Abstract
Purpose
Anaeromyxobacter is a typical representative genus of dissimilatory metal-reducing microbes. However, the community structure and metabolic function of Anaeromyxobacter have rarely been reported because of the limited number of Anaeromyxobacter isolations. Therefore, this study aimed to investigate the community structure and succession of Anaeromyxobacter in a Fe(III)-reducing enriched culture of paddy soils.
Materials and methods
A 40-day anaerobic incubation of paddy soils enriched with ferrihydrite and goethite was conducted to investigate the response of the community structure and succession of Anaeromyxobacter to iron oxide addition.
Results and discussion
The dominant Anaeromyxobacter in paddy soils were potentially capable of Fe(III) reduction. Ferrihydrite enrichment increased the absolute abundance of Anaeromyxobacter by 0.01 × 108 to 3.2 × 108 copies g−1 soil, while goethite enrichment increased the absolute abundance of Anaeromyxobacter by 0.004 × 108 to 1.8 × 108 copies g−1 soil. Iron oxide enrichment significantly influenced the richness of Anaeromyxobacter during the later stages of incubation but had a negligible influence on the evenness. Nonetheless, Fe(II) accumulation was stimulated by ferrihydrite enrichment after paddy soil was incubated for 5 days, whereas goethite had a negligible effect on Fe(II) accumulation. Redundancy analysis revealed that Anaeromyxobacter community succession was closely correlated with the processes of Fe(III) reduction.
Conclusions
Exogenous ferrihydrite addition showed a greater influence than goethite on the Anaeromyxobacter community during anaerobic incubation of paddy soils. The difference in inherent amorphous iron oxide content in paddy soils was also decisive in the distinct community structure and succession of Anaeromyxobacter in paddy soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The redox process of iron (Fe) oxides in paddy soil is a prominent biogeochemical process when the soil redox potential decreases to the range of − 100–100 mV after submergence (Johnston et al. 2014; Weber et al. 2006). Fe(III) reduction in submerged paddy soil is considered a microbial-mediated biogeochemical process, which is important in the global element cycle and remediation of polluted soils (Burgin et al. 2011; Hori et al. 2010; Li et al. 2012a; He et al. 2018). It is widely accepted that microorganisms are the main drivers mediating dissimilatory Fe(III) reduction (Lovley 2006; Xu et al. 2014).
Anaeromyxobacter, a typical representative genus of dissimilatory Fe(III)-reducing microbes, can use acetate or H2 as electron donor to reduce Fe(III) to Fe(II) and gain energy to maintain growth in the process (He & Sanford 2003; Treude et al. 2003; Lin et al. 2007). Many studies have indicated that Anaeromyxobacter community is strongly related to the Fe(III) reduction process in various environments (Hori et al. 2007 and 2010; Zhu et al. 2011). Although it has been widely isolated for example, from paddy soils, river sediments, and compost, Anaeromyxobacter is less dominant in such habitats, resulting in a lack of knowledge regarding the metabolic function of Anaeromyxobacter. Except for the critical role in Fe(III) reduction, Anaeromyxobacter could alternatively take halogenated phenols, nitrate, nitrous oxide, fumarate, oxygen, U (VI), As(V), and Se (IV) as electron acceptors competing with Fe(III) (Wu et al. 2006; Sanford et al. 2007; Thomas et al. 2009; Strycharz et al. 2010; He and Yao 2011; Sanford et al. 2012; Tonomura et al. 2015). This suggests the potential role of Anaeromyxobacter in the bioremediation of heavy metals, radioactive metals, and organic halide pollution. Therefore, investigating the succession and diversity of Anaeromyxobacter could improve understanding of the metabolic versatility of Anaeromyxobacter.
Because iron oxides are the terminal electron acceptor of the electron transport chain, the abundance and species of iron oxide greatly affect the process of microbial Fe(III) reduction (Qu et al. 2004; Bonneville et al. 2009). Amorphous iron oxide and lepidocrocite as representatives of weakly crystalline iron oxides are easily reduced by Fe(III)-reducing bacteria, whereas microbial reduction of goethite, hematite, and their aluminum substitutes as representatives of crystalline iron oxides is more difficult (Qu et al. 2004). The different species of iron oxide can influence the bacterial community structure in paddy soil. Li et al. (2012b) enriched bacteria in Geobacter and Clostridium genus with ferrihydrite and found that Clostridium spp. was the dominant population in goethite enrichment. Although Fe(III)-reducing bacterial strains were isolated and identified in the enriching culture with different species of iron oxides, only a few studies had investigated the difference of the Anaeromyxobacter community during anaerobic incubation with ferrihydrite and goethite (Hori et al. 2010; Ding et al. 2015; Hori et al. 2015).
In addition, the abundance and species of iron oxides in paddy soil vary greatly due to the differences of parent materials and anthropogenic management of paddy soil (Yuan et al. 2016). The succession and diversity of Anaeromyxobacter community over flooding time in different types of paddy soils remain unclear. Moreover, the success of the Anaeromyxobacter community in response to the exogenous addition of the different species of iron oxide also requires further research. Therefore, this study investigated the changes of Anaeromyxobacter community in the enriched culture of different paddy soils with ferrihydrite and goethite based on the qPCR and high-throughput sequencing technology. Furthermore, the relationship between the Anaeromyxobacter community and the process of Fe(III) reduction was also analyzed to better understand the biogeological mechanism of Fe(III) reduction in paddy soil.
2 Materials and methods
2.1 Soil sampling
Soil samples were collected from the upper 20 cm of two paddy fields located in the Experimental Station of Hunan Academy of Agriculture Science, Changsha, Hunan province (HN; 28°12′26″N, 113°5′36″E; collected in August 2014) and Huilong town, Qionglai city, Chengdu, Sichuan province (SC; 30°18′8″N, 103°40′53″E, collected in April 2018), respectively. After rice was harvested, five sites were selected in each paddy field along an S-shaped pattern for the collection of a composite sample. Subsequently, the visible plant residues in the samples were removed. The soil samples were then air-dried, filtered through a 1-mm sieve, and stored at room temperature. The basic chemical properties of the two soil samples were determined by standard methods (Table 1) (Page et al. 1982).
2.2 Synthesis of iron oxide
Ferrihydrite and goethite were used in the present study, which represented an amorphous iron oxide and crystalline iron oxide, respectively. Both iron oxides were synthesized according to the methods of Schwertmann and Cornell (1991). The lattice structure of the iron oxides was analyzed by X-ray diffractometer (D8 ADVANCE A25, Bruker, Germany) and is shown in Fig. S1 (Electronic Supplementary Material–ESM).
2.3 Treatments and anaerobic incubation experiments
Two series of enriched cultures of paddy soils were conducted through the addition of ferrihydrite or goethite. Paddy soil without iron oxide supplement was considered as the control treatment (CK). A series sample of exactly 3000 g of soil was mixed with 3 mL ferrihydrite or goethite suspension or 3 mL distilled water in 10-mL sterile serum bottles. The concentration of added iron oxides was 5 mg Fe per gram air-dried soil. Subsequently, serum bottles were covered with rubber stoppers, purged with nitrogen gas (N2) for 5 min, sealed with aluminum covers, and then incubated in a controlled environment incubator in darkness at 25 °C for 40 days. During the anaerobic incubation, three bottles of slurry samples were randomly selected from each treatment as three biological replicates and stored at − 80 °C for extraction of total soil DNA on days 1, 5, 10, 20, 30, and 40. Meanwhile, three bottles were randomly selected from each treatment to determine the Fe(II) content using the o-phenanthroline colorimetric method as previously described (He and Qu 2008).
2.4 Total microbial DNA extraction
After the slurry samples were thawed and thoroughly mixed, 800 μL of the slurry was pipetted into a weighed 2-mL centrifuge tube containing glass beads. The tube containing the slurry sample was weighed again to determine the weight of the collected slurry, and then centrifuged (D3024R, Scilogex, USA) at 4 °C at 5500 rpm (2870×g) for 15 min to remove the liquid. Thereafter, the rest of the soil sample was subjected to DNA extraction with the Soil DNA Kit (D5625-01, Omega Bio-Tek, Norcross, GA, USA), and the extracted total DNA was stored at − 80 °C.
2.5 Quantitative polymerase chain reaction
To determine the absolute abundance of Anaeromyxobacter, the extracted soil total DNA in triplicate was used as template for the quantitative polymerase chain reaction (qPCR), and Fac12-66F/Fac12-432R (Treude et al. 2003) was used for the specific primer of Anaeromyxobacter. The qPCR was performed in triplicate in a 25-μL mixture containing 1 μL of DNA template, 0.5 μL of each primer, 0.5 μL of ROX Reference Dye (TaKaRa Bio, Otsu, Japan), 12.5 μL of SYBR Premix Ex TaqTM (TaKaRa Bio, Otsu, Japan), and 10 μL of sterile ddH2O. Subsequently, the qPCR using a StepOnePlusTM Real-Time PCR Instrument (Thermo Fisher Scientific, USA) was started with pre-denaturation at 95 °C for 3 min, and followed by 40 cycles of denaturation at 95 °C for 20 s then annealing at 55 °C for 40 s and extension at 72 °C for 45 s.
2.6 Community analysis
Partial sequence of the bacterial 16S rRNA gene, targeting the V3-V4 hypervariable region, was amplified with primer pairs 341F/806R. Sequencing was performed on an Illumina HiSeq PE250 platform (Novogene Bioinformatics Institute, Beijing, China). All the raw sequence data were filtrated to remove the low-quality sequence reads, such as reads containing ambiguous bases and/or those shorter than 200 bp. The effective sequences were clustered into operational taxonomic units (OTUs) with a threshold of 97% similarity, and then classified taxonomically via the Silva database (http://www.arb-silva.de).
2.7 Data analysis
The OTUs belonging to the Anaeromyxobacter genus (named A-OTUs) were selected, and the relative abundance of each A-OTU was calculated as the ratio of its sequence reads to that of the total bacteria. The diversity of the Anaeromyxobacter community was assessed by the Marglef index and the evenness index based on the A-OTU. The similarity of the Anaeromyxobacter community, based on the A-OTU, was determined by a principal component analysis (PCA) using CANOCO 4.5 (http://www.canoco5.com). The phylogenetic tree analysis was constructed using MEGA 6.0 with the neighbor-joining method (www.megasoftware.net). A redundancy analysis was conducted to analyze the relationship between the Anaeromyxobacter and environmental factors using CANOCO 4.5 software.
3 Results
3.1 Absolute abundance of Anaeromyxobacter
The variation in the absolute abundance of Anaeromyxobacter with incubation time was considerably different between the HN and SC paddy soils (Fig. 1). Iron oxide enrichment resulted in significant increases in the absolute abundance of Anaeromyxobacter in both soils, which was ranked in the order of ferrihydrite > goethite > CK. In HN soil, the absolute abundance of Anaeromyxobacter increased near linearly from 0.12 × 108 to 0.87 × 108 copies g−1 soil along with the incubation time from 1 to 40 days of incubation. Ferrihydrite enrichment enhanced the absolute abundance of Anaeromyxobacter to between 0.14 × 108 and 1.68 × 108 copies g−1 soil, and goethite enrichment enhanced the absolute abundance of Anaeromyxobacter to between 0.13 × 108 and 1.31 × 108 copies g−1 soil. The promotion of iron oxides on the growth of Anaeromyxobacter was more apparent at 20–40 days of incubation relative to the early stage (p < 0.05). In SC soil, abundance of Anaeromyxobacter increased rapidly and peak at day 5, then decreased to minimum on day 10, and increased slightly until the end of the incubation. Compared with the CK treatment, the absolute abundance of Anaeromyxobacter at days 5–10 of incubation was significantly enhanced by 0.6–1.5 times in the ferrihydrite enrichment and 0.3–0.9 times in the goethite enrichment (p < 0.05).
Absolute abundance of A-OTUs in the paddy soils during anaerobic enrichment culture with different species of iron oxides (CK, the original soil without adding iron oxides; F, soil with added ferrihydrite; G, soil with added goethite; paddy soils were collected from Hunan Province (HN) and Sichuan province (SC), respectively; n = 3 for each treatment, the same below)
3.2 Community structure and relative abundance of Anaeromyxobacter
According to the result of high-throughput sequencing, Anaeromyxobacter genus accounted for 0.03–2.31% and 0.86–5.19% of the total bacteria in the HN and SC paddy soil, respectively, which was assigned to 23 and 36 microbial OTUs, respectively (Fig. 2). The variation trend of relative abundance over incubation time was parallel to that of absolute abundance. For the HN paddy soil, the relative abundance of Anaeromyxobacter gradually rose to a peak of 2.84% on day 30 of the incubation and then declined slightly. Compared with the CK treatment, iron oxide addition showed evident influence to the growth of Anaeromyxobacter during 30–40 days of incubation (p < 0.05). The relative abundance of Anaeromyxobacter during 30–40 days of ferrihydrite-enriched incubation was 1.29–1.34 times higher than the CK treatment, while that in the goethite-enriched incubation was 1.05–1.12 times higher than that of the CK treatment. A-OTU_29 was the dominant species and occupied 39–73% of Anaeromyxobacter genus after 10 days of incubation. It was closely related to uncultured Anaeromyxobacter, which was identified in the environment of goethite as the sole electron acceptor (Fig. 3). Coincidently, the growth of A-OTU_29 at 20–40 days was stimulated in the goethite enrichment but inhibited in the ferrihydrite enrichment. Another dominant population, A-OTU_476 was closely related to uncultured Anaeromyxobacter, which has been identified in the uranium-contaminated underground water and sediments, indicating the potential of metal-reducing ability.
Phylogenetic tree analysis of Anaeromyxobacter on the 16S rDNA sequences. Bold font OTUs represent the A-OTUs based on high-throughput sequencing. Values > 50% (1000 replications) are shown at the branches. GenBank accession numbers are in the parentheses. The scale bar represents 5% sequence divergence. Hydrogenobacter thermophilus (H. thermophilus) is used as the outgroup
For SC paddy soils, the relative abundance of Anaeromyxobacter in the CK treatment increased rapidly to the peak of 4.65% on day 5, but decreased to 4.01% on day 10, and then fluctuated in the range of 4.38–4.46%. Ferrihydrite enrichment resulted in slight increases in relative abundance of Anaeromyxobacter to 4.45–5.19% during 5–20 days of incubation. For goethite enrichment, the relative abundance of Anaeromyxobacter was decreased to 3.66% at 40 days of incubation. Like A-OTU_476, A-OTU_125 was closely related to uncultured Anaeromyxobacter with potential capacity of metal reduction. A-OTU_44 was another dominant species of Anaeromyxobacter that occupied 39–73% of the Anaeromyxobacter genus after 1 day of incubation in SC paddy soil. It was closely related to uncultured Anaeromyxobacter, which was identified in the environment of ferrihydrite as the sole electron acceptor. The growth of A-OTU_44 was stimulated with ferrihydrite enrichment but suppressed with goethite enrichment. A-OTU_9 was also related to Anaeromyxobacter that was capable of ferrihydrite reduction.
3.3 α-Diversity index of Anaeromyxobacter
The Marglef and evenness diversity of Anaeromyxobacter could characterize the richness and uniformity of Anaeromyxobacter communities, respectively (Fig. 4). For the HN paddy soil, the Marglef index of Anaeromyxobacter gradually increased within the first 10 days of anaerobic incubation and was then maintained at approximately 3.0 during the subsequent incubation time. Compared with the CK treatment, goethite enrichment led to a remarkable increase in the Marglef index by 24.50% on day 30 of incubation (p < 0.05), whereas ferrihydrite enrichment caused no significant difference on the Marglef index during the entire incubation. For SC paddy soil, the Marglef index of Anaeromyxobacter fluctuated in the range of 3.8–4.5 during the anaerobic incubation. Ferrihydrite enrichment showed significant stimulation on the richness of Anaeromyxobacter, and the Marglef index on day 1 and day 20 of incubation in the ferrihydrite enrichment was 0.58 and 0.55 higher than that of the CK treatment (p < 0.05). There was no evident influence of goethite enrichment on the richness of Anaeromyxobacter.
The Marglef and evenness index of Anaeromyxobacter in paddy soils during anaerobic enrichment culture with different species of iron oxides (vertical bars represent standard errors of the means (n = 3), different small letters above the column indicate a significant difference between the three treatments at the p < 0.05 level, the same below)
The evenness index of Anaeromyxobacter in HN paddy soil decreased rapidly within the first 10 days of anaerobic incubation, and then remained at a stable level. For SC paddy soil, the evenness index of Anaeromyxobacter remained at approximately 0.3 after 5 days of anaerobic incubation. Nonetheless, iron oxide enrichment led to few differences in the evenness index of Anaeromyxobacter at an identical time of incubation.
3.4 PCA analysis for the community structure of Anaeromyxobacter
PCA was performed to illustrate the differences in the community structure of Anaeromyxobacter between all samples (Fig. 5). Axis 1 explained 96.4% and 67.2% of variation in the HN and SC soil, respectively, and axis 2 explained 1.4% and 9.6%, respectively. For the HN soil, samples incubated for 1, 5, and 10 days in the enrichment and CK treatment were clustered into three distinct groups according to the incubation time, implying that the form of iron oxide had a limited influence on the diversity during the first 10 days of incubation. During the subsequent part of the incubation (20–40 days), samples in the ferrihydrite enrichment were clustered far from samples in the CK and goethite enrichment. For SC paddy soil, samples were clustered in the ordination plot in three distinct groups: 1 day, 5/10 days, and 20/30/40 days, which suggest a clear succession of Anaeromyxobacter with incubation time. During 20–40 days of incubation, samples in CK and the goethite enrichment were similar, whereas samples in ferrihydrite enrichment were still scattered.
Principal component analysis (PCA) plot based on the relative abundances of A-OTUs in paddy soils during anaerobic enrichment culture with different species of iron oxides (the number followed by the abbreviations of the treatments represents the anaerobic incubation time. One sample point represents the average of biological replicates (n = 3))
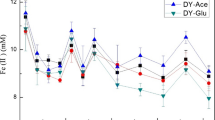
3.5 Dynamics of Fe(II) concentration
After the HN and SC paddy soils were submerged, Fe(II) both in the enrichment and CK treatment was accumulated rapidly during the 1–20 days of anaerobic incubation and then gradually increased to the maximum concentration of 8.64 and 7.45 mg g−1 on day 40 (Fig. 6). Compared with the CK treatment, ferrihydrite addition in both HN and SC soil increased the Fe(II) accumulation significantly by 8.3–27.6% and 15.4–27.5% during the middle and later stages of anaerobic incubation (p < 0.05). Goethite enrichment stimulated Fe(II) accumulation to a minor extent during the late stages of anaerobic incubation and no significant differences were found between the CK and goethite enrichment in both HN and SC soils.
4 Discussion
4.1 Differences in the Anaeromyxobacter community between paddy soils
The succession of microbial community structure could be significantly different according to the physicochemical properties of paddy soils (Unger et al. 2009). In our study, the abundance and diversity index of Anaeromyxobacter in the SC paddy soil was significantly higher than that in the HN paddy soil. This could be because the inherent amorphous iron oxide content in the SC paddy soil was higher than that in the HN paddy soil (Table 1). Amorphous iron oxide has a large specific surface area and high solubility and is thus easily reducible by microorganisms (Bonneville et al. 2004; Bosch et al. 2010). Most iron-reducing bacteria are preferentially enriched in anaerobic enrichment culture with amorphous iron oxide (Li et al. 2012b; Zhang et al. 2013) (Fig. S2-ESM). Thus, a higher content of amorphous iron oxide might stimulate the growth of Anaeromyxobacter capable of Fe(III) reduction. Another possible explanation could be attributed to the lower initial pH value of the SC paddy soil (Table 1). Lower pH value can enhance the stability of iron oxide (Bonneville et al. 2004), which can supplement the easily reducible iron oxide for Anaeromyxobacter. In addition, Qu et al. (2004) found that the crystalline iron oxide in paddy soil could be reduced by iron-reducing bacteria during the late stages of anaerobic incubation. Studies have also suggested that some Anaeromyxobacter can couple the respiratory metabolism of goethite with acetate oxidation (Hori et al. 2010). In the present study, we found that A-OTU_29 was potentially capable of goethite reduction, and A-OTU_44 and A-OTU_9 were potentially capable of ferrihydrite reduction (Thomas et al. 2009; Hori et al. 2010). Thus, A-OTU_29 was awakened after day 5 when the inherent amorphous iron was reduced in the HN soil (Figs. 2 and 6). However, A-OTU_29 was less prominent in the SC soil because the inherent amorphous iron was finally reduced until day 30 of incubation, whereas A-OTU_44 and A-OTU_9 were identified throughout the incubation of SC soil.
4.2 Effect of iron oxide enrichment on Anaeromyxobacter community
The ferrihydrite addition can supply sufficient easily reducible electron acceptor to Fe(III)-reducing bacteria (Li et al. 2012b; Qu et al. 2004). In the present study, the Anaeromyxobacter community in the SC soil preferred to take ferrihydrite as an electron acceptor. Thus, it is not surprising that ferrihydrite enrichment stimulated the growth of Anaeromyxobacter during 5–10 days of incubation in SC soil. The relative abundance of A-OTU_44 and A-OTU_9 in SC soil was also increased to a minor extent. With the gradual reduction of amorphous iron oxide in the later stages of incubation, the competition of Fe(III)-reducing bacteria for amorphous iron oxide in paddy soil would be intensified (You et al. 2011). Therefore, ferrihydrite enrichment enhanced the absolute abundance of Anaeromyxobacter after 20 days of incubation in the HN soil. Accordingly, the diversity of Anaeromyxobacter increased when the inherent amorphous iron reduced. The relative abundance of A-OTU_29 clearly declined in the ferrihydrite-enriched culture in the HN soil because it was less competitive with ferrihydrite as the electron acceptor.
Goethite cannot easily be used by Fe(III)-reducing bacteria because of its high mineral crystal size, small specific surface area, and low solubility (Cornell and Schwertmann 2003; Bosch et al. 2010). However, the relative abundance of A-OTU_29 in the HN soil was increased under goethite enrichment. The community structure of Anaeromyxobacter in goethite-enriched cultures was close to that of the CK treatment, whereas the community structure of Anaeromyxobacter at the later stage of incubation in ferrihydrite-enriched cultures was significantly different to the CK and goethite treatments. This suggests that ferrihydrite had a greater influence on the community structure of Anaeromyxobacter than goethite during the anaerobic incubation of paddy soils.
4.3 Potential correlation of Anaeromyxobacter community with Fe(III) reduction in the Fe(III)-enriched culture
Redundancy analysis (Fig. 7) and Pearson correlation analysis (Table 2) were used to reflect the correlation between the community structure of Anaeromyxobacter and the process of Fe(III) reduction. For the two paddy soils, the relative abundance of Anaeromyxobacter showed a significant positive correlation with the Fe(II) accumulation (p < 0.05), whereas the evenness index of Anaeromyxobacter showed a significant negative correlation with the Fe(II) accumulation, suggesting that Anaeromyxobacter was closely related to the Fe(III) reduction process. Previous studies also confirmed that Anaeromyxobacter played an important role in the process of Fe(III) reduction in the flooded paddy soils (Treude et al. 2003; Wu et al. 2006). The enhanced Fe(II) accumulation under the ferrihydrite enrichment was attributed to the enhanced absolute abundance of Anaeromyxobacter in both the HN and SC soils. Furthermore, although the dominant Anaeromyxobacter strains in the HN paddy soil were competitive with goethite as the electron acceptor and its abundance and Marglef index increased to a minor extent, the reduced Fe(II) might cover the surface of crystalline goethite (Roden and Urrutia 2002; Roden 2003). This prevented the reduction of goethite by the Anaeromyxobacter during the later flooding period and might explain why there was little difference of Fe(II) accumulation between the goethite enrichment and CK treatments. Moreover, Anaeromyxobacter is representative of respiratory Fe(III) reducers and can consume low-weight organic acids in paddy soil to reduce Fe(III) to Fe(II). This could explain why the diversity index and relative abundance of Anaeromyxobacter showed a significant correlation with the pH value of soil slurry. Additionally, even though there were limited isolations of Anaeromyxobacter in paddy soils, the less dominant Anaeromyxobacter were still metabolically important. Although this study provided fundamental information for understanding the role of Anaeromyxobacter in Fe(III)-enriched culture, further efforts should be focused on the isolation and characterization of Anaeromyxobacter strains that are contributors to the process of element cycling.
Redundancy analysis (RDA) for the relationship between the community of Anaeromyxobacter and the process of Fe(III) reduction in HN and SC paddy soils. Three symbol types were used: circle for the CK treatment, square for the ferrihydrite treatment, and triangle for the goethite treatment. Species parameters are represented by the arrows with blue line that include the absolute abundance (AA), relative abundance (RA), Marglef index (dMa), and evenness index (E) of Anaeromyxobacter, respectively. Environmental parameters are represented by the arrows with a red line, including Fe(II) concentration [Fe(II)] and soil pH
5 Conclusions
This study investigated the succession of the Anaeromyxobacter community during anaerobic incubation of paddy soils enriched with two species of iron oxides. We revealed that ferrihydrite and goethite could enhance the absolute abundance of Anaeromyxobacter in paddy soils. The indigenous iron oxide in the soils could affect the succession of the Anaeromyxobacter community. Ferrihydrite had a greater influence than goethite on the Anaeromyxobacter community during the later stages of anaerobic incubation. The succession of Anaeromyxobacter community was closely related to the process of Fe(III) reduction. These results could provide a theoretical basis for understanding the ecological distribution and metabolic function of Anaeromyxobacter, which plays a vital role in the electron transport chain in paddy soils.
References
Bonneville S, Cappellen PV, Behrends T (2004) Microbial reduction of iron (III) oxyhydroxides: effects of mineral solubility and availability. Chem Geol 212:255–268
Bonneville S, Behrends T, Van Cappellen P (2009) Solubility and dissimilatory reduction kinetics of iron (III) oxyhydroxides: a linear free energy relationship. Geochim Cosmochim Acta 73:5273–5282
Bosch J, Heister K, Hofmann T, Meckenstock RU (2010) Nanosized iron oxide colloids strongly enhance microbial iron reduction. Appl Environ Microbiol 76:184–189
Burgin AJ, Yang WH, Hamilton SK, Silver WL (2011) Beyond carbon and nitrogen: how the microbial energy economy couples elemental cycles in diverse ecosystems. Front Ecol Environ 9:44–52
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences and uses. Wiley, Hoboken
Ding L, Su J, Xu H, Jia Z, Zhu Y (2015) Long-term nitrogen fertilization of paddy soil shifts iron-reducing microbial community revealed by RNA-13C-acetate probing coupled with pyrosequencing. ISME J 9:721–734
He J, Qu D (2008) Dissimilatory Fe (III) reduction characteristics of paddy soil extract cultures treated with glucose or fatty acids. J Environ Sci (China) 20:1103–1108
He Q, Sanford RA (2003) Characterization of Fe(III) reduction by chlororespiring Anaeromxyobacter dehalogenans. Appl Environ Microbiol 69:2712–2718
He Q, Yao K (2011) Impact of alternative electron acceptors on selenium (IV) reduction by Anaeromyxobacter dehalogenans. Bioresour Technol 102:3578–3580
He Z, Zhang Q, Feng Y, Luo H, Pan X, Gadd GM (2018) Microbiological and environmental significance of metal-dependent anaerobic oxidation of methane. Sci Total Environ 610:759–768
Hori T, Noll M, Igarashi Y, Friedrich MW, Conrad R (2007) Identification of acetate-assimilating microorganisms under methanogenic conditions in anoxic rice field soil by comparative stable isotope probing of RNA. Appl Environ Microbiol 73(1):101–109
Hori T, Müller A, Igarashi Y, Conrad R, Friedrich MW (2010) Identification of iron-reducing microorganisms in anoxic rice paddy soil by 13C-acetate probing. ISME J 4:267–278
Hori T, Aoyagi T, Itoh H, Narihiro T, Oikawa A, Suzuki K, Ogata A, Friedrich MW, Conrad R, Kamagata Y (2015) Isolation of microorganisms involved in reduction of crystalline iron(III) oxides in natural environments. Front Microbiol 6:1–16
Johnston SG, Burton ED, Aaso T, Tuckerman G (2014) Sulfur, iron and carbon cycling following hydrological restoration of acidic freshwater wetlands. Chem Geol 371:9–26
Li X, Liu T, Zhang N, Ren G, Li F, Li Y (2012a) Effect of Cr(VI) on Fe (III) reduction in three paddy soils from the Hani terrace field at high altitude. Appl Clay Sci 64:53–60
Li HJ, Peng JJ, Li HB (2012b) Diversity and characterization of potential H2-dependent Fe (III)-reducing bacteria in paddy soils. Pedosphere 22:673–680
Lin B, Hyacinthe C, Bonneville S, Braster M, Van Cappellen P, Röling WF (2007) Phylogenetic and physiological diversity of dissimilatory ferric iron reducers in sediments of the polluted Scheldt estuary, Northwest Europe. Environ Microbiol 9:1956–1968
Lovley DR (2006) Dissimilatory Fe(III)- and Mn(IV)-reducing prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The Prokaryotes. Springer, New York, pp 635–658
Page AL, Miller RH, Keeny OR (1982) Chemical and microbiological properties. In: Buxton DR (ed) Methods of soil analysis, 2nd edn. American Society of Agronomy Press, Madison, pp 199–776
Qu D, Ratering S, Schnell S (2004) Microbial reduction of weakly crystalline iron(III) oxides and suppression of methanogenesis in paddy soil. Bull Environ Contam Toxicol 72:1172–1181
Roden EE (2003) Fe (III) oxide reactivity toward biological versus chemical reduction. Environ Sci Technol 37:1319–1324
Roden EE, Urrutia MM (2002) Influence of biogenic Fe(II) on bacterial crystalline Fe (III) oxide reduction. Geomicrobiol J 19:209–251
Sanford RA, Wu Q, Sung Y, Thomas SH, Amos BK, Prince EK, Loffler FE (2007) Hexavalent uranium supports growth of anaeromyxobacter dehalogenans and geobacter spp. with lower than predicted biomass yields. Environ Microbiol 9:2885–2893
Sanford RA, Wagner DD, Qingzhong W, Chee-Sanford JC, Thomas SH, Claribel CG, Gina R, Arturo MD, Krishnani KK, Ritalahti KM (2012) Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc Natl Acad Sci U S A 109:19709–19714
Schwertmann U, Cornell RM (1991) Iron oxides in the laboratory: preparation and characterization, 2nd edn. WILEY-VCH: Verlagsgesellschaft, Weinheim
Strycharz SM, Gannon SM, Boles AR, Franks AE, Nevin KP, Lovley DR (2010) Reductive dechlorination of 2-chlorophenol by Anaeromyxobacter dehalogenans with an electrode serving as the electron donor. Environ MicrobioI Rep 2:289–294
Thomas SH, Padilla-Crespo E, Jardine PM, Sanford RA, Löffler FE (2009) Diversity and distribution of Anaeromyxobacter strains in a uranium-contaminated subsurface environment with a nonuniform groundwater flow. Appl Environ Microbiol 75:3679–3687
Tonomura M, Ehara A, Suzuki H, Amachi S (2015) Draft genome sequence of Anaeromyxobacter sp. strain PSR-1, an arsenate-respiring bacterium isolated from arsenic-contaminated soil. Genome Announc 3:e00472–e00415
Treude N, Rosencrantz D, Liesack W, Schnell S (2003) Strain FAc12, a dissimilatory iron-reducing member of the Anaeromyxobacter subgroup of Myxococcales. FEMS Microbiol Ecol 44:261–269
Unger IM, Kennedy AC, Muzika R (2009) Flooding effects on soil microbial communities. Appl Soil Ecol 42:1–8
Weber KA, Achenbach LA, Coates JD (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–764
Wu Q, Sanford RA, Löffler FE (2006) Uranium(VI) reduction by Anaeromyxobacter dehalogenans strain 2CP-C. Appl Environ Microbiol 72:3608–3614
Xu Y, He Y, Feng X, Liang L, Xu J, Brookes PC, Wu J (2014) Enhanced abiotic and biotic contributions to dechlorination of pentachlorophenol during Fe (III) reduction by an iron-reducing bacterium Clostridium beijerinckii Z. Sci Total Environ 473:215–223
You J, Xia S, Wang B, Qu D (2011) Effect of flooding time on community structure and abundance of Geobacteraceae in paddy soil. Wei Sheng Wu Xue Bao 51:796–804
Yuan HY, Ding LJ, Wang N, Chen SC, Deng Y, Li XM, Zhu YG (2016) Geographic distance and amorphous iron affect the abundance and distribution of Geobacteraceae in paddy soils in China. J Soils Sediments 16:2657–2665
Zhang J, Zhang Y, Chang J, Quan X, Li Q (2013) Biological sulfate reduction in the acidogenic phase of anaerobic digestion under dissimilatory Fe (III)–reducing conditions. Water Res 47:2033–2040
Zhu C, Xia SH, Wang BL, Qu D (2011) Variation of Anaeromyxobacter community structure and abundance in paddy soil slurry over flooding time. Afr J Agric Res 6:6107–6118
Acknowledgments
The authors are also grateful for the anonymous reviewers for their quality comments. We thank Alex Boon, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
Support for this paper was provided by the National Natural Science Foundation of China (Grant No: 41571239).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jizheng He
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 166 kb).
Rights and permissions
About this article
Cite this article
Wang, K., Jia, R., Li, L. et al. Community structure of Anaeromyxobacter in Fe(III) reducing enriched cultures of paddy soils. J Soils Sediments 20, 1621–1631 (2020). https://doi.org/10.1007/s11368-019-02529-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02529-7