Abstract
Purpose
Nano silicon particles (nSiO2) is one of the most widely used industrial engineered nanomaterials (ENMs). The extensive applications of nSiO2 may pose potential risks to aquatic ecosystems and human health. Humic acid (HA) is a major component of soil and water that exists widely in the natural environment and adsorbs to the surface of nanoparticles, which affects the fate and transport of ENMs in soil. Therefore, it triggers the necessity to study the chemical reaction of HA controlling the sedimentation and transport of nSiO2.
Materials and methods
The sedimentation kinetics and transport breakthrough curves of nSiO2 with/without HA in water-saturated porous media were studied in two electrolyte (NaCl and CaCl2) solutions. The likely mechanisms were explored with both multiple technologies and numerical modeling including TEM-EDX, particle size distribution, zeta potentials, and the two-site kinetic attachment model (TSKAM).
Results and discussion
Our experimental results showed that the existence of HA generally increased the suspensivity and the transportability of nSiO2 in NaCl and CaCl2 solutions in packed sand columns at acidic pH. This result was attributed to the HA adsorption leading to the more negatively charged surface and the smaller size of nSiO2 aggregates. However, the formation of coordination complexes associated with larger cluster among nSiO2 between HA and Ca2+ contributed to the increased sedimentation of nSiO2 at alkaline pH. Subsequently, the presence of HA inhibited the transport of nSiO2 in CaCl2 solution at pH 9.0. Comparably, in NaCl at pH 9.0, HA showed the negligible effect on the nSiO2 deposition in sand. Both the attachment and detachment parameters, which were obtained from fitting the breakthrough curves of ENMs using the TSKAM, could be used to well describe the transport behavior of nSiO2 with HA under various conditions. In particular, the irreversible attachment parameters at site 2 on sand were positively related to the retention of nSiO2 with HA.
Conclusions
The fate and transport of nSiO2 can be distinctly affected by HA depending on the ion composition, ion strength, and pH in soil. This study will provide insights for assessing the mobility of nSiO2 with HA in subsurface soil and aquatic environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With a rapid development in the nanotechnology industry, there are increasing concerns about the production and application of engineered nanomaterials (ENMs) (USEPA, 2007). Nano silica (nSiO2), as one of industrial inorganic ENMs, are widely used in various fields like paint (Diener et al. 2013), rubber (Cochrane and Lin 1993), plastics (Cho and Sul 2001), pigments (Yuan et al. 2008), biology, and medicine (Li et al. 2012). It is inevitably released into the soil and water environments during process and usage which result in the potential hazard to human health and environment (Oberdörster et al. 1994; Adams et al. 2006; Nel et al. 2006). Some studies have been carried out on the toxic effects of nSiO2 on animals, microorganisms, plants, and human being cells (Fruijtier-Polloth 2012). Although, there is no evidence from limited animal studies that nSiO2 induce reproductive or developmental toxicity. nSiO2 may enter the body in particulate or dissolved form, which depended on aggregate size of silica particles and pH (Cho et al. 2009). Also, the nSiO2 pose potential hazards to human by penetrating the intracellular targets in the lung and systemic circulation (Napierska et al. 2010).
Because these ENMs could be released into the environment during application or transportation, more and more researchers are paying attention to the fate and transport of ENMs. The mutual electrostatic reactions between receptors and ENMs may result in the difference in deposition of ENMs (Jiang et al. 2010; Li et al. 2012), which were affected by many factors in natural environment: ionic species, ionic strength (Solovitch et al. 2010), bacteria, pH, surfactant, and organic matter. Except that, the surface roughness of soil (Redman et al. 2001), charge heterogeneity of ENMs (Johnson and Elimelech 1995), and variability in the characteristics of colloid (Bolster et al. 1999) contributed to the different retention of ENMs in soil. As a kind of natural organic matter (NOM), humic acid (HA) is formed by complex biochemical reactions of animals and plants debris which present in the groundwater and soil environments. HA is consist of large amount of carboxylic and phenolic functional groups (Amirbahman and Olson 1995). Therefore, HA adsorption can alter the ENMs surface properties, and consequently impacts their transport patterns: nanoparticle zero-valent iron (nZVI) (Johnson et al. 2009), fullerenes (Espinasse et al. 2007; Loon and Menachem 2008), titanium oxide (TiO2) (Ben-Moshe et al. 2010; Doshi et al. 2008; Fisher-Power and Cheng 2018; Chen et al. 2018), zinc oxide (ZnO) (Jiang et al. 2010), natural colloids (clays) (Kretzschmar et al. 1999; Hahn and O’meliae 2004), biochar (Wang et al. 2013), and hydroxyapatite nanoparticles (Wang et al. 2012b). For instance, NOM showed distinct effects on the transport behavior of rutile TiO2 in quartz sands (Chen et al. 2012). NOM was found to facilitate the transport of rutile TiO2 at low pH but have no effect under alkaline condition. The combined factors of HA and bacteria favor the transport of nTiO2 (Guggenberger et al. 2008). Additionally, HA also could facilitate the transportability of bare nZVI (Jung et al. 2014). As known, the distinct physicochemical properties of ENMs and the subtly different compositions of soil and water contributed to the different transport behavior (Bayat et al. 2015). Currently, the particle size and phosphate have been reported to influent the nSiO2 transport in water-saturated porous sand (Wang et al. 2012a; Liu et al. 2017). However, there is li information available on the mobility or transport behavior of nSiO2 with HA.
In this study, the HA effect on the sedimentation and transport of nSiO2 in the water-saturated sand medium were researched in monovalent (Na+) and divalent (Ca2+) electrolyte solutions. Besides, the zeta potentials and hydrodynamic radius of nSiO2 with HA were measured correspondingly. The underlying mechanism controlling the transport behavior of nSiO2 with HA was described using a two-site kinetic attachment model (TSKAM) to imitate their breakthrough curves (BTCs) in sand porous media.
2 Materials and methods
2.1 Preparation of nSiO2 suspension
The nSiO2 with a purity of 99.9 % is obtained from the Aladdin Reagent Company. The nSiO2 samples characterized by powder X-ray diffraction (XRD, D8-Focus, Bruker AXS Co., Ltd., Germany) are spherical particles. The particle size of nSiO2 was ~ 15 × 20 nm, which was the mean values based on the measurements from five different locations performed using transmission electron microscopy (TEM, FEI Tecnai G2 F20 S-Twin, USA). Its characteristic surface area was 200 m2 g−1, which was estimated by a nitrogen multi-point BET isotherm.
Before transport experiments, 50 mg L−1 nSiO2 suspension was prepared in NaCl or CaCl2 electrolyte solution containing different HA (purchased from Sigma-Aldrich company) concentrations (0–3 mg L−1) at certain pH. The tested nSiO2 and HA concentrations were consistent with those reported in the literature (Redman et al. 2002; Chen et al. 2012; Chowdhury et al. 2012; Wang et al. 2012a, b). Previous researchers used pH as high as 10.0 in order to minimize surface charge heterogeneity and also to ensure attachment of silica nanoparticles under unfavorable conditions (Bradford et al. 2009; Zhang et al. 2010; Wang et al. 2012a). Thus, the pH values of 6.0 and 9.0 were chosen in this study, which were consistent with other studies on ENMs transport in the presence of HA (Chen et al. 2012; Wang et al. 2012b). And then the nSiO2 suspensions were homogenized by a magnetic stirrer for 1 min and sonicated for another 30 min for better dispersivity. In addition, all the chemical reagents (AR) used in this study were obtained from the Chinese medicine group.
2.2 Measurements of zeta potentials and hydraulic radius
A set of nSiO2 (50 mg L−1) suspensions with or without HA (1 and 3 mg L−1) in electrolyte solutions were adjusted to pH values of 6.0 and 9.0 using a diluted HCl or NaOH solution. Then, samples were sonicated for 30 min before further analysis. Finally, a Zeta-sizer Nano ZS90 (Malvern Instruments Ltd., Malvern, UK) was employed to exam the hydrodynamic radius of particles and the dynamic light scattering (DLS) measurement was used to determine their zeta potential at room temperature.
2.3 Sedimentation experiments
Sedimentation experiments were conducted to investigate the dynamics of the aggregation process using a spectrophotometer (UV-2450, Shimadzu Scientific Instrument, Japan). Briefly, after ultrasonication for 30 min, the suspensions of nSiO2 with or without HA were placed in a cuvette of spectrophotometer. The samples were read at 5-min intervals over 2 h and the concentration of nSiO2 in cuvette was directly determined using a UV-Vis spectrophotometer based on pre-established calibration curves (Fig. S1a, Electronic Supplementary Material - ESM) in supporting information (SI) at 250 nm, where HA show almost no adsorption effect on detection of nSiO2 (Fig. S1b - ESM). Sedimentation curve was analyzed by calculating the concentration ratio (C/C0) of nSiO2 as a function of time, in which C0 and C are the concentrations of SiO2 at initial and the certain time of sedimentation, respectively. Supposed that no dissolution of the nSiO2 happened in any of the solutions. Thus, any decrease in nSiO2 concentration over time was on the account of sedimentation.
2.4 Column transport experiments
Quartz sand used in all the transport experiments was pretreated (Chen et al. 2015). Glass chromatography columns (2.5 cm inner diameter and 20 cm long) were filled evenly with the pretreated quartz sands as the porous media, which were covered with the 80-μm nylon net film at both ends. Once filled, the columns were saturated with deionized water for more than 24 h. The procedural details were mentioned in previous studies (Xu et al. 2017). Briefly, 4 pore volumes (PVs) of electrolyte solution was pumped upward to columns at a constant flow rate (1 mL min−1). It has been reported that the addition of HA in the presence of Ca2+ resulted in decreased critical coagulation concentration of silicon nanoparticles from 0.4 to 0.1 M (Liu et al. 2011). Therefore, 100–300 mM NaCl or CaCl2 were used as electrolyte solutions in this study. Then, another 4 PVs of particle suspension which was homogenized by a magnetic stirrer at the same time, was introduced at the same flow rate (phase I). After that, 4 PVs of electrolyte solution was pumped toward columns during phase II and 4 PVs of deionized water was flushed as well until no nSiO2 detected in column effluents during phase III. Finally, the breakthrough curves (BTCs) of C/C0 (where the concentrations of nSiO2 in influent (C0) and effluent (C) passing through columns) were determined as a function of PV. The corresponding physical parameters for transport experiments were summarized and presented in Table S1 (see ESM). The Si analysis was based on the silicon molybdenum yellow method by colorimetry (Liu et al. 2017).
2.5 Modeling
The mass transfer of ENMs between the aqueous and solid phases has been successfully described using an improved form of the advection-dispersion equation (ADE) and the TSKAM (Bradford et al. 2003; Schijven and ŠimŮnek 2002). Currently, there is little information available on describing the transport behavior of nSiO2 with HA in porous media using TSKAM. In this work, TSKAM was used to simulate the breakthrough curves (BTCs) of nSiO2 with HA in water-saturated sand using the HYDRUS-1D code (Simunek et al. 2016), which used a nonlinear least-squares optimization routine based on the Levenberg-Marquardt algorithm to fit nSiO2 transport parameters (Chen et al. 2015; Esfahani et al. 2014; Xu et al. 2018). The corresponding equations are defined in text in the SI.
3 Results and discussion
3.1 Zeta potential of nSiO2 with and without HA
The zeta potential (ZP) of NPs is a significant influence factor for particle aggregation or stability in solution. As the representative, NaCl and CaCl2 electrolyte solutions were chosen to investigate the zeta potentials of nSiO2 with HA at certain pH values (6.0 and 9.0). The surface charges of nSiO2 became less negative as increasing the ion concentration (IC) of electrolyte because of the charge shielding effect and the compression of the surface double layer. In NaCl, the nSiO2 surface was more negatively charged as the HA concentration increased (Fig. 1a, b). Specifically, the ZP of nSiO2 in 200 mM NaCl solution was − 13.93 mV in the absence of HA and − 17.87 mV in the presence of 3 mg L−1 HA at pH 6.0. The ZPs at pH 9.0 (Fig. 1b) were more negative than that at pH 6.0 (Fig. 1a). It attributed to that a larger charge density was formed on the surface of nSiO2 after deprotonation of surface hydroxyl (Svecova et al. 2008) under the alkaline pH. Furthermore, the ZPs of nSiO2 were negligibly changed with the increase of HA, due to subtle adsorption of HA at high pH.
Considering the specific absorption occurred which refers to the Ca2+ adsorption on the surface (Schulman 1960), the individual nSiO2 surface became more positive in CaCl2 (Fig. 1c–d) than that in NaCl at the same pH (Fig. 1a–b). In the presence of HA, the negative charge on the nSiO2 surface increased (Fig. 1c–d), since Ca2+ adsorbed to the surface of nSiO2 can react with HA to form coordination complexes (Liu et al. 2011).
3.2 Sedimentation kinetics of nSiO2 with and without HA
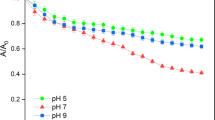
The sedimentation kinetics of nSiO2 with/without HA were investigated in Na+ and Ca2+ solutions at pH 6.0 and 9.0 (Fig. 2). In 300 mM NaCl solutions at pH 6.0, the percentage normalized concentration (C/C0) of nSiO2 without HA dropped down quickly in the first 30 min and then remained 38 % for 2 h. With the addition of 1 mg L−1 HA, the C/C0 (45 %) increased slightly and presented a little better stability compared to individual nSiO2 (Fig. 2a). This was because of the stronger electrostatic repulsion due to the more negatively charged surface with the increase in HA (Fig. 1a). As a result, the particle size decreased gradually with increasing the HA as the intensity-weighted particle size distribution in NaCl at pH 6.0 shown in Fig. 3a. When pH increased from 6.0 to 9.0, the stability of nSiO2 with and without HA was significantly improved (Fig. 2b vs. Fig. 2a). It might be due to the more negatively charged at pH 9.0 (Fig. 1b) than that at pH 6.0 (Fig. 1a). HA had subtle effects on the stability of nSiO2 in NaCl solution. As supported, the particle size distribution showed negligible change (Fig. 3b), due to the subtle absorption of HA onto nSiO2.
Notably, in 50 mM CaCl2 solution at pH 6.0, HA significantly improved the dispersivity of nSiO2 suspension (Fig. 2c). In detailed, the individual nSiO2 continuously settled down and only 30 % of nSiO2 remained in suspension. However, this value increased to 80 % finally with the addition of HA, which was contributed to slightly lower absolute values of surface charge (Fig. 1c). This result was consistent with the smaller sizes of particles with HA as shown in Fig. 3c. For instance, the size of individual nSiO2 in 50 mM CaCl2 was mainly distributed around 1484 nm, whereas that was around 712 nm after addition of 1 mg L−1 HA. Conversely, HA did inhibit the dispersion of nSiO2 in 1 mM CaCl2 at pH 9.0. As shown in Fig. 2d, about 82 % of individual nSiO2 remained stable in suspension, whereas C/C0 of nSiO2 decreased to 73 % with the addition of HA. At pH 9.0, the individual nSiO2 in 1 mM CaCl2 was mainly around 90 nm, while the particles size was distributed at 160 nm after the HA present in solution (Fig. 3d). This result appeared the HA increased the size of nSiO2 in Ca2+ solution at alkaline pH, which can be supported by the formation of larger coordination complexes among nSiO2 by a bridge between HA and Ca2+ (Liu et al. 2011). In order to verify this, TEM images were taken to show that the regular nanoparticles of SiO2 were relatively distributed in NaCl solution after HA was added (Fig. 4a). Nevertheless, with the addition of HA, the large aggregates were formed in Ca2+ solution (Fig. 4b). These aggregates were composed of the agglomerated nanoparticles with unclear and irregular surface (Fig. 4c), which were consisted of Si, O, and Ca elements (Fig. 4d). This result suggested that large nSiO2 aggregates were formed with interplay between HA and Ca2+.
3.3 Transport of nSiO2 with and without HA
The BTCs of nSiO2 with and without HA in packed quartz sands simulated by a TSKAM were shown in Figs. 5 and 6, and the related model parameters were concluded in Table 1 under various experimental conditions. Note that the R2 values obtained from fitting the models were all greater than 0.97, which suggested that the model showed a good simulation in the nSiO2 transport in the presence of HA.
Generally, the transport parameters (k1, k1d, k2) from TSKAM were first-order coefficients [T−1]. It was shown in Table 1 that the irreversible straining coefficient (k2) at site 2 on sand is obviously less than the reversible attachment and detachment coefficients (k1 and k1d) at site 1 in all experiments. This implied that the retention of nSiO2 was reversible during phase I and II. As expected, nSiO2 could be detected in effluent during phase III regardless the existence of HA (Figs. 5 and 6). Furthermore, the values of k1 were clearly greater than those of k1d, even though the k1 had a magnitude similar to k1d. As a result, the values of k1d/k1 were lower than 1 for nSiO2 with and without HA. It indicated that the attachment interaction between nSiO2 (with or without HA) and quartz sand was stronger than the detachment. It was consistent with the transport behavior of silver (Liang et al. 2013) and zinc oxide (Zhu et al. 2005) NPs in soil. Noted that the k1d/k1 values for nSiO2 with HA in NaCl and CaCl2 solutions were more than that without HA at pH 6.0 (Table 1). Accordingly, the transport of nSiO2 was facilitated by the addition of HA under acidic condition (Fig. 5a vs. b and d vs. e), which was consistent with the facilitated transport of hydroxyapatite by HA (Wang et al. 2012b). However, the different phenomenon was observed at pH 9.0. Especially, in NaCl solution, the k1d/k1 values for nSiO2 with HA were almost similar as those without HA at pH 9.0 (Table 1), which was consistent with the negligible difference between nSiO2 with and without HA (Fig. 6a vs. b). This was possibly because HA showed an insignificant effect on the stability (Fig. 2b) and particle size of nSiO2 (Fig. 3b) in Na+ solution at pH 9.0, due to low adsorption of HA on SiO2. This result was consistent with slight change in zeta potentials of nSiO2 after HA was added in NaCl solution at pH 9.0 (Fig. 1c). In CaCl2 at pH 9.0, the k1d/k1 values for nSiO2 with HA were less than those without HA (Table 1). This suggested that the detachment at site 1 on sand was decreased by the presence of HA. As previously discussed, the larger coordination complexes among nSiO2 by a bridge of HA-Ca2+ (Liu et al. 2011) might contribute to the increased deposition of nSiO2 with HA in sand at alkaline pH (Fig. 6c vs. d).
In addition, high pH decreased the values of k2 regardless of the HA existence. For example, with the increase in NaCl (100–300 mM), the k2 value for nSiO2 with 1 mg L−1 HA was in the range of 0.007~0.022 min−1 and 0.006~0.009 min−1 at pH 6.0 and 9.0 (Table 1), respectively. It was also consistent with the variation of k1d/k1. It indicated the straining process of nSiO2 at site 2 on sand decreased. The reason could be that the hydrodynamic size became larger as the pH decreased in NaCl (Fig. 3a vs. b). In CaCl2 solution, zeta potential experimental results suggested that the surfaces of nSiO2 with and without HA were positively charged at pH 6.0 and negatively charged at pH 9.0 (Fig. 1c vs. d). In contrast, quartz sand was usually negatively charged (Liu et al. 2017). The charge heterogeneity resulted in an increase of straining at site 2 at pH 6.0 compared to that at pH 9.0. This increased the irreversible attachment interaction of nSiO2 in CaCl2 at pH 6.0. As a result, the low pH favors the deposition of nSiO2 independent of HA.
Given that values of k1 and k2 for individual nSiO2 increased with increasing the IS (Table 1). This suggested that the greater retention of nSiO2 was related to the depth of the secondary minimum (Toloni et al. 2014; Tufenkji and Elimelech 2005). However, the different change trend of parameters took place after the addition of HA: k1 decreased and k2 increased with increasing the IS. Also noted, k2 with HA was less than that without HA under the same conditions. For example, the k2 for individual nSiO2 increased from 0.011 to 0.042 min−1 with the increase of Na+ at pH 6.0, while that with the HA ranged from 0.007 to 0.022 min−1 (Table 1). This result was consistent with the facilitated transport of nSO2 with HA due to the decreased irreversible retention resulting from HA adsorption (Fig. 5a vs. c and d vs. e). However, in Ca2+ at pH 9.0, k1 with HA was greater than that without HA, which was again due to the formation of larger coordination complexes among nSiO2 by a bridge between HA and Ca2+ (Liu et al. 2011). As supported, the value of k2 was slightly increased by the presence of HA at alkaline pH (Table 1). This indicated that the HA increased the irreversible deposition of nSiO2 in CaCl2 solution at pH 9.0 (Fig. 6c vs. d). To elucidate the direct influence of HA on the transport of nSiO2 in soils containing calcium, additional experiment was conducted: HA was injected to the SiO2 and Ca2+ pre-deposited sand column at pH 9.0. The breakthrough curves (BTCs) of particulate nSiO2 was presented in Fig. S2 (ESM). The shape of the BTCs for nSiO2 in phases I and II was similar to that for individual nSiO2 in CaCl2 at pH 9.0 (Fig. 6c). However, a certain amount of nSiO2 with HA in CaCl2 influent was flushed out from sand column by DI water during phase III (Fig. 6d). However, almost no nSiO2 with HA could be detected during phase III (Fig. S2 - ESM). This result demonstrated that the pre-deposited nSiO2 in sand was irreversible, which was difficult to leach from sand column, but prone to deposition by the formation of larger complexes between HA and Ca2+.
4 Conclusions
The sediment and transport behavior of nSiO2 with HA in porous media was investigated in the system. The impacts of IC, HA concentration, and pH were studied in great detail. HA could adsorb to the surface of nSiO2, which lead to the more negatively charged surface and smaller particle size of nSiO2, thus improved the suspension stability of nSiO2 and facilitated their transport in NaCl and CaCl2 at acidic pH. This effect was strengthened with the increase of HA level. However, HA can form coordination complexes with calcium ions which adsorbed to the nSiO2 surface in CaCl2 electrolyte at alkaline pH and resulted in the larger complexes, thus increased the sedimentation rate and inhibited the transport of nSiO2. Particularly, due to the specific absorption occurred between Ca2+ and nSiO2, the surface of nSiO2 was positively charged at larger calcium concentrations. The two-site kinetic attachment model fitting results showed that the combined values of the attachment/detachment coefficients at site 1 and the straining coefficient at site 2 are responsible for the retention of nSiO2 with HA in porous sand media. Owing to numerous complex substances in the soil and underground water system, further work will be required on the transport of nSiO2 in soil to better understand the transport behaviors of nSiO2. Overall, the information presented herein may be useful for assessing the environmental exposure and risk of nSiO2 in natural system with the enrichment of HA and for eventually developing regulations for such ENMs.
References
Adams LK, Lyon DY, Alvarez PJ (2006) Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res 40(19):3527–3532
Amirbahman A, Olson TM (1995) Deposition kinetics of humic matter-coated hematite in porous media in the presence of Ca2+. Colloids Surf A Physicochem Eng Asp 99(1):1–10
Bayat AE, Junin R, Shamshirband S, Chong WD (2015) Transport and retention of engineered Al2O3, TiO2, and SiO2 nanoparticles through various sedimentary rocks. Sci Rep 5(1):1–12
Ben-Moshe T, Dror I, Berkowitz B (2010) Transport of metal oxide nanoparticles in saturated porous media. Chemosphere 81(3):387–393
Bolster CH, Mills AL, Hornberger GM, Herman JS (1999) Spatial distribution of deposited bacteria following miscible displacement experiments in intact cores. Water Resour Res 35(6):1797–1807
Bradford SA, Simunek J, Bettahar M, van Genuchten MT, Yates SR (2003) Modeling colloid attachment, straining, and exclusion in saturated porous media. Environ Sci Technol 37(10):2242–2250
Bradford SA, Kim HN, Haznedaroglu BZ, Torkzaban S, Walker SL (2009) Coupled factors influencing concentration-dependent colloid transport and retention in saturated porous media. Environ Sci Technol 43(18):6996–7002
Chen G, Liu X, Su C (2012) Distinct effects of humic acid on transport and retention of TiO2 rutile nanoparticles in saturated sand columns. Environ Sci Technol 46(13):7142–7150
Chen M, Xu N, Cao X, Zhou K, Chen Z (2015) Facilitated transport of anatase titanium dioxides nanoparticles in the presence of phosphate in saturated sands. J Colloid Interface Sci 451:134–143
Chen M, Xu N, Christodoulatos C, Wang D (2018) Synergistic effects of phosphorus and humic acid on the transport of anatase titanium dioxide nanoparticles in water-saturated porous media. Environ Pollut 243:1368–1375
Cho JW, Sul KI (2001) Characterization and properties of hybrid composites prepared from poly (vinylidene fluoride–tetrafluoroethylene) and SiO2. Polymer 42(2):727–736
Cho M, Cho WS, Choi M, Kim SJ, Han BS, Kim SH, Kim HO, Sheen YY, Jeong J (2009) The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol Lett 189:177–183
Chowdhury I, Cwiertny DM, Walker SL (2012) Combined factors influencing the aggregation and deposition of nano-TiO2 in the presence of humic acid and bacteria. Environ Sci Technol 46(13):6968–6976
Cochrane H, Lin CS (1993) The influence of fumed silica properties on the processing, curing, and reinforcement properties of silicone rubber. Rubber Chem Technol 66(1):48–60
Diener L, Wick P, Kaiser JP (2013) Nanoparticles in paints: A new strategy to protect façades and surfaces? J Phys Conf Ser 429:12036–12045
Doshi R, Braida W, Christodoulatos C, Wazne M, Gregory O (2008) Nano-aluminum: transport through sand columns and environmental effects on plants and soil communities. Environ Res 106(3):296–303
Esfahani A, Firouzi A, Sayyad G, Kiasat A (2014) Transport and retention of polymer-stabilized zero-valent iron nanoparticles in saturated porous media: effects of initial particle concentration and ionic strength. J Ind Eng Chem 20(5):2671–2679
Espinasse B, Hotze EM, Wiesner MR (2007) Transport and retention of colloidal aggregates of C60 in porous media: effects of organic macromolecules, ionic composition, and preparation method. Environ Sci Technol 41(21):7396–7402
Fisher-Power L, Cheng T (2018) Nanoscale titanium dioxide (nTiO2) transport in natural sediments: importance of soil organic matter and Fe/Al oxyhydroxides. Environ Sci Technol 52(5):2668–2676
Fruijtier-Polloth C (2012) The toxicological mode of action and the safety of synthetic amorphous silica—a nanostructured material. Toxicology 294(2–3):61–79
Guggenberger G, Rodionov A, Shibistova O, Grabe M, Kasansky OA, Fuchs H, Mikheyeva N, Zrazhevskaya G, Flessa H (2008) Storage and mobility of black carbon in permafrost soils of the forest tundra ecotonein Northern Siberia. Glob Chang Biol 14(6):1367–1381
Hahn MW, O’meliae CR (2004) Deposition and reentrainment of brownian particles in porous media under unfavorable chemical conditions: some concepts and applications. Environ Sci Technol 38(1):210–220
Jiang X, Tong M, Li H, Yang K (2010) Deposition kinetics of zinc oxide nanoparticles on natural organic matter coated silica surfaces. J Colloid Interface Sci 350(2):427–434
Johnson PR, Elimelech M (1995) Dynamics of colloid deposition in porous media: blocking based on random sequential adsorption. Langmuir 11(3):801–812
Johnson RL, Johnsinm GO, Nurmi JT, Tratnyek PG (2009) Natural organic matter enhanced mobility of nano zerovalent iron. Environ Sci Technol 43(14):5455–5460
Jung B, O’Carroll D, Sleep B (2014) The influence of humic acid and clay content on the transport of polymer-coated iron nanoparticles through sand. Sci Total Environ 496:155–164
Kretzschmar R, Borkovec M, Grolimund D, Elimelech M (1999) Mobile subsurface colloids and their role in contaminant transport. Adv Agron 66(08):121–193
Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI (2012) Cheminform abstract: mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev 41(7):2590–2605
Liang Y, Bradford S, Simunek J, Heggen M, Vereecken H, Klumpp E (2013) Retention and remobilization of stabilized silver nanoparticles in an undisturbed loamy sand soil. Environ Sci Technol 47(21):12229–12237
Liu X, Wazne M, Chou T, Xiao R, Xu S (2011) Influence of Ca2+ and Suwannee River Humic Acid on aggregation of silicon nanoparticles in aqueous media. Water Res 45(1):105–112
Liu C, Xu N, Feng G, Zhou D, Cheng X, Li Z (2017) Hydrochars and phosphate enhancing the transport of nanoparticle silica in saturated sands. Chemosphere 189:213–223
Loon CK, Menachem E (2008) Interaction of fullerene (C60) nanoparticles with humic acid and alginate coated silica surfaces: measurements, mechanisms, and environmental implications. Environl Sci Technol 42(20):7607–7614
Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH (2010) The nanosilica hazard: another variable entity. Part Fibre Toxicol 7:39–70
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311(5761):622–627
Oberdörster G, Ferin J, Lehnert BE (1994) Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect 102(suppl 5):173–179
Redman JA, Grant SB, Olson TM, Estes MK (2001) Pathogen filtration, heterogeneity, and the potable reuse of wastewater. Environ Sci Technol 35(9):1798–1805
Redman AD, Macalady DL, Ahmann D (2002) Natural organic matter affects arsenic speciation and sorption onto hematite. Environ Sci Technol 36(13):2889–2896
Schijven JF, ŠimŮnek J (2002) Kinetic modeling of virus transport at the field scale. J Contam Hydrol 55(1–2):113–135
Schulman JH (1960) Colloid chemistry. Annu Rev Phys Chem 11(1):169–186
Simunek J, van Genuchten MT, Sejna M (2016) Recent developments and applications of the HYDRUS computer software packages. Vadose Zone J 15(7). https://doi.org/10.2136/vzj2016.04.0033
Solovitch N, Labille J, Rose J, Chaurand P, Borchneck D, Wiecner MR, Bottero JY (2010) Concurrent aggregation and deposition of TiO2 nanoparticles in a sandy porous media. Environ Sci Technol 44(13):4897–4902
Svecova L, Cremel S, Sirguey C, Simonnot MO (2008) Comparison between batch and column experiments to determine the surface charge properties of rutile TiO2 powder. J Colloid Interface Sci 325(2):363–370
Toloni I, Lehmann F, Ackerer P (2014) Modeling the effects of water velocity on TiO2 nanoparticles transport in saturated porous media. J Contam Hydrol 171:42–48
Tufenkji N, Elimelech M (2005) Breakdown of colloid filtration theory: role of the secondary energy minimum and surface charge heterogeneities. Langmuir 21(3):841–852
USEPA (2007) Nanotechnology white paper. Prepared for the U.S. Environmental Protection Agency by members of the nanotechnology workgroup, a group of EPA’s science policy council science policy council. U.S. Environmental Protection Agency, Washington, DC
Wang C, Bobba AD, Attinti R, Lazouskaya V, Wang LP, Jin Y (2012a) Retention and transport of silica nanoparticles in saturated porous media: effect of concentration and particle size. Environ Sci Technol 46(13):7151–7158
Wang D, Bradford SA, Harvey RW, Gao B, Cang L, Zhou D (2012b) Humic acid facilitates the transport of ARS-labeled hydroxyapatite nanoparticles in iron oxyhydroxide-coated sand. Environ Sci Technol 46(5):2738–2745
Wang D, Zhang W, Zhou D (2013) Antagonistic effects of humic acid and iron oxyhydroxide grain-coating on biochar nanoparticle transport in saturated sand. Environ Sci Technol 47(10):5154–5161
Xu X, Xu N, Cheng X, Guo P, Chen Z, Wang D (2017) Transport and aggregation of rutile titanium dioxide nanoparticles in saturated porous media in the presence of ammonium. Chemosphere 169:9–17
Xu N, Cheng X, Zhou K, Xu X, Li Z, Chen J (2018) Facilitated transport of titanium dioxide nanoparticles via hydrochars in the presence of ammonium in saturated sands: effects of pH, ionic strength, and ionic composition. Sci Total Environ 612:1348–1357
Yuan J, Xing W, Gu G, Wu L (2008) The properties of organic pigment encapsulated with nano-silica via layer-by-layer assembly technique. Dyes Pigments 76(2):463–469
Zhang W, Morales VL, Cakmak ME, Salvucci AE, Geohring LD, Hay AG, Parlange JY, Steenhuis TS (2010) Colloid transport and retention in unsaturated porous media: effect of colloid input concentration. Environ Sci Technol 44(13):4965–4972
Zhu Z, Andelman T, Yin M, Chen T, Ehrlich S, O’Brien S (2005) Synchrotron x-ray scattering of Zno nanorods: periodic ordering and lattice size. J Mater Res 20(4):1033–1041
Acknowledgments
We also greatly appreciate the support from the Jiangsu Collaborative Innovation Center of Technology and Material for Water Treatment.
Funding
This research was funded by the National Natural Science Foundation of China (grant nos. 21777110 and 21377090).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Dong-Mei Zhou
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 95 kb)
Rights and permissions
About this article
Cite this article
Zhang, M., Li, D., Ye, Z. et al. Effect of humic acid on the sedimentation and transport of nanoparticles silica in water-saturated porous media. J Soils Sediments 20, 911–920 (2020). https://doi.org/10.1007/s11368-019-02444-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02444-x