arsenic fractions extracted by three SEPs, where mobile arsenic fraction reads as follows: FW1 + FW2 for Wenzel SEP, FL1 + FL2 for Larios SEP, and FS1 + FS2 + FS4 for Shiowatana SEP. Data was submitted to least significant difference (LSD) test at 95% confidence limit; different lowercase letters indicate significant difference (P < 0.05) among mobile arsenic fractions
3.3 Arsenic uptake by amaranth and spinach
Arsenic in soil affected plant growth. Excessive arsenic in soil could inhibit the growth of plants to some extent, resulting in dwarf plants and a decrease in biomass yield (Table 4). It was also observed that in the pot experiment, amaranth and spinach sown in soils S8, S9, and S10 stopped growth after germination and died gradually. It was speculated that the main reason was that the arsenic content in these soils exceeded the tolerance limit of amaranth and spinach to arsenic. The large amount of reactive oxygen species produced due to arsenic stress could not be effectively eliminated by their own antioxidant system, resulting in disorder of their metabolism, destruction of cell structure, and eventually tending to growth stagnation or even death (Chakraborty et al. 2016; Tauqeer et al. 2016; Smolinska and Szczodrowska 2017). In addition, it was noteworthy that, unlike soil S8 and S9, the total arsenic content in soil S10 was not high, close to that in soil S7 and much lower than these in soils S2 and S5. However, the growth of the two plants in these soils was quite different, the former eventually died, and the latter three survived despite their growth being inhibited to varying degrees. The reason was related to the high content of potential bioavailable arsenic fractions in soil sample S10 due to its short history of arsenic pollution (Table 3 and Table 4; Fig. 1). Furthermore, compared with the soil samples where the two plants could grow normally, soil samples S8 and S9 leading to eventual plant death and soil samples S2 and S5 with significant inhibition on plant growth also contained high content of potential bioavailable arsenic. These findings indirectly demonstrated that the bioavailability and associated risk of arsenic in soil was greatly dependent to its fractions, especially the mobile fractions, rather than its total content (Martínez-Sánchez et al. 2011; Sundaray et al. 2011; Wan et al. 2017).
The soil-to-plant transfer factor (TF) is defined by the ratio of metal concentration in the plants to that in the rhizosphere soil, which is often used to indicate metal uptake by plants from the soil. Except for plant deaths in soil samples S8, S9, and S10, the TFs for arsenic uptake by amaranth and spinach were 0.034–0.172 and 0.039–0.219, respectively, comparable to these reported by some studies on arsenic uptake by the two plants (Yao et al. 2009; Kar et al. 2013; Bergqvist et al. 2014; Wan et al. 2017). The soil-to-plant transfer factor can be used as a direct index of soil metal bioavailability under given soil metal concentration and indicator plants, and the higher the TF is, the higher the content of bioavailable metal in soil. As demonstrated by many studies, the bioavailable content of metal was highly dependently to the content of mobile metal fractions instead of its total content (Adamo and Zampella 2008; Bashir et al. 2009; Alan and Kara 2019); this study found that the TFs of amaranth and spinach were significantly correlated to the portion of mobile arsenic fractions in soil rather than total arsenic content (Fig. S2). In addition, the study also indicated that there was no statistical difference in measuring bioavailable arsenic between amaranth and spinach; however, the measurement of bioavailable arsenic by spinach was little higher than that by amaranth with respect to each soil sample. As other studies have pointed out (Marrugo-Negrete et al. 2015), plants were usually different from each other in metal uptake ability due to species difference; the above results might be attributed to the fact that spinach is better than amaranth in arsenic uptake. These results also confirmed the limitation of metal bioavailability determined by bioassays, that is, the results are generally species-specific, which may be quite different due to the use of various biological species (Rehman et al. 2016).
3.4 Bioavailability of arsenic fractions defined by three SEPs
The bioavailability of different arsenic fractions can be evaluated by analyzing their contribution to arsenic uptake by plants: the higher the contribution of the fractions was, the higher the bioavailability of the fractions (Anawar et al. 2008; Martínez-Sánchez et al. 2011). Pearson correlation analysis showed that there was a statistically significant positive correlation between all arsenic fractions and arsenic uptake by spinach (Fbio-spinach) and by amaranth (Fbio-amaranth) (Table 5). In each SEP, the arsenic fractions extracted in the first step (i.e., FW1, FL1 and FS1) and the second step (i.e., FW2, FL2 and FS2) were highly correlated with Fbio-spinach and Fbio-amaranth, respectively, which was consistent with our recognition of the high mobility of water-soluble and exchangeable metal fractions (Martínez-Sánchez et al. 2011; Kar et al. 2013; Wan et al. 2017). Moreover, in the Shiowatana SEP, besides FS1 and FS2, FS4 also showed a high correlation with the arsenic uptake by the two plants. The mobility of metal fractions in soil generally decreased in the order of water-soluble, exchangeable, carbonate-bound, reducible, oxidizable, and residual fractions, and the possibility of their utilization by plants decreased accordingly (Sungur et al. 2016; Vaananen et al. 2018). Similar results were concluded from Pearson correlation analysis: the higher the mobility of arsenic fractions was, the higher the correlation between them and the arsenic uptake by the two plants, indicating their high bioavailability. However, Pearson correlation analysis ignores the intrinsic correlation between different arsenic fractions and is not enough to identify or even misjudge sometime which fractions are the main sources of bioavailable arsenic determined by the two plants, which need further partial correlation analysis to clarify.
Partial correlation analysis showed that the arsenic fraction extracted in the second step of each SEP, not the one extracted in the first step, had the highest correlation with the bioavailable arsenic determined by the two plants (Table 5), although the later was more mobile than the former. This might be ascribed to the very low content of arsenic fraction extracted in the first step of each SEP (Table 3), with most of them below 0.5 mg kg−1 and far lower than that of arsenic fraction extracted in the second step. Different from the other two SEPs, in the Shiowatana SEP, in addition to FS1 and FS2, FS4 was also highly correlated with the bioavailable arsenic determined by the two plants, with the correlation degree second only to that of FS2 (Table 5), indicating that this arsenic fraction was also an important source of bioavailable arsenic and had certain bioavailability (Batjargal et al. 2010; Masto et al. 2015). Therefore, from the point of bioavailability, the arsenic bound to carbonate had better be extracted as an independent fraction, such as FS4 in the Shiowatana SEP.
A very low positive or even negative correlation was found between reducible arsenic (i.e., FW3 and FW4, FL3, FL5 and FL6, and FS3) and the bioavailable arsenic determined by the two plants through partial correlation analysis. This is because, on the one hand, iron oxide is an important immobilizer of arsenic in soil (Warren et al. 2003; Hartley et al. 2004), and the reducible arsenic, which is mainly composed of the arsenic associated with Fe/Al oxides, has a low mobility naturally. On the other hand, high redox potential and weak acidity of the soil are not conducive to the bio-utilization of the reducible arsenic (Masscheleyn et al. 1991).
Similarly, because, like sulfide, organic matter in soil could promote the immobilization of metal(loid)s and reduce the mobility of arsenic and other metal(loid)s in soil (Reis et al. 2010), the oxidizable arsenic (i.e., FL4) was also negatively correlated with the bioavailable arsenic determined by the two plants (Table 5).
The residual arsenic was the most stable among all arsenic fractions, which was not bioavailable under natural environmental, and accordingly, it was negatively correlated with the bioavailable arsenic determined by the two plants (Table 5).
3.5 Quantitative estimation of bioavailable arsenic by three SEPs
Only part of the metal(loid)s entering the soil, i.e., bioavailable metal fractions, will have adverse effects on ecosystems and organisms (Alexander 1995; Alexander 2000). The determination of these fractions is the key to environmental risk assessment. As discussed earlier, although the bioassay is relatively accurate and intuitive, it is time-consuming and species-specific, which often fails to meet the needs of large-scale sample analysis for site risk assessment. Here, based on the results of three SEPs and the bioavailable arsenic content in soil determined by spinach and amaranth, a quantitative estimation method of bioavailable arsenic in soil was established, which could provide an alternative for the rapid determination of bioavailable arsenic in soil.
The bioavailable arsenic could be estimated through two paths using the arsenic fractions determined by the three SEPs. First, empirical equations were established by linear regression analysis involving the contributions of all mobile arsenic fractions (Table 6). Second, empirical equations were established by stepwise linear regression analysis only considering the mobile arsenic fractions with statistically significant contribution to bioavailable arsenic fraction (Table 6). It could be found through these equations that mobile fractions were the premise and basis for the estimation of bioavailability. All the three SEPs could provide reliable approximate estimation of bioavailable arsenic fraction through their defined and measured mobile arsenic fractions. Among them, because it could effectively extract the arsenic bound to carbonate, the Shiowatana SEP performed better than the other two SEPs in predicting the bioavailable arsenic. The contribution of mobile fractions to bioavailable ones is closely not only to its mobility but also to its content. For example, although the first arsenic fraction extracted in each SEP (mainly water-soluble arsenic) was the most mobile among all arsenic fractions, it had no statistically significant contribution to the bioavailable arsenic due to its very low content and was not introduced into the stepwise linear regression prediction equations. It could be inferred that in mild arsenic-contaminated sites, both the exchangeable and the carbonate-bound arsenics might become main contributors to the ecological risk instead of water-soluble arsenic due to their much higher content than that of water-soluble arsenic. Only in the case of severe pollution could the high mobility of water-soluble arsenic play a role and become a significant contributor to threatening ecological environment and human health, due to their greatly increased content in soil.
4 Conclusion
Although Wenzel SEP performed slightly worse than Larios SEP and Shiowatana SEP, all three SEPs had acceptable accuracy and reproducibility in arsenic fractionation.
Among various arsenic fractions defined by the three SEPs, both the first and second fractions (i.e., water-soluble and exchangeable arsenic fractions) of each SEP, together with the fourth arsenic fraction (i.e., carbonate-bound arsenic) of Shiowatana SEP, showed high correlation with the arsenic uptake by spinach and amaranth, indicating that they all were potential bioavailable arsenic fractions. However, their contribution to bioavailable fractions depended on not only their mobility but also their content.
Due to independent extraction of carbonate-bound arsenic besides water-soluble and exchangeable arsenic fractions, Shiowatana SEP performed better than the other two SEPs in identifying and extracting the bioavailable arsenic, indicating that the design of arsenic-specific SEPs should pay more attention to the extraction of carbonate-bound arsenic.
All three SEPs could provide approximate estimation of bioavailable fractions. However, the Shiowatana SEP was more comprehensive in identifying the source of bioavailable arsenic, indicating that it might be more suitable for the risk assessment of arsenic-polluted sites based on arsenic fractionation.
References
Adamo P, Zampella M (2008) Chapter nine – chemical speciation to assess potential toxic metals’ (PTMs’) bioavailability and geochemical forms in polluted soils. Environment Geochem 91:175–212
Adamo P, Iavazzo P, Albanese S, Agrelli D, De Vivo B, Lima A (2014) Bioavailability and soil-to-plant transfer factors as indicators of potentially toxic element contamination in agricultural soils. Sci Total Environ 500-501:11–22
Alan M, Kara D (2019) Assessment of sequential extraction methods for the prediction of bioavailability of elements in plants grown on agricultural soils near to boron mines in Turkey. Talanta 200:41–50
Alborés AF, Cid BP, Gómez EF, López EF (2000) Comparison between sequential extraction procedures and single extractions for metal partitioning in sewage sludge samples. Analyst 125:1353–1357
Alexander M (1995) How toxic are toxic chemicals in soil? Environ Sci Technol 29:2713–2717
Alexander M (2000) Aging, bioavailability, and overestimation of risk from environmental pollutants. Environ Sci Technol 34:4259–4265
Anawar HM, Garcia-Sanchez A, Santa Regina I (2008) Evaluation of various chemical extraction methods to estimate plant-available arsenic in mine soils. Chemosphere 70:1459–1467
Bacon JR, Davidson CM (2008) Is there a future for sequential chemical extraction? Analyst 133:25–46
Bagherifam S, Brown T, Fellows C, Naidu R (2019) Bioavailability of arsenic and antimony in terrestrial ecosystems: a review. Pedosphere 29:681–720
Bashir F, Kashmiri MA, Shafiq T, Tariq M (2009) Heavy metals uptake by vegetables growing in sewage irrigated soil: relationship with heavy metal fractionation in soil. Chem Speciat Bioavailab 21:199–209
Batjargal T, Otgonjargal E, Baek K, Yang JS (2010) Assessment of metals contamination of soils in Ulaanbaatar, Mongolia. J Hazard Mater 184:872–876
Bergqvist C, Herbert R, Persson I, Greger M (2014) Plants influence on arsenic availability and speciation in the rhizosphere, roots and shoots of three different vegetables. Environ Pollut 184:540–546
Berthelot Y, Valton E, Auroy A, Trottier B, Robidoux PY (2008) Integration of toxicological and chemical tools to assess the bioavailability of metals and energetic compounds in contaminated soils. Chemosphere 74:166–177
Caussy D (2003) Case studies of the impact of understanding bioavailability: arsenic. Ecotoxicol Environ Saf 56:164–173
CCME (2007) A protocol for the derivation of water quality guidelines for the protection of aquatic life 2007. CCME (Canadian Council of Ministers of the Environment), Winnipeg
Chakraborty P, Chakraborty S, Ramteke D, Chennuri K (2014) Kinetic speciation and bioavailability of copper and nickel in mangrove sediments. Mar Pollut Bull 88:224–230
Chakraborty K, Bishi SK, Goswami N, Singh AL, Zala PV (2016) Differential fine-regulation of enzyme driven ROS detoxification network imparts salt tolerance in contrasting peanut genotypes. Environ Exp Bot 128:79–90
Dong H, Lin Z, Wan X, Feng L (2017) Risk assessment for the mercury polluted site near a pesticide plant in Changsha, Hunan, China. Chemosphere 169:333–341
Ehlers GA, Loibner AP (2006) Linking organic pollutant (bio)availability with geosorbent properties and biomimetic methodology: a review of geosorbent characterisation and (bio)availability prediction. Environ Pollut 141:494–512
Ehlers LJ, Luthy RG (2003) Contaminant bioavailability in soil and sediment. Environ Sci Technol 37:295A–302A
EuropeanCommission (2009) European Union risk assessment report. Voluntary risk assessment of copper and its compounds. Environmental part. Scientific Committee of Health and Environmental Risks. CAS No. 7440-50-8, EINECS No: 231-159-6
Filgueiras AV, Lavilla I, Bendicho C (2002) Chemical sequential extraction for metal partitioning in environmental solid samples. J Environ Monit 4:823–857
Gleyzes C, Tellier S, Astruc M (2002) Fractionation studies of trace elements in contaminated soils and sediments: a review of sequential extraction procedures. Trac-Trend Anal Chem 21:451–467
Hartley W, Edwards R, Lepp NW (2004) Arsenic and heavy metal mobility in iron oxide-amended contaminated soils as evaluated by short- and long-term leaching tests. Environ Pollut 131:495–504
Ianni C, Bignasca A, Magi E, Rivaro P (2010) Metal bioavailability in marine sediments measured by chemical extraction and enzymatic mobilization. Microchem J 96:308–316
Islam S, Rahman MM, Duan L, Islam MR, Kuchel T, Naidu R (2017) Variation in arsenic bioavailability in rice genotypes using swine model: an animal study. Sci Total Environ 599-600:324–331
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107
Kalnicky DJ, Singhvi R (2001) Field portable XRF analysis of environmental samples. J Hazard Mater 83:93–122
Kapaj S, Peterson H, Liber K, Bhattacharya P (2006) Human health effects from chronic arsenic poisoning--a review. J Environ Sci Health A Tox Hazard Subst Environ Eng 41:2399–2428
Kar S, Das S, Jean J-S, Chakraborty S, Liu C-C (2013) Arsenic in the water–soil–plant system and the potential health risks in the coastal part of Chianan Plain, Southwestern Taiwan. J Asian Earth Sci 77:295–302
Keshavarzifard M, Moore F, Sharifi R (2019) The influence of physicochemical parameters on bioavailability and bioaccessibility of heavy metals in sediments of the intertidal zone of Asaluyeh region, Persian Gulf, Iran. Geochemistry 79:178–187
Kumpiene J, Giagnoni L, Marschner B, Denys S, Mench M, Adriaensen K, Vangronsveld J, Puschenreiter M, Renella G (2017) Assessment of methods for determining bioavailability of trace elements in soils: a review. Pedosphere 27:389–406
Larios R, Fernandez-Martinez R, Rucandio I (2012) Comparison of three sequential extraction procedures for fractionation of arsenic from highly polluted mining sediments. Anal Bioanal Chem 402:2909–2921
Larios R, Fernández-Martínez R, Rucandio I (2013) Assessment of a sequential extraction procedure for arsenic partitioning and application to samples from different pollution sources. Anal Methods 5:4096–4104
Lee SH, Park H, Koo N, Hyun S, Hwang A (2011) Evaluation of the effectiveness of various amendments on trace metals stabilization by chemical and biological methods. J Hazard Mater 188:44–51
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Marrugo-Negrete J, Durango-Hernandez J, Pinedo-Hernandez J, Olivero-Verbel J, Diez S (2015) Phytoremediation of mercury-contaminated soils by Jatropha curcas. Chemosphere 127:58–63
Martínez-Sánchez MJ, Martínez-López S, García-Lorenzo ML, Martínez-Martínez LB, Pérez-Sirvent C (2011) Evaluation of arsenic in soils and plant uptake using various chemical extraction methods in soils affected by old mining activities. Geoderma 160:535–541
Masscheleyn PH, Delaune RD, Patrick WH (1991) Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ Sci Technol 25:1414–1419
Masto RE, Sarkar E, George J, Jyoti K, Dutta P, Ram LC (2015) PAHs and potentially toxic elements in the fly ash and bed ash of biomass fired power plants. Fuel Process Technol 132:139–152
Mester Z, Cremisini C, Ghiara E, Morabito R (1998) Comparison of two sequential extraction procedures for metal fractionation in sediment samples. Anal Chim Acta 359:133–142
Müller G, Gastner M (1971) The ‘Karbonat-Bombe’, a simple device for the determination of carbonate content in sediment, soils, and other materials. Neues Jahrbuch Minearalogie 10:466–469
Perin G, Fabris R, Manente S, Wagener AR, Hamacher C, Scotto S (1997) A five-year study on the heavy-metal pollution of Guanabara Bay sediments (Rio de Janeiro, Brazil) and evaluation of the metal bioavailability by means of geochemical speciation. Water Res 31:3017–3028
Rehman ZU, Khan S, Qin K, Brusseau ML, Shah MT, Din I (2016) Quantification of inorganic arsenic exposure and cancer risk via consumption of vegetables in southern selected districts of Pakistan. Sci Total Environ 550:321–329
Reis AT, Rodrigues SM, Davidson CM, Pereira E, Duarte AC (2010) Extractability and mobility of mercury from agricultural soils surrounding industrial and mining contaminated areas. Chemosphere 81:1369–1377
Remon E, Bouchardon JL, Le Guedard M, Bessoule JJ, Conord C, Faure O (2013) Are plants useful as accumulation indicators of metal bioavailability? Environ Pollut 175:1–7
Rosado D, Usero J, Morillo J (2016) Ability of 3 extraction methods (BCR, Tessier and protease K) to estimate bioavailable metals in sediments from Huelva estuary (southwestern Spain). Mar Pollut Bull 102:65–71
Semple KT, Doick KJ, Jones KC, Peter B, Andrew C, Hauke H (2004) Defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated. Environ Sci Technol 38:228A–231A
Shiowatana J, McLaren RG, Chanmekha N, Samphao A (2001) Fractionation of arsenic in soil by a continuous-flow sequential extraction method. J Environ Qual 30:1940–1949
Silva V, Loredo J, Fernandez-Martinez R, Larios R, Ordonez A, Gomez B, Rucandio I (2014) Arsenic partitioning among particle-size fractions of mine wastes and stream sediments from cinnabar mining districts. Environ Geochem Health 36:831–843
Singh R, Singh S, Parihar P, Singh VP, Prasad SM (2015) Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol Environ Saf 112:247–270
Smolinska B, Szczodrowska A (2017) Antioxidative response of Lepidium sativum L. during assisted phytoremediation of Hg contaminated soil. New Biotechnol 38:74–83
Srithongkul C, Wongsaipun S, Krongchai C, Santasup C, Kittiwachana S (2019) Investigation of mobility and bioavailability of arsenic in agricultural soil after treatment by various soil amendments using sequential extraction procedure and multivariate analysis. Catena 181:104084. https://doi.org/10.1016/j.catena.2019.104084
Srithongkul C, Krongchai C, Santasup C, Kittiwachana S (2020) An investigation of the effect of operational conditions on a sequential extraction procedure for arsenic in soil in Thailand. Chemosphere 242:125230. https://doi.org/10.1016/j.chemosphere.2019.125230
Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments--a case study: Mahanadi basin, India. J Hazard Mater 186:1837–1846
Sungur A, Soylak M, Yilmaz S, Ozcan H (2016) Heavy metal mobility and potential availability in animal manure: using a sequential extraction procedure. J Mater Cycles Waste Manag 18:563–572
Tauqeer HM, Ali S, Rizwan M, Ali Q, Saeed R, Iftikhar U, Ahmad R, Farid M, Abbasi GH (2016) Phytoremediation of heavy metals by Alternanthera bettzickiana: growth and physiological response. Ecotoxicol Environ Saf 126:138–146
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particular trace elements. Anal Chem 51:844–851
Turner A, Olsen YS (2000) Chemical versus enzymatic digestion of contaminated estuarine sediment: relative importance of Iron and manganese oxides in controlling trace metal bioavailability. Estuar Coast Shelf Sci 51:717–728
U.S. EPA (2007) Aquatic life ambient freshwater quality criteria - copper. 2007 revision, EPA-822-T-07-001, Office of Water
Ure AM, Quevauviller P, Muntau H, Griepink B (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int J Environ Anal Chem 51:135–151
Vaananen K, Leppanen MT, Chen X, Akkanen J (2018) Metal bioavailability in ecological risk assessment of freshwater ecosystems: from science to environmental management. Ecotoxicol Environ Saf 147:430–446
Wan X, Dong H, Feng L, Lin Z, Luo Q (2017) Comparison of three sequential extraction procedures for arsenic fractionation in highly polluted sites. Chemosphere 178:402–410
Wang S, Mulligan CN (2006) Occurrence of arsenic contamination in Canada: sources, behavior and distribution. Sci Total Environ 366:701–721
Wang H, Liu R, Wang Q, Xu F, Men C, Shen Z (2016) Bioavailability and risk assessment of arsenic in surface sediments of the Yangtze River estuary. Mar Pollut Bull 113:125–131
Warren GP, Alloway BJ, Lepp NW, Singh B, Bochereau FJM, Penny C (2003) Field trials to assess the uptake of arsenic by vegetables from contaminated soils and soil remediation with iron oxides. Sci Total Environ 311:19–33
Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano DC (2001) Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta 436:309–323
Whitacre S, Basta N, Stevens B, Hanley V, Anderson R, Scheckel K (2017) Modification of an existing in vitro method to predict relative bioavailable arsenic in soils. Chemosphere 180:545–552
Xie JJ, Yuan CG, Shen YW, Xie J, He KQ, Zhu HT, Zhang KG (2019) Bioavailability/speciation of arsenic in atmospheric PM2.5 and their seasonal variation: a case study in Baoding city, China. Ecotoxicol Environ Saf 169:487–495
Yang S, Zhou D, Yu H, Wei R, Pan B (2013) Distribution and speciation of metals (Cu, Zn, Cd, and Pb) in agricultural and non-agricultural soils near a stream upriver from the Pearl River, China. Environ Pollut 177:64–70
Yao LX, Li GL, Dang Z, He ZH, Zhou CM, Yang BM (2009) Arsenic uptake by two vegetables grown in two soils amended with as-bearing animal manures. J Hazard Mater 164:904–910
Zhang W, Wang WX (2018) Arsenic biokinetics and bioavailability in deposit-feeding clams and polychaetes. Sci Total Environ 616-617:594–601
Zhang S, Wang Y, Pervaiz A, Kong L, He M (2018) Comparison of diffusive gradients in thin-films (DGT) and chemical extraction methods for predicting bioavailability of antimony and arsenic to maize. Geoderma 332:1–9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest exists in the submission of this manuscript, and all authors approve extremely of the publication of this manuscript and the research not involving Human Participants or Animals. All the authors listed have known and approved the manuscript that is enclosed. I would like to declare on behalf of my co-authors that the work described was original research that has not been previously submitted to the Journal of Soils and Sediments and not under consideration for publication elsewhere, in whole or in part.
Additional information
Responsible editor: Maria Manuela Abreu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 254 kb)
Rights and permissions
About this article
Cite this article
Du, X., Gao, L., Xun, Y. et al. Comparison of different sequential extraction procedures to identify and estimate bioavailability of arsenic fractions in soil. J Soils Sediments 20, 3656–3668 (2020). https://doi.org/10.1007/s11368-020-02694-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02694-0