Abstract
Purpose
Acid soils often have low P availability limiting plant growth, which is addressed by application of inorganic fertilizers and neutralizing agents. However, little is known about the effect of addition of inorganic P and neutralizing agents on P pools in acid sulfate soils under submerged or moist conditions.
Materials and methods
Sulfuric acid sulfate soil (pH < 4) was amended with two neutralizing agents (NaOH or Ca(OH)2) to achieve soil pH 4 or 5.5, without or with addition of inorganic P equivalent to 20 kg ha−1. Soils were incubated at 25 °C in either submerged or moist conditions (100% of maximum water holding capacity). After 2 weeks, soil P pools (labile P, moderately labile P, non-labile P and residual P) and Fe and Al oxides were determined.
Results and discussion
Adjustment of pH had little effect on the measured parameters. Labile, moderately labile and non-labile P pools were higher with P addition than without P addition. With P addition, labile and non-labile P pools were up to twofold higher in submerged incubation than in moist incubation. Labile P, non-labile P and residual P represented 70%, 15% and 15% in submerged incubation and 40%, 40% and 30% in moist incubation, respectively. In submerged incubation, Fe oxides were higher in soils amended with neutralizing agents than in the original soil which can be explained by the higher pH.
Conclusions
A high proportion of added P was available after 2 weeks of application particularly in submerged incubation. The pH increase had little effect on P availability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Acid sulfate soils (ASS) are defined as soils in which sulfuric acid may be produced, is being produced or has been produced in amounts that have a lasting effect on main soil characteristics (Fanning et al. 2017). Acid sulfate soils are commonly found where conditions are conducive for sulfate reduction such as water logged, sulfate and organic matter–rich coastal and inland wetlands or floodplain areas, and rivers or lakes (Fitzpatrick et al. 2008). In Australia, it has been estimated that ASS occupy about 4 million ha in coastal areas and 1.5 million ha in inland areas (Fitzpatrick et al. 2008). Areas containing ASS are often used for paddy rice cultivation where they may be under oxidized and reduced conditions (Fanning et al. 2010). ASS materials typically contain iron sulfides such as FeS or pyrite (FeS2) formed in reactions of hydrogen sulfide with dissolved Fe2+ under anaerobic conditions (Blunden 2000). Acid sulfate soils are chemically stable under reducing conditions. However, entry of air such as upon drainage can lead to pyrite oxidation which can result in formation of sulfuric acid and pH < 4 or less in soil and leachate (Andriesse and Van Mensvoort 2006).
As a macronutrient, phosphorus (P) plays a critical role in plants for processes such as photosynthesis, respiration and carbohydrate metabolism (Hawkesford and Barraclough 2011). In soil, P is often poorly available to plants because only a very small proportion (< 1%) of total soil P is immediately available in the soil solution. The majority of soil P is in the form of poorly available inorganic (e.g. precipitated with Al, Fe or Ca) or organic compounds. In ASS, a large proportion of soil P is likely to be bound with Fe or Al hydroxides (Iuliano et al. 2007). Fe and Al minerals are sensitive to changes in redox potential and soil pH. Changes in redox potential during flooding and draining/oxidation influence Fe forms and may therefore also affect P availability. When less soluble ferric (FeIII) compounds are reduced to more soluble Fe2+ under anaerobic/submerged conditions, P associated with ferric compounds is released into the soil solution (Peretyazhko and Sposito 2005). Under oxidizing/dry conditions on the other hand, Fe2+ is converted to hydrated ferric oxide minerals (or in acid sulfate soils, Fe oxyhydroxy sulfate minerals) which can occlude P (Ponnamperuma 1972; Fitzpatrick et al. 2017).
P availability in acid soils can be enhanced by application of P fertilizer or pH increase (Antoniadis et al. 2015). However, due to the binding of P on Fe minerals in acid soils, even soluble P fertilizer can become insoluble and less available to plants shortly after application (Ryan et al. 1985). It has been suggested that neutralizing agents (e.g. NaOH or Ca(OH)2) increase P availability by exchange of OH− with PO43− from Fe hydroxide compounds and by reducing the positive surface charge of Fe minerals, thereby weakening P retention (Oburger et al. 2011).
However, the pH increase induced by neutralizing agents in acid soils can also influence Fe forms. At pH > 3.5, hydrolysis of Fe3+ results in precipitation of hydroxyl-iron species such as Fe(OH)2+, which bind P in the soil solution (Penn and Camberato 2019). In addition, it has been reported that Ca(OH)2 may limit P availability by precipitation of relatively insoluble calcium phosphates (Curtin and Syers 2001). Van Mensvoort et al. (1985) suggested that liming of flooded ASS could reduce P availability by formation of insoluble Al phosphate compounds.
Although low P availability in acid soils is commonly addressed by adding neutralizing agents (e.g. lime) and application of inorganic P, little is known about the interactions between neutralizing agents and P availability in sulfuric soils under submerged or drained conditions. This limits the ability to understand how to promote plant growth in sulfuric soils which may provide benefits to remediation (Gardner et al. 2018). Further, due to the high amounts of Fe and dramatic changes in pH in submerged and moist ASS, P availability in ASS may be affected by soil water content to a greater extent than in other acid soils. This study aimed to (i) determine the influence of pH increase by two different neutralizing agents (NaOH and Ca(OH)2) on P pools and Fe oxides in ASS under submerged and moist conditions and to (ii) investigate the effect of inorganic P addition on P pools and Fe oxides with or without pH adjustment. We hypothesized that (i) the effect of pH increase on soluble P and Fe will be greater in soil amended with inorganic P than without P addition; (ii) without P addition, available P will be lower in moist incubation than in submerged incubation, due to oxidation and precipitation of Fe3+; and (iii) P availability will be lower in soils amended with Ca(OH)2 compared to NaOH due to formation of calcium phosphate minerals.
2 Materials and methods
Sulfuric acid sulfate soil (pH < 4) was collected from Gillman in the Barker Inlet, South Australia (34° 49′ 47.25″ S; 138° 32′ 40.24″ E). Properties of the soil were as follows: pH 3.16 (1:1 soil:water), EC1 5123 mS cm−1, sand 89%, silt 7%, clay 4%, total P 0.30 mg g−1, total Fe 21.9 mg g−1, total organic carbon 18.9 mg g−1 and maximum water holding capacity (WHC) 140 mg g−1. The soil had the following properties used for acid sulfate soil characterization: acid neutralizing capacity 0% CaCO3, actual acidity 51 mmol H+ kg−1, HCl-soluble sulfur 11.5 mg g−1 and chromium reducible sulfur 17 mg g−1 (for further details and methods, see Thomas 2011). The soil was stored at room temperature (water content 0.01 g g−1) before starting the experiment. For details of the site and soil classification, see Kölbl et al. (2019).
2.1 Experimental design
The soil was sieved to < 2 mm and mixed with reverse osmosis (RO) water at a 1:2 ratio to form a slurry. There were two incubation conditions: submerged or 100% WHC, referred to as submerged and moist, respectively. The ten amendment treatments differed in soil pH (4 or 5.5), neutralizing agent (NaOH or Ca(OH)2) and the addition of inorganic P equivalent to 20 kg P ha−1. This P rate was used because it is commonly applied in cropped soil. The amendment treatments were as follows (Table 1): control without or with P addition (ControlP0, Control+P), pH increase to 4 with NaOH without or with P addition (Na4P0, Na4+P), pH increase to 5.5 with NaOH without or with P addition (Na5.5P0, Na5.5+P) (pH increase to 5.5 with NaOH, P added), pH increase to 4 with Ca(OH)2 without P or with P addition (Ca4P0, Ca4+P) and pH increase to 5.5 with Ca(OH)2 without P or with P addition (Ca5.5P0, Ca5.5+P). Soil pH was increased to 4.0 ± 0.5 or 5.5 ± 0.5 by drop-wise addition of 0.5 N NaOH or Ca(OH)2 and thorough mixing. Soil pH was measured 24 h after adjustment, and if the target pH was not reached, NaOH or Ca(OH)2 was added again. After reaching the target pH, soils were dried in a fan-forced oven at 30 °C for 36 h until the water content was 100% WHC. Then, soil pH was measured again in a 1:1 soil:water slurry. Final pH values were 4.0 ± 0.1 or 5.5 ± 0.1. Soil equivalent to 20 g dry weight was placed in 70-mL plastic containers. In treatments with P addition, inorganic P (as KH2PO4) was thoroughly mixed at 20 kg P ha−1 (equivalent to 769 mg P kg−1 soil−1). Soil was incubated at 25 °C in the dark for 2 weeks, at two different water contents. The 2-week incubation was chosen because in a preliminary experiment with this soil which was conducted over 10 weeks, P pools changed little after 2 weeks. For submerged incubation, RO water was added so that the soil surface was covered by a 2-cm layer of water which was maintained by adding water throughout the following 2 weeks. The vials were closed tightly to minimize entry of air. For moist incubation, soil was incubated at 100% WHC. This water content was selected based on Jayalath et al. (2016) who found that oxidation/acidification in ASS was maximal at 100% WHC. Throughout the moist incubation, 100% WHC was maintained by checking the water content by weight regularly and adding water if required. All treatments were sampled after 2 weeks. There were four replicates per treatment.
2.2 Analyses
Soil pH was measured in a 1:1 soil:water slurry. Soil maximum water holding capacity was measured using a sintered glass funnel connected to a 1-m water column (Wilke 2005). Soil texture was determined by the hydrometer method (Gee and Or 2002). To determine total P, soil was digested with nitric acid-perchloric acid at a 4:1 ratio and total P in the digest was determined by the phosphovanado-molybdate method (Hanson 1950). Total Fe was determined after concentrated nitric acid dissolution (Zarcinas et al. 1996). The extracts were filtered and analysed for Fe by inductively coupled plasma optical emission spectroscopy (ICP-OES; Agilent, Mulgrave, Australia). In the submerged treatment, soil pH and redox potential were measured by inserting the probes into the soil. In the moist treatments, the electrode was inserted into the soil to measure redox potential; soil pH was determined in a 1:1 soil:water slurry ratio. A chemical fractionation scheme developed by Ivanoff et al. (1998) was used to determine soil P pools, with a few modifications. Briefly, soil (1 g dry soil equivalent) was sequentially extracted with 0.5 M NaHCO3, 1 M HCl and 0.5 M NaOH to separate labile, moderately labile P (MLP) and non-labile P (NLP) pools, respectively. After shaking (16 h, 4 h and 16 h with 0.5 M NaHCO3, 1 M HCl and 0.5 M NaOH, respectively), centrifugation and filtration, P in the extracts was determined by the malachite green method (Ohno and Zibilske 1991). To determine residual P, the remaining soil pellet was digested with nitric acid-perchloric acid at a 4:1 ratio and residual P in the digest was determined by the phosphovanado-molybdate method (Hanson 1950). The Fe and Al oxides in soil were determined using the extraction procedure of Holmgren (1967). To 0.5 g dry soil equivalent, 0.5 g sodium dithionite and 25 mL of 0.75 M sodium citrate solution was added and shaken for 16 h at 25 °C. After shaking, 25 ml RO water containing three drops of Superfloc solution was added. Then, the mixture was shaken vigorously for 5 s and centrifuged to obtain extracts free of soil particles. The supernatant was filtered and diluted 10-fold and acidified with 0.2% v/v nitric acid. Then, Fe, Ca, Al and Na concentrations in the extracts were determined using ICP-OES.
2.3 Data analysis
There were four replicates per treatment. Before conducting analysis of variance (ANOVA), the data was checked for normality (W test) and was log-transformed to achieve normal distribution. Data of submerged and moist treatments was analysed separately by two-way ANOVA with P addition and pH adjustment as factors (GenStat 15th edition; VSN Int., Ltd., UK). Tukey’s multiple comparison tests at 95% confidence interval were used to determine significant differences among treatments (P addition × pH adjustment) separately for submerged and moist incubations. To compare submerged and moist treatments, paired t test was carried out for each amendment treatment separately (GenStat 15th edition; VSN Int., Ltd., UK).
3 Results
The initial soil pH of ControlP0 was < 3.3, indicating pyrite oxidation had occurred leading to production of sulfuric (pH < 4) materials (Table 2). Redox potential values ranged from 400 mV to around 650 mV in all samples at the start of the incubation. Labile P, MLP and NLP were higher with P addition than without P addition.
After 2 weeks, soil pH or redox potential values were not or only slightly influenced by incubation water content (Table 3). Labile P was 10- to 100-fold higher in +P treatments than 0P treatments, and it was higher in submerged than in moist incubation except in Ca5.5P0 where it was 20% higher in moist incubation (Fig. 1a, b). In submerged incubation, labile P in 0P treatments was 30% higher than ControlP0 in Na4P0 and Ca4P0. In +P treatments, labile P was higher than Control+P only in Ca4+P and Ca5.5+P where it was about 70% higher. In moist incubation, labile P differed little among 0P treatments except in Ca5.5P0 where it was about 40% higher than ControlP0. With P addition, labile P was about 30% lower than Control+P in Na5.5+P, Ca4+P and Ca5.5+P.
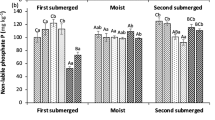
Labile P (a, b) and moderately labile P (c) pools in treatments with different amendments (for treatment names, see Table 1) after 2 weeks of submerged (a, c) or moist incubation (b). Columns with different letters are significantly different (n = 4, ± standard error, P ≤ 0.05). Means with asterisk are significantly higher than the incubation with the other water content. Moderately labile P was detected only in submerged incubation
MLP in submerged incubation (Fig. 1c) was four to sevenfold higher in +P treatments than in 0P treatments with the greatest difference in Ca5.5. It differed little among 0P treatments. Compared to Control+P, MLP was 30% lower in Na4+P and 20% higher in Ca5.5+P. MLP was not detectable in moist incubation treatments.
NLP in submerged incubation was about fourfold higher in +P than in 0P treatments (Fig. 2a). In general, NLP differed a little between pH-adjusted treatments and their respective control. In +P treatments, NLP was about twofold higher in submerged than in moist incubation (Fig. 2b). Without P addition, NLP was not affected by incubation water content except in ControlP0 and Ca5.5P0 where it was higher in moist than in submerged incubation. In moist incubation, NLP was higher than ControlP0 only in Ca5.5P0 where it was 30% higher. NLP differed a little among +P treatments.
Non labile P (a, b) and residual P (c, d) pools in treatments with different amendments (for treatment names, see Table 1) after 2 weeks of submerged (a, c) or moist incubation (b, c). Columns with different letters are significantly different (n = 4, ± standard error, P ≤ 0.05). Means with asterisk are significantly higher than the incubation with the other water content
Residual P (RP) in submerged incubation without P addition compared to ControlP0 was about 20% higher in pH-adjusted treatments, except in Ca4P0 where it was twofold higher (Fig. 2c). Compared to 0P treatments, RP with P addition was lower in Na4P+ and Ca4P+, but higher in Na5.5P+ and Ca5.5P+. With P addition, RP was about 30% higher in Na5.5P+, Ca4P+ and Ca5.5P+ than in Control+P. Incubation water content had an effect on RP only in Ca treatments where it was higher in submerged than in moist incubation in Ca4, but lower in Ca5.5. In moist incubation, RP differed a little between pH-adjusted treatments and the respective controls in except in Ca5.5P0 where it was 30–80% higher (Fig. 2d).
Iron oxides were up to threefold higher in submerged than in moist incubation except in ControlP0 where it was twofold higher in moist than in submerged incubation (Fig. 3a, b). P addition had little effect on Fe oxides. Only in submerged incubation compared to the respective treatment without P, Fe oxides were twofold higher in Na4+P and 50% lower in Ca4+P. In submerged incubation, compared to the controls, Fe oxides were about threefold higher in pH-adjusted treatments. In moist incubation, there was no consistent treatment effect on Fe oxides. Al oxide concentration was not affected by incubation water content, pH adjustment or P addition (Fig. 3c, d). There was no correlation between P pools and Fe/Al oxides.
Fe oxides (a, b) and Al oxides (c, d) in treatments with different amendments (for treatment names, see Table 1) after 2 weeks of submerged (a, c) or moist incubation (b, d). Columns with different letters are significantly different (n = 4, ± standard error, P ≤ 0.05). Means with asterisk are significantly higher than the incubation with the other water contents
In submerged incubation without P addition, RP was the largest percentage of P pools (around 70% of measured P), followed by NLP (about 20%) (Table 4). Only about 5% of measured P was in labile P (LP). With P addition on the other hand, LP was the largest pool (about 70%) whereas RP and NLP together were only about 30%. Moderately labile P was a small pool irrespective of P addition, less than 10% of measured P. In moist incubation without P addition, the proportion of RP, NLP and LP was similar as in submerged incubation (70%, 20% and 5% of measured P, respectively). With P added in moist incubation, about 40% of measured P was LP, 20–30% NLP and 30–40% RP. However, in submerged incubation, about 65% of measured P was LP, 15% NLP and less than 10% RP.
4 Discussion
This experiment showed that in sulfuric ASS, a large proportion of the added soluble P remained in available form, for at least over 2 weeks. Labile P, MLP and NLP were generally higher with P added than without P addition. P addition resulted in a 10-fold increase in the proportion of LP in total P detected, particularly in submerged incubation. Some of the added P was converted into NLP, likely through binding to Fe/Al oxides on soil particles. The lack of increase in RP with P addition suggests that the 2-week incubation was not long enough to convert P into very stable P forms. In general, P pools were influenced by P addition and incubation water content (submerged or moist) whereas pH adjustment had little effect.
Without P addition, P pools differed little between submerged and moist incubations. Therefore, the second hypothesis (without P addition, available P will be lower in moist incubation compared to submerged incubation, due to oxidation and precipitation of Fe3+) has to be declined. Labile P was slightly higher in submerged incubation than in moist incubation, and MLP was detectable only in submerged incubation. This suggests that submerged incubation enhanced mobilization of native soil P, possibly through reduction of Fe oxides to which P was bound (Zhang et al. 2003). However, Fe oxides were lower in submerged than in moist incubation only in ControlP0. Native soil P may have been mobilized by organic acid anions produced from decomposition of native OM at low oxygen concentrations which replaced P from binding sites (Ponnamperuma 1972). Redox potentials measured in the soil were not low, but low oxygen concentrations could occur in microsites, e.g. close to the bottom of the containers.
Labile P can be transformed into MLP or NLP by adsorption to soil minerals such as clays and Fe and Al oxides or by formation of salts with Ca, Fe or Al (Smeck 1985). With P added, LP and NLP in submerged incubation were up to twofold higher than those in moist incubation and MLP was detected only in submerged incubation. Thus, in submerged incubation, a greater proportion of the added P remained soluble than in moist incubation, although a greater proportion was also immobilized as NLP than in moist incubation. This apparent contradiction can be explained by the different sizes of the three pools in submerged incubation. Labile P was about fivefold greater than NLP and 10-fold greater than MLP. Thus, the increase in MLP and NLP had little impact on LP concentration. The higher MLP and NLP in submerged incubation than in moist incubation with added P may be due to greater diffusion of the added P throughout the soil which increased the likelihood of contact with soil particles and formation of stable P pools.
Increasing soil pH had no consistent effect on P pools without P addition. With P added in submerged incubation, LP was about 50% higher with Ca addition than the control which is in agreement with the first hypothesis (the effect of pH increase on soluble P and Fe will be greater in soil amended with inorganic P than without P addition). Although labile P was slightly lower in moist incubation than in submerged incubation, Fe oxides were higher in submerged than in moist incubation (except in ControlP0). Therefore, the lower labile P in moist incubation cannot be explained by enhanced formation of Fe oxides and thus greater surface area for P binding. Ca addition can result in formation of Ca phosphates (Curtin and Syers 2001), but this apparently did not happen in this soil because LP was higher with Ca addition than with Na addition or without pH adjustment. Thus, the third hypothesis (P availability will be lower in soils limed with Ca(OH)2 compared to NaOH due to formation of calcium phosphate minerals) has to be declined. The higher LP with Ca addition may be due to replacement of sorbed P by OH− (McDowell et al. 2003). This did not occur with NaOH addition, likely because less OH− was added with the latter. In moist incubation, pH adjustment to 5.5 with Ca(OH)2 increased RP compared to the control which indicates formation of very stable P forms. However, the higher RP did not affect NLP or LP, because the increase in RP was quite small (about 40 mg kg−1).
In submerged incubation, Fe oxides were about threefold higher in pH-adjusted treatments compared to the controls. This can be explained by the lower solubility of Fe oxides at pH > 4 and the redox potential between 400 and 600 mV which reduces Fe solubility compared to lower redox potentials (Cook and Olive 2012). Iron oxides were higher in submerged incubation than in moist incubation which indicates that Fe reduction was not enhanced in submerged conditions, possibly because the redox potential was similar as in moist incubation. In moist incubation, sulfate ions could have been released by pyrite by oxidation (Johnson and Hallberg 2005). Sulfate ions may react with Na or Ca added with the neutralizing agents and enhance the formation of Ca/Na hydrous sulfate coatings on soil particles. These coatings can reduce the rate of oxygen diffusion and thereby limit oxidation of Fe minerals (Blowes et al. 1991).
5 Conclusions
This study showed that added soluble P remained available 2 weeks after addition and little had entered the more stable P pools. This suggests that added P was not quickly bound on Fe minerals in the acid sulfate soil used in this study. Soil water content influenced P pools only with P addition where a greater proportion of the added P remained soluble in submerged compared to moist incubation. Increasing the pH had little effect on P pools. The results suggest P amendment to acid sulfate soils could be an effective strategy to increase available P and promote plant growth. However, high rates of P fertilizer addition may also increase P loss via runoff or seepage. To further assess the effect of P addition and water content on P pools in ASS, studies with a range of P concentrations over longer periods (several months) are needed.
References
Andriesse W, Van Mensvoort M (2006) Acid sulfate soils: distribution and extent. Encyclop Soil Sci 1:14–19
Antoniadis V, Hatzis F, Bachtsevanidis D, Koutroubas S (2015) Phosphorus availability in low-P and acidic soils as affected by liming and P addition. Commun Soil Sci Plant Anal 46:1288–1298
Blowes DW, Reardon EJ, Jambor JL, Cherry JA (1991) The formation and potential importance of cemented layers in inactive sulfide mine tailings. Geochem Cosmochim Acta 55:965–978
Blunden BG (2000) Management of acid sulfate soils by groundwater manipulation. PhD thesis, University of Woolongong, Australia
Cook WG, Olive RP (2012) Pourbaix diagrams for the iron–water system extended to high-subcritical and low-supercritical conditions. Corros Sci 55:326–331
Curtin D, Syers J (2001) Lime-induced changes in indices of soil phosphate availability. Soil Sci Soc Am J 65:147–152
Fanning D, Rabenhorst MC, Balduff D, Wagner D, Orr R, Zurheide P (2010) An acid sulfate perspective on landscape/seascape soil mineralogy in the US Mid-Atlantic region. Geoderma 154:457–464
Fanning DS, Rabenhorst MC, Fitzpatrick RW (2017) Historical developments in the understanding of acid sulfate soils. Geoderma 308:191–206
Fitzpatrick R, Powell B, Marvanek S (2008) Atlas of Australian acid sulfate soils inland acid sulfate soil systems across Australia. Austral Soil Res Inform Syst (ASRIS) 249:75–89
Fitzpatrick R, Shand P, Mosley L (2017) Acid sulfate soil evolution models and pedogenic pathways during drought and reflooding cycles in irrigated areas and adjacent natural wetlands. Geoderma 308:270–290
Gardner W, Fitzpatrick R, Hindhaugh C (2018) Restoration of wetlands: successes and failures on scalds comprising an iron oxide clogged layer with areas of acid sulfate soils. Plant Soil 433:289–307
Gee G, Or D (2002) Particle size analysis. In: Methods of soil analysis, part 4. Physical methods Soil Science Society of America Book Series, vol 5, pp 255–293
Hanson W (1950) The photometric determination of phosphorus in fertilizers using the phosphovanado-molybdate complex. J Sci Food Agric 1:172–173
Hawkesford MJ, Barraclough PB (2011) The molecular and physiological basis of nutrient use efficiency in crops (eds). Wiley-Blackwell, Chichester, UK.
Holmgren GG (1967) A rapid citrate-dithionite extractable iron procedure 1. Soil Sci Soc Am J 31:210–211
Iuliano M, Ciavatta L, De Tommaso G (2007) On the solubility constant of strengite. Soil Sci Soc Am J 71:1137–1140
Ivanoff D, Reddy K, Robinson S (1998) Chemical fractionation of organic phosphorus in selected histosols. Soil Sci 163:36–45
Jayalath N, Mosley L, Fitzpatrick R, Marschner P (2016) Addition of organic matter influences pH changes in reduced and oxidised acid sulfate soils. Geoderma 262:125–132
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14
Kölbl A, Bucka F, Marschner P, Mosley L, Fitzpatrick F, Schulz S, Lueders T, Koegel-Knabner I (2019) Consumption and alteration of different organic matter sources during remediation of a sandy sulfuric soil. Geoderma 347:220–232
McDowell R, Mahieu N, Brookes P, Poulton P (2003) Mechanisms of phosphorus solubilisation in a limed soil as a function of pH. Chemosphere 51:685–692
Oburger E, Jones DL, Wenzel WW (2011) Phosphorus saturation and pH differentially regulate the efficiency of organic acid anion-mediated P solubilization mechanisms in soil. Plant Soil 341:363–382
Ohno T, Zibilske LM (1991) Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci Soc Am J 55:892–895
Penn CJ, Camberato JJ (2019) A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 9:120
Peretyazhko T, Sposito G (2005) Iron (III) reduction and phosphorous solubilization in humid tropical forest soils. Geochim Cosmochim Acta 69:3643–3652
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96
Ryan J, Curtin D, Cheema M (1985) Significance of iron oxides and calcium carbonate particle size in phosphate sorption by calcareous soils. Soil Sci Soc Am J 49:74–76
Smeck NE (1985) Phosphorus dynamics in soils and landscapes. Geoderma 36:185–199
Thomas B (2011) Coastal acid sulfate soil processes in Barker inlet, South Australia. PhD thesis University of Adelaide
Van Mensvoort M, Lantin R, Brinkman R, Van Breemen N (1985) Toxicities of wetland soils wetland soils: characterization, classification, and utilization. International Rice Research Institute, Los Baños, pp 123–138
Wilke B-M (2005) Determination of chemical and physical soil properties. In: Margesin R, Schinner F (eds) Monitoring and assessing soil bioremediation. Springer, pp 47–95
Zarcinas BA, McLaughlin MJ, Smart MK (1996) The effect of acid digestion technique on the performance of nebulization systems used in inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal 27:1331–1354
Zhang Y, Lin X, Werner W (2003) The effect of soil flooding on the transformation of Fe oxides and the adsorption/desorption behavior of phosphate. J Plant Nutr Soil Sci 166:68–75
Acknowledgements
Sonia Mayakaduwage receives a Beacon of Enlightenment postgraduate scholarship from the University of Adelaide.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhenli He
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mayakaduwage, S., Alamgir, M., Mosley, L. et al. Phosphorus pools in sulfuric acid sulfate soils: influence of water content, pH increase and P addition. J Soils Sediments 20, 1446–1453 (2020). https://doi.org/10.1007/s11368-019-02521-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02521-1