Abstract
Purpose
Soil organic carbon enrichment by addition of organic amendments (OAs) is a common agricultural and gardening practice. Such amendments can cause ambiguous environmental effects; it could enhance the sorption of pesticides by increasing soil organic carbon content, and on the contrary, dissolved organic matter (DOM) from OAs could facilitate their leaching. This study evaluated the influence of OAs, mixed waste compost, and dried goat organic manure on the sorption of organophosphates, dichlorvos, and chlorpyrifos.
Materials and methods
Soil (15 cm depth) was collected from an agricultural field and stored. Dissolved organic matter (DOM) extracted from the amendments and the amended soils was characterized by fluorescence spectroscopy and Fourier transform infrared spectroscopy (FT-IR). Initially, studies were carried out to evaluate the effect of DOM from organic amendments (OA-DOM) and dissolved humic acids (HAs) as model DOM on the sorption of selected pesticides. In the later part, OAs (2.5 and 5% w/w) were added to the soil, and sorption experiments were carried out using amended soil to understand the combined effects of insoluble and soluble organic carbon fraction. As dichlorvos sorption was found to be very low, desorption experiments were conducted only for chlorpyrifos using 0.01 M CaCl2 and DOM solutions.
Results and discussion
The spectroscopic characterization of OA-DOM revealed that it mainly contained large amounts of highly humified and aromatic material. OA-DOM and HAs had a similar effect on pesticide sorption leading to a slight but not significant increase in dichlorvos sorption while a substantial reduction in chlorpyrifos sorption was observed. Surface tension analysis highlighted that OA-DOM and HAs might have caused greater solubilization of chlorpyrifos, thus reducing sorption. Further, it also promoted greater desorption of adsorbed chlorpyrifos. These results seem to be related to the humified and aromatic nature of OA-DOM and HAs, determining the interactions between hydrophobic chlorpyrifos and DOM. On the contrary, the addition of OAs to soil promoted greater chlorpyrifos and dichlorvos sorption, but a clear correlation between increase in soil organic carbon and pesticide sorption could not be established.
Conclusions
The study highlighted that the net effect of OA application was an increase in pesticide sorption that depended on the nature of DOM and pesticide properties. The interactions of hydrophobic chlorpyrifos with DOM can lead to a significant reduction in sorption to such an extent that the sorption in the presence of substantial DOM concentration can be less than the sorption without it.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pesticides applied to the agricultural fields can leach into the groundwater and also reach lakes, rivers, etc. through surface runoff. Naturally occurring sorption process especially by the soil colloidal fraction can attenuate these losses. Soil organic matter, and specifically humic substances, is the primary adsorbent for pesticides (Cox et al. 2000). Hence, soil with low organic matter may result in high pesticide mobility. Addition of organic amendments (OAs) and organic waste recycling such as compost amendment are common practices to enrich soils with low organic matter and also to increase structural stability. Studies have been conducted to prove the benefits of OAs in preventing pesticide losses by runoff or leaching (Albarrán et al. 2004; Yu et al. 2009). In addition, OAs can also bring about changes in pesticide degradation and movement in soils, depending on the type of the OAs and their effect on soil microbial activity (Pan et al. 2008).
Recent studies have shown that the addition of organic wastes can bring about modification in water retention capacity (Chalhoub et al. 2013), hydraulic conductivities (Schneider et al. 2009), adsorption, biodegradation, and transport of organic contaminants such as pesticides (Pot et al. 2011; Filipović et al. 2014). The incorporation of OA to soils introduces dissolved organic matter (DOM) in addition to the solid organic matter (Kalbitz et al. 2000; Cambier et al. 2014). DOM is a mixture of diverse and complex compounds with varied molecular weights and chemical structures that may exert multiple interactions with organic pollutants, governing their distribution in soils (Barriuso et al. 2011). Humic substances (HS) constitute the largest fraction of DOM and play an important role in interacting with pesticides. In a study conducted with two commercially available humic acids from terrestrial and aquatic sources, it was reported that arsenite and arsenate species bind to humic acids at all pH values (Buschmann et al. 2006). In another study conducted on triorganotin compounds with dissolved Aldrich and Suwanee river humic acids, association of triorganotin compounds (cations of biocides tributyltin and triphenyltin) with dissolved humic acids was studied (Arnold et al. 1998). Both of these studies highlighted the complexation and association of humic substances with different cationic species. A previous study by Martin-Neto et al. (2001) explained the interactive mechanism between herbicides such as atrazine and its metabolite hydroxy atrazine with soil humic substances. Studies have also been conducted to understand the interaction of pesticides with humic acids from other sources in either soil-water-pesticide or water-pesticide systems (Almendros 1995; Iglesias-Jiménez et al. 1997; Brigante et al. 2010; Scaglia et al. 2016). Therefore, HS can be used as model systems to explain the interactive mechanism between pesticides and DOM (Prosen et al. 2007).
The extent to which DOM affects the sorption and transport of organic pollutants is poorly understood due to some controversial and contrary results. It has been reported that DOM can lead to reduction in pesticide sorption because of the competitive sorption for sites taking place between the pesticide and the DOM. This reduction in pesticide sorption can also occur as a result of interactions taking place between DOM and pesticide in the soil-water systems (Graber et al. 2001; Song et al. 2008; Cabrera et al. 2014) and moreover through cumulative or cosorption of pesticides by DOM (Wang et al. 2010). Contrary to that, a study by Thevenot et al. (2008) reported an increase in mobility of non-ionic pesticides in the presence of DOM which can increase the risk of groundwater contamination. On the other hand, some studies also suggested that DOM had little or no effect on the adsorption and desorption of herbicides (Spark and Swift 2002; Barriuso et al. 2011). Such contradictory results could be due to differences in the nature of soils, DOM, and pesticide characteristics.
The organophosphate pesticides (OPs) are used on a large scale in agriculture, municipal hygiene, and domestic pest control (Zheng et al. 2007). Some of these OPs are highly toxic and used for pest control on a large scale. The wider usage of OPs can cause serious concerns over food safety and environmental pollution (Deng et al. 2015). Dichlorvos (0,0-dimethyl-2,2-dichlorovinyl phosphate; C4H7Cl2PO4) and chlorpyrifos [0,0-diethyl 0-(3,5,6-trichloro-2-pyridyl) phosphorothioate] are most commonly used OPs. Dichlorvos is a relative low toxic OP and is generally applied in lawns, gardens, and around homes to kill insects (Golash and Gogate 2012). It is capable of moving faster through soil into shallow groundwaters and into surface waters through runoff and eventually causing environmental damage (Calamari and Zhang 2002; Zulin et al. 2002). Chlorpyrifos is also extensively used OP and it has phosphorus linked to a sulfur by a double bond (P=S). Worldwide, it is used to control the attack of chewing and sucking insects, pests, and mites on economically vital crops such as citrus fruits, vegetables, potatoes, coffee, tea, cotton, wheat, rice, etc. (Thengodkar and Sivakami 2010). OPs cause adverse effect on the human nervous system by inhibiting activities of the enzymes, acetyl-cholinesterase and cholinesterase, necessary for the efficient neuron function and other process (Bai et al. 2009). These pesticides can contaminate water sources such as lakes, ponds, and groundwater leading to toxicity in humans, animals, and birds.
Compost and farmyard manure such as goat organic manure are commonly used soil organic amendments. Goat organic manure is a very recognized OA in semi-arid regions with tropical climate (Kihanda et al. 2004). Hence, it becomes necessary to evaluate the effect of such OAs on pesticide behavior in order to optimize their use. Moreover, the evaluated sorption coefficients can be used for simulating transport in organically amended soils or DOM containing soil-water system in developed pesticide transport models (Gaonkar et al. 2016a, b).
As such, to the best of our knowledge, no studies have been carried out to evaluate the effects of DOM from mixed waste compost and dried goat manure on the sorption of dichlorvos and chlorpyrifos pesticides. Moreover, little work has been reported on the effect of DOM on pesticide sorption considering both soil-water system as well as amended soils. The aim of the present study was to assess the influence of soil organic amendments: mixed waste compost and dried goat manure on the sorption of dichlorvos and chlorpyrifos emphasizing on the role of DOM. In the first phase of the study, the extracted OA-DOMs were characterized and used to assess the effect of DOM on sorption of selected organophosphate pesticides in a soil-water system. Further, the influence of humic acids, which act as model DOM on sorption of these pesticides, was also evaluated. The latter phase focuses on the effect of DOM on pesticide sorption when OAs were directly added to the soil, incubated, and used to carry out pesticide sorption studies. The outcome of this study would improve our understanding on the influence of OAs on pesticide leaching through the soil profile and provide information in evaluating the risk of groundwater contamination in amended soils. The results from this study are useful to comprehend the sorption of pesticides that show non-linearity due to the presence of organic amendments. Further, a more realistic assessment of environmental risk was performed by providing sorption coefficients at varying concentrations taking into accounts the impact of DOC and soil organic carbon variations. In the absence of this, the theoretical equation would be used which is not capable to capture the added effect of new organic matter.
2 Materials and methods
2.1 Pesticides and other chemicals
Technical grade dichlorvos and chlorpyrifos of high purity were procured from a local company. Stock solutions of chlorpyrifos (1 g L−1) in methanol and dichlorvos (5 g L−1) in distilled water were prepared. The working solutions were freshly prepared by diluting with solution containing 0.01 M CaCl2 as a background electrolyte (methanol content < 1% for chlorpyrifos). High purity analytical standards of dichlorvos and chlorpyrifos were purchased from Sigma-Aldrich, USA, and used to prepare external standards for pesticide analysis. Solvents methanol and n-hexane used were of HPLC grade procured from Rankem, India. The physical and chemical properties of the pesticides are as shown in Online Resource Table S1 (Electronic Supplementary Material).
2.2 Soil and organic amendments
The soil in this study was obtained from an agricultural field in Ludhiana, Punjab where wheat and paddy were predominant crops from 15 cm depth below the soil surface (root zone) vertically by continuous coring. The area is occupied by Indo-Gangetic alluvium and experiences tropical and semi-arid climatic conditions. According to USDA textural soil classification method, the collected soil is sandy soil (Soil Science Division Staff 2017). The soil can be classified as alluvial soil based on the classification by Indian council for agricultural research (Bhattacharyya et al. 2013). The soil collected was air-dried to a constant mass, sieved (< 2 mm), and stored for subsequent studies. The soil was checked for any background pesticide contamination which was found to be below detectable limit. The soil characteristics such as bulk density, specific gravity, porosity, pH, permeability, cation exchange capacity (CEC), % fractions were determined as per standard methods mentioned in Bureau of Indian Standards (BIS 1987). Soil pH were measured in a 1:5 (w/w) soil/deionized water mixture and the soil organic carbon content was determined by Shimadzu TOC-TNM-SSM analyzer. The cation exchange capacity (CEC) of soil was measured based on the ammonium acetate (pH = 7) method. The physicochemical properties of the soil are listed in Online Resource Table S2 (Electronic Supplementary Material).
Sodium salt of humic acid (HA) containing 50 to 60% humic acids was purchased from Acros Organics and used as a model DOM. Two organic amendments were selected for this study, a compost (C) from mixed wastes (46% vegetable waste, 27% dewatered septage, 11% wood chips, 8% food waste, 4% each of coir pith and cow dung) from a local composting facility and dried goat organic manure (OM) from a nearby farmyard. The two OAs (C and OM) were added to 5 kg of air-dried and sieved (< 2 mm) natural soil (S) sample (2.5 and 5% w/w on a dry basis) and mixed thoroughly. The amended soil was incubated for 45 days at ambient temperature conditions (26.4 ± 1.9 °C) in the absence of sunlight before use. The moisture content (70% of field capacity) was maintained constant throughout this incubation period by addition of distilled water as and when necessary. After the incubation period, soils were dried, sieved, and used for adsorption studies. The properties of the amendments and amended soils are listed in Table 1.
2.3 Pesticide sorption studies
Sorption studies were carried out using the equilibrium batch method. Autoclaved soil of 5 ± 0.01 g was treated with 100 mL of chlorpyrifos solutions with initial concentrations (C0) ranging from 0.1 to 10 mg L−1 or with 100 mL of dichlorvos with C0 of 0.25 to 100 mg L−1 in 0.01 M CaCl2 solutions. During the first phase of the study, the first set of sorption experiments was carried out using different HA concentrations as background solutions instead of 0.01 M CaCl2 to assess the effect of HA-DOM on pesticides. In the second set of experiments, compost DOM (C-DOM) and dried goat organic manure DOM (OM-DOM) were used as background solutions to assess the effect of OA-DOM on pesticides. Second phase of the sorption studies was carried out using amended soils with 0.01 M CaCl2 as background solution. The details of the batch sorption experiments are given in Table 2. The soil-water suspensions were shaken at 25 ± 2 °C for 24 h in glass conical flasks and then centrifuged at 10,000 rpm at the same temperature. Initially, it was observed from the kinetic sorption experiments that pseudo-equilibrium was reached within 24 h and that no measurable degradation occurred during this period (data not shown). Flasks containing pesticide blanks, i.e., without soil and containing only the pesticide dissolved in background solution, were placed as degradation controls during the sorption experiments.
In addition, blanks containing soil, but without pesticide solution, were treated in the same way, as laboratory method blanks. Degradation controls, blanks, and replicate samples were placed and analyzed with each round of samples for quality control. The collected samples were extracted with 10 mL of n-hexane solution in a standard separating funnel with Teflon stopper. The water layer was decanted carefully and the supernatant was further extracted twice with 5 mL of n-hexane. Finally, the extracted samples were filtered through anhydrous sodium sulfate to remove trace amounts of water. Preliminary extraction methods were performed and good improvement efficiency was found in multiple extractions with n-hexane. The obtained recoveries were constant and greater than 90%. Hence, no corrections for recovery were required. The extracted samples were analyzed by gas chromatograph (GC) with electron capture detector (ECD), PerkinElmer Clarus 500 equipped with an auto-sampler, an on-column, split/split less capillary injection system, and Elite-35 capillary column (30 m × 0.53 mm × 0.5 mm film thickness). The injector and detector temperatures were maintained at 250 and 350 °C, respectively. The GC oven temperature was initially held at 100 °C for 2 min, then increased from 100 to 280 °C at a rate of 15 °C per min, and held at 280 °C for 2 min. One microliter of liquid samples was injected at a split ratio of 1:10. The dichlorvos and chlorpyrifos peaks were obtained at the retention times of 9.71 and 14.07 min, respectively. The difference between initial concentration (C0) and final concentration (Ce) is the amount of pesticide sorbed (Cs). The mean value is reported in all the graphs shown.
The entire reaction mixture in the conical flask was centrifuged after adsorption study and the supernatant was decanted carefully. The amount of decanted supernatant was replaced with the same amount of 0.01 M CaCl2 and OA-DOM for desorption studies. The flasks were then kept in an orbital shaker to attain desorption equilibrium. After attaining pseudo desorption equilibrium, sample was withdrawn from the controlled flasks and analyzed for pesticide concentration.
2.4 Data analysis
Dichlorvos sorption data was found to fit to Langmuir (1918) isotherm given by Eq. (1).
qmax represents the maximum adsorption capacity and b represents the Langmuir constant.
The data from the chlorpyrifos sorption and desorption experiments were fitted to the Freundlich equation represented by Eqs. (2) and (3), respectively.
where S and D (mg kg−1) are the amount of pesticide sorbed and remaining sorbed per unit mass of adsorbent, respectively. Ce (mg L−1) is the equilibrium concentration of pesticide in solution and Kf or Kfd (mg1 − n kg−1 Ln) and n or nd are the Freundlich sorption or desorption capacities and sorption or desorption intensities, respectively. The Hysteresis index (HI) was calculated as represented by Eq. (4).
2.5 Characterization of DOM
Soil and the amendments were treated with a solution of 0.01 M CaCl2 (1:20 w/v) and shaken for 10–15 min at room temperature for DOM extraction. The samples were then centrifuged at 8000 rpm for 10 min and filtered with 0.45-μm polycarbonate filters and stored at 4 °C until use. The dissolved organic carbon (DOC) in the extracted DOM samples was measured using a Shimadzu Total Organic Carbon (TOC) analyzer and absorption was measured at 254 nm with SpectraMax M3 FL-Spectrophotometer. Using the DOC value and absorption data at 254 nm wavelength, specific UV absorbance (SUVA) was calculated. Fluorescence spectra of the DOM extracts were obtained at emission wavelengths from 300 to 480 nm under excitation at 254 nm using a SpectraMax M3 FL-Spectrophotometer. The sample extracts were acidified with 2 N HCl (pH = 2) and the fluorescence spectra were recorded to identify the possible interferences due to protonated groups. In order to avoid the interferences due to changes in concentrations, the samples were diluted with double distilled water to bring the optical density below 0.1 cm−1. The fluorescence emission spectra obtained was corrected by multiplying the fluorescence intensity with factor eA, where A is the absorbance in cm−1 at the excitation wavelength. The complexity and condensation of the organic molecules can be expressed in terms of Humification index (HIX) defined by Eq. (5) (García-Jaramillo et al. 2014).
where W1 is the wavelength (nm) and IW1 is the fluorescence intensity at this wavelength. The HIX can be normalized and can be expressed as Eq. (6):
DOM was extracted from OAs (1:20 extraction on a dry mass basis) and analyzed by Fourier transform infrared spectroscopy (FT-IR). The extracts obtained were freeze dried and analyzed using Perkin Elmer (PE) Spectrum 100 FTIR.
2.6 Statistical analysis
The differences in between the samples and under different conditions compared to control were tested using a one-way analysis of variance (ANOVA; Tukey’s test at 95% confidence level). These analyses were carried out with SPSS 16.0.
3 Results and discussion
3.1 DOM characterization
The characterization of soluble organic matter from the soil and amendments provides valuable information of its structural and functional properties and thus can help us in better understanding of the pesticide–DOM interactions. The DOM was characterized using fluorescence and Fourier transform infrared (FTIR) spectroscopy.
Spectroscopic studies indicated the structural differences between the amendments in addition to differences in the DOC concentrations (Table 1). The maximum fluorescence intensity for natural soil DOM, C-DOM, and OM-DOM was observed at 375, 415, and 420 nm, respectively. The maximal intensity at wavelengths greater than 400 nm (towards the red region) indicated that the fluorescence for C-DOM and OM-DOM was dominated by condensed molecules, presumably aromatic, typical for humic materials (Zsolnay et al. 1999). It can be compared to the fluorescence spectrum of HA-DOM where the maximal intensity was located at 450 nm indicating the highly aromatic nature.
There was not much reduction in HIX and SUVA on acidification of C-DOM and OM-DOM in comparison to HA-DOM. Reduction in DOM fluorescence intensity by acidification was mainly because of the withdrawal of electrons from aromatic structures (ð-electron system) and changes occurring in the spatial structure of DOM upon protonation. The strong reaction to protonation of HA-DOM indicated a higher number of carboxylic groups. The SUVA and HIX of soil, OAs, and amended soil are represented in Online Resource Fig. S1 (Electronic Supplementary Material). The value of HIX is proportional to the increasing complexity of the organic compounds, e.g., increasing number of aromatic rings, degree of condensation and conjugation, as well as C/H ratio (Zsolnay 2003). The HIX values of the C-DOM (3.28) and OM-DOM (3.36) indicated its complex and aromatic nature.
In the second phase of this study, a major change in the fluorescence spectra of DOM extracts from the amended soils was observed especially in the region 300–400 nm (Online Resource Fig. S2, Electronic Supplementary Material). This indicated a rapid soil adsorption of the DOC fraction fluorescing at lower wavelengths (less condensed and less humified material) as observed by Cox et al. (2004). A large fraction of highly complex and humified organic material remains in the solution and is more stable to mineralization while having a lower affinity for mineral surfaces than the smaller DOC molecules. In addition, DOC polymerization (humification) may have also occurred which can be largely confirmed from the increase in the spectral intensity in the red region. Overall, it led to an increase in HIX of amended soils. There was no significant change in the values of DOC extracted from the soils amended with 2.5 and 5% OAs (p > 0.05) (Table 1), suggesting that longer time may be needed for the DOC from the amended soils to pass into the soil solution.
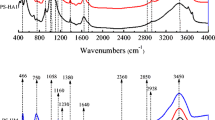
The FT-IR spectra (Fig. 1) revealed that OM-DOM has a strong absorption band at 3325 cm−1 representing O–H groups while the compost DOM spectra show a shallow and slightly shifted band at 3188 cm−1, mainly associated with O–H stretching of bonded and non-bonded hydroxyl groups. It has also been associated with phenol, alcohol, carboxyl groups, N-H amines, and amides (El Ouaqoudi et al. 2014). The presence of phenol and carboxyl groups in C-DOM is further supported by the band at 1406 and 1374 cm−1, which is associated with the COO− stretching of carboxylic acids. The stronger bands at 3325 and 1036 cm−1 suggest that OM-DOM is abundant in oxygen containing functional groups such as phenolic and carboxylic groups. Hydrophobic organic compounds can be held in colloidal dispersion by hydrophilic sites such as carboxylic acid, phenolic, and hydroxyl groups (Hassett and Anderson 1979). The low intensity peaks at 2920, 2888, and 2851 cm−1 in both DOMs indicate lower aliphatic moieties. In addition, for C-DOM, it implied a decrease in low molecular weight carbohydrates (amylose, etc.) and an increase of aromatic condensation with the presence of recalcitrant biomolecules such as long chain fatty acids and waxes (Wei et al. 2007; Smidt and Meissl 2007). The lower DOC extracted from C-DOM could be related to this part of the structure, richer in recalcitrant compounds, and hydrophobic compounds such as lignin (Thevenot et al. 2009). The band at 1611 cm−1 which has high intensity in OM-DOM compared to C-DOM represents aromatic C=C in addition to C=O and C–N from ketones and amides, respectively. Numerous bands were observed in the range 480–1800 cm−1 in compost DOM and attributed to the vibrations of the organic (proteins and polysaccharides) and inorganic components (carbonates and silicates) (Kovac et al. 2002). A clear distinction between many of the peaks was difficult because of the overlapping of bands in this region.
The FT-IR spectra revealed structural differences between C-DOM and OM-DOM. The aromaticity of DOM determines its association with hydrophobic contaminants (Uhle et al. 1999). These interactions between DOM and organic compounds may be through hydrogen bonding, ion exchange, van der Waals, hydrophobic bonding, and charge transfer (Katagi 2006). Further studies are required to elucidate the type of interactions associating DOM with the pesticides in the soil-water mixture.
3.2 Effect of OA-DOM and HA-DOM on pesticide sorption
The first phase of the sorption study was conducted in a system consisting of OA-DOM and pesticides. It was observed that dichlorvos sorption on natural soil was very low. A L-type isotherm was observed which was attributed to a decrease in site availability with increased dichlorvos concentration (Fig. 2a, c). Only about 11 to 13% of dichlorvos was sorbed at higher dichlorvos concentrations. The low sorption of dichlorvos in natural soil is due to its high solubility in water and low soil organic carbon. The low sorption observed in the case of dichlorvos did not allow us to obtain any desorption result. The dichlorvos equilibrium sorption data was fitted using Langmuir isotherm and the parameters are represented in Table 3. On the other hand, hydrophobic chlorpyrifos was strongly adsorbed to the soil and Freundlich isotherm was found to be best fit for the equilibrium data. The sorption of the selected pesticides was evaluated in the presence of OA-DOM. It did not significantly affect dichlorvos sorption (p > 0.05) (Fig. 2a). C-DOM and OM-DOM marginally increased dichlorvos sorption generally in the order S < S-OMDOM < S-CDOM. Sorption in the presence of C-DOM was slightly greater than OM-DOM only at higher dichlorvos concentrations.
The increase in dichlorvos sorption in the presence of DOM might be due to availability of more sorption sites. The DOM undergoes sorption on to the soil providing additional sites and contributing to increase in the organic matter content in the solid phase, facilitating dichlorvos sorption. Organic carbon normalized adsorption capacity (Koc) values tend to increase in the presence of DOM (Table 3). This effect is probably more important for the hydrophilic pesticides (Barriuso et al. 2011).
On the other hand, there was a substantial and significant decrease in chlorpyrifos sorption in the presence of extracted DOM in the order S > S-CDOM > S-OMDOM (p = 0.01) as observed in Fig. 2b. As explained earlier, the humic acids or humified fraction of DOM plays an important role in determining the sorption characteristics. This effect of OA-DOM on pesticides can also be explained further by conducting sorption experiments replacing OA-DOM in the solution with HA-DOM.
The effect of humic acids on the sorption of selected pesticides, dichlorvos, and chlorpyrifos is represented in Fig. 2c, d, respectively. There was an overall increase in dichlorvos sorption with increasing HA concentration similar to observation made in the presence of OA-DOM. This increase in dichlorvos sorption was significant when the HA concentration increased beyond 250 mg L−1 and only at higher dichlorvos concentrations. This can be confirmed with an increase in Koc values with increasing HA concentrations at an equilibrium concentration of 50 mg L−1 (Table 3). On the contrary, in case of chlorpyrifos, a significant decrease in sorption was observed. There was a slight decrease in chlorpyrifos sorption at HA concentrations up to 250 mg L−1. But a substantial reduction was observed in chlorpyrifos sorption when the HA concentration further increased (p < 0.0001). However, no significant differences in sorption were observed at very low chlorpyrifos concentrations. Chlorpyrifos sorption (Kf values and calculated Koc values) for soil decreased in the presence of increasing dissolved HA concentrations (Table 3), e.g., Koc values were 20,621 without any HA addition, 13,080 with 100 mg L−1 HA, 11,999 with 250 mg L−1 HA, 6981 with 500 mg L−1 HA, and 5405 with 1000 mg L−1 at an equilibrium concentration (Ce) of 1.5 mg L−1. The effective sorption coefficients determined by considering the effect of DOC may improve the prediction of contaminant transport models.
The pesticide sorption characteristics are governed by pesticide properties as well as the nature and source of DOM. In this study, the selected pesticides have contrasting solubility and polarity. Chlorpyrifos being a very hydrophobic compound has a high affinity for the soil as well as DOM. A study conducted by Huang and Lee (2001) using DOM from poultry, swine, and cow waste-derived lagoon effluents obtained similar results where a reduction in chlorpyrifos sorption was reported.
One of the possible reasons for reduced chlorpyrifos sorption and low Koc values at higher HA concentrations may be the increase in apparent solubility of chlorpyrifos and can be confirmed from the surface tension measurements at different HA concentrations (Online Resource Fig. S3, Electronic Supplementary Material). The surface tension of water was significantly lowered in the presence of higher HA-DOM (p < 0.02). A slight reduction in surface tension was also observed in the presence of OA-DOM (data not shown). Often, it has been reported that DOM and HAs have similar action as surfactants, and hence, they have the ability to lower the surface tension and increase the solubility of hydrophobic organic compounds (HOC) such as chlorpyrifos (Guetzloff and Rice 1996; Cho et al. 2002). An increase in the apparent aqueous solubility of chlordane, DDT, PCBs, and chlorodioxins in the presence of humic substances has been previously reported (Chiou et al. 1987; Johnson-Logan et al. 1992). The reduced sorption may also be attributed to the complex formation of HAs with organic compounds or competitive sorption (Williams et al. 2000; Flores-Céspedes et al. 2002). Moreover, HA and DOM association with the soil surface may lead to negative effect in terms of direct blockage of sorption sites for chlorpyrifos by the sorbed HA and lead to some modification of the surface characteristics resulting in decreased affinity for the pesticide molecule. The probable mechanism in such case may be that HA is bound to the soil through the hydrophobic regions on its surface with the orientation of hydrophilic and ionizable groups towards the solution phase. This would make the soil/solution interface more hydrophilic leading to preferential sorption of water molecules instead of hydrophobic compounds. Although the reduction in surface tension observed pointed towards higher solubilization of chlorpyrifos by DOM, the reduction in sorption as a result of other effects or a combination of effects cannot be neglected. This was because although DOM sorption was found to be low, but still it was substantial and hence its effect cannot be ignored. Keeping the initial DOC concentrations constant during the experiments, HA-DOM was found to be least sorbed with 25 to 30% sorption. OM-DOM was sorbed about 36 to 39% while C-DOM sorption on the soil was highest with 43 to 48% sorption.
3.3 Desorption experiments
Chlorpyrifos was strongly sorbed to the soil. Only about 15% of the sorbed concentration was desorbed in a single desorption step with 0.01 M CaCl2 solution. When the desorption was carried out in multiple steps, about 45% of the sorbed concentration was desorbed in six steps (Online Resource Fig. S4, Electronic Supplementary Material). The desorption data was fitted by Freundlich isotherm and the parameters are represented in Table 4. When the desorption was carried out using 0.01 M CaCl2, the desorption capacity (Kdf) was lower and hysteresis index (HI) was higher for a system containing HA and chlorpyrifos compared to the system without HA signifying relatively easy desorption in HA containing systems.
Additionally, desorption experiments were also carried out using HA-DOM to evaluate its potential to desorb the sorbed chlorpyrifos molecules from the natural soil. Solutions containing different HA concentrations were used to desorb the initially sorbed CPF molecules (Online Resource Fig. S5, Electronic Supplementary Material). The single step percentage of desorbed chlorpyrifos increased from 15 to 31% as HA-DOM concentration increased from 100 to 1000 mg L−1. Thus, there was a greater chlorpyrifos desorption from soil by HA-DOM solution compared to 0.01 M CaCl2, as indicated by lower Freundlich desorption capacities (Kfd) (Table 4). Chlorpyrifos exhibits strong partitioning onto soils from aqueous solutions because of its nonpolar behavior. The greater capability of HA-DOM compared to 0.01 M CaCl2 in desorbing chlorpyrifos from the soil agrees with the negative effect of HA on chlorpyrifos sorption. As discussed earlier, the stable interactions in solution between HA and chlorpyrifos or by processes taking place at the soil/solution interface such as competition for sorption sites on soil may lead to the greater desorption observed. Similar findings were reported for herbicide desorption mainly attributed to competitive sorption between DOM and pesticide without any resorption of the herbicide on the adsorbed DOM (Businelli 1997). The HI value for 0.01 M CaCl2 and DOM containing solutions was close to 1, indicating that no hysteresis was observed in desorption with these solutions.
OA-DOM characterized mainly by humified and complex molecules led to a slight but not a significant increase in sorption of hydrophilic pesticide dichlorvos whereas it can lead to significant reduction in sorption of hydrophobic pesticide chlorpyrifos. The humified OA-DOM has similar effect on pesticide sorption as that of dissolved HAs.
3.4 Effect of OA addition to soil on pesticide sorption and desorption
In the latter phase, the OAs were mixed with the soil, incubated, and then sorption studies were conducted. The addition of OAs can provide both insoluble and soluble carbon to the soil system. Moreover, according to previous studies (Gebremariam et al. 2012a, b; Tiwari and Guha 2012), the strong adsorption of chlorpyrifos was strongly correlated only to soil organic matter and not to any other soil properties. The increase in dichlorvos sorption observed with the addition of OAs was not significant (Fig. 3a). The adsorption increased in the following order: S < S + 2.5% C = S + 5% C = S + 2.5% OM < S + 5% OM. The isotherms obtained for dichlorvos sorption in the amended soil were of L-type and followed Langmuir isotherm. Although there was an overall increase in sorption upon the addition of OAs, a clear correlation with increasing OC content of the soil could not be established. The difference in the sorption observed between the OM and compost amended soil might be because of the different characteristics of these OAs and their potential for providing additional sites. The difference in dichlorvos sorption between amended and unamended soil was observed only at high concentrations. The isotherm parameters are represented in Table 5.
An increase in chlorpyrifos sorption was observed with OA addition to soil in the following order: S < S + 2.5% OM = S + 2.5% C < S + 5% OM < S + 5% C. Addition of higher percentage of OAs (5%) significantly increased chlorpyrifos sorption (p < 0.0001). Compost had a major effect on sorption than OM probably because of higher soil OC content. Contrary to dichlorvos, difference in sorption characteristics between natural and amended soil was observed for chlorpyrifos even at lower concentrations (Fig. 3b). These results were contrary to the sorption results obtained in first phase soil-water sorption studies in the presence of OA-DOM where a reduction in sorption was observed. DOM released into solution from the amended soils could also have a similar effect on chlorpyrifos. However, it should be noted that the DOC levels in the amended soil were lower than those in the extracted DOM solutions and this would reduce the DOM effects. The addition of insoluble carbon fraction to the system also seems to have impacted pesticide sorption. Moreover, in spite of an increase in percentage of OA addition to soil, there was hardly any change in the DOC concentration (Table 1). However, repeated OA addition to soil in fields can lead to increase in DOM concentration over time thus affecting chlorpyrifos sorption. Thus, it appeared that although the overall effect of OA addition to soil increases the soil organic carbon and hence the pesticide sorption, the DOM interactions with hydrophobic chlorpyrifos can reduce sorption onto the soil. The sorption can be reduced to such an extent that the sorption in the presence of substantial concentration of OA-DOM can be less than the sorption without it. Slight hysteresis was observed in case of compost amended soil and at 5% OM amended soil. In general, it can be stated that it was difficult for chlorpyrifos to leach from amended soils compared to unamended soils (Online Resource Table S3, Electronic Supplementary Material).
4 Conclusions
The application of OAs, mixed waste compost, and dried goat manure greatly influenced the soil sorption of the hydrophobic pesticide such as chlorpyrifos. In addition, it also significantly influenced the chlorpyrifos desorption and leaching from soil. Only a slight impact was observed on hydrophilic pesticide such as dichlorvos, leading to an increase in dichlorvos sorption mainly because of the additional sites provided by the sorbing DOM and no interactions taking place between DOM and dichlorvos. However, the OA-DOM characterized by large humification index consisting mainly of complex and condensed molecules of aromatic nature significantly decreased the chlorpyrifos sorption. The interactions between DOM and hydrophobic chlorpyrifos mainly in the solution and to some extent at soil/solution interface were the probable reasons for the reduction in chlorpyrifos sorption. This effect of OA-DOM was found to be similar to that caused by HA-DOM on pesticide sorption because of aromatic nature of the OAs. In agreement with the sorption results, chlorpyrifos desorption was significantly increased by OA-DOM as well as HA-DOM when compared to 0.01 M CaCl2 solution and no hysteresis was observed.
The increased soil organic carbon content because of amendment addition to soil increased retention of both the pesticides. It appeared that, although the net effect of OA application was an increase in pesticide sorption, interactions between the OA-DOM and hydrophobic pesticide resulted in some reduction in sorption that depended on the nature and concentration of OA-DOM. Thus, it can be concluded that the pesticide properties, nature and concentration of DOM were the main factors determing the interactions taking place between DOM and pesticide interactions thus governing the pesticide sorption on a particular soil.
Evaluating the change in sorption characteristics in the presence of OAs represents a valuable contribution to the understanding of the attenuation phenomena of the organic contaminants off-site migration in the environment. However, non-equilibrium sorption is ought to be observed at the field scale. The effects of DOM especially at the concentrations used in this study are more relevant at a local scale depending on its availability at short time–space scale. Fate and transport simulation models of pesticides in the environment require locally determined sorption values as input data and this study provides a valuable contribution in that aspect. Moreover, this study can help in improving and designing better management strategies for the rate and timing of soil organic amendments in the agricultural fields containing alluvial soil in the region considered in this study.
References
Albarrán A, Celis R, Hermosín MC et al (2004) Behaviour of simazine in soil amended with the final residue of the olive-oil extraction process. Chemosphere 54:717–724
Almendros G (1995) Sorptive interactions of pesticides in soils treated with modified humic acids. Eur J Soil Sci 46:287–301
Arnold CG, Ciani A, Müller SR, Amirbahman A, Schwarzenbach RP (1998) Association of triorganotin compounds with dissolved humic acids. Environ Sci Technol 32:2976–2983
Bai Y, Chen J, Mu H, Zhang C, Li B (2009) Reduction of dichlorvos and omethoate residues by O2 plasma treatment. J Agric Food Chem 57:6238–6245
Barriuso E, Andrades MS, Benoit P, Houot S (2011) Pesticide desorption from soils facilitated by dissolved organic matter coming from composts: experimental data and modelling approach. Biogeochemistry 106:117–133
Bhattacharyya T, Pal DK, Mandal C et al (2013) Genesis and classification of soils in Chattisgarh Basin view project. Soils of India: historical perspective, classification and recent advances. Curr Sci 104:1308–1323
Brigante M, Zanini G, Avena M (2010) Effect of humic acids on the adsorption of paraquat by goethite. J Hazard Mater 184:241–247
Buschmann J, Kappeler A, Lindauer U, Kistler D, Berg M, Sigg L (2006) Arsenite and arsenate binding to dissolved humic acids: influence of pH, type of humic acid, and aluminum. Environ Sci Technol 40:6015–6020
Businelli D (1997) Pig slurry amendment and herbicide coapplication effects on s-triazine mobility in soil: an adsorption-desorption study. J Environ Qual 26:102–108
Cabrera A, Cox L, Spokas K et al (2014) Influence of biochar amendments on the sorption-desorption of aminocyclopyrachlor, bentazone and pyraclostrobin pesticides to an agricultural soil. Sci Total Environ 470–471:438–443
Calamari D, Zhang L (2002) Environmental risk assessment of pesticides on aquatic life in Xiamen, China. Toxicol Lett 128:45–53
Cambier P, Pot V, Mercier V, Michaud A, Benoit P, Revallier A, Houot S (2014) Impact of long-term organic residue recycling in agriculture on soil solution composition and trace metal leaching in soils. Sci Total Environ 499:560–573
Chalhoub M, Coquet Y, Vachier P (2013) Water and bromide dynamics in a soil amended with different urban composts. Vadose Zone J 12:0. https://doi.org/10.2136/vzj2012.0056
Chiou CT, Kile DE, Brinton TI, Malcolm RL, Leenheer JA, MacCarthy P (1987) A comparison of water solubility enhancements of organic solutes by aquatic humic materials and commercial humic acids. Environ Sci Technol 21:1231–1234
Cho H-H, Park J-W, Liu CCK (2002) Effect of molecular structures on the solubility enhancement of hydrophobic organic compounds by environmental amphiphiles. Environ Toxicol Chem 21:999–1003
Cox L, Celis R, Hermosín MC, Cornejo J, Zsolnay A, Zeller K (2000) Effect of organic amendments on herbicide sorption as related to the nature of the dissolved organic matter. Environ Sci Technol 34:4600–4605
Cox L, Fernandes MC, Zsolnay A, Hermosín MC, Cornejo J (2004) Changes in dissolved organic carbon of soil amendments with aging: effect on pesticide adsorption behavior. J Agric Food Chem 52:5635–5642
Deng S, Chen Y, Wang D, Shi T, Wu X, Ma X, Li X, Hua R, Tang X, Li QX (2015) Rapid biodegradation of organophosphorus pesticides by Stenotrophomonas sp. J Hazard Mater 297:17–24
El Ouaqoudi FZ, El Fels L, Winterton P et al (2014) Study of humic acids during composting of ligno-cellulose waste by infra-red spectroscopic and thermogravimetric/thermal differential analysis. Compost Sci Util 22:188–198
Filipović V, Coquet Y, Pot V, Houot S, Benoit P (2014) Modeling the effect of soil structure on water flow and isoproturon dynamics in an agricultural field receiving repeated urban waste compost application. Sci Total Environ 499:546–559
Flores-Céspedes F, González-Pradas E, Fernández-Pérez M, Villafranca-Sánchez M, Socías-Viciana M, Ureña-Amate MD (2002) Effects of dissolved organic carbon on sorption and mobility of imidacloprid in soil. J Environ Qual 31:880–888
Gaonkar OD, Kumar GS, Nambi IM (2016a) Numerical investigations on pesticide fate and transport in an unsaturated porous medium for a coupled water and pesticide management. Environ Earth Sci 75:1232
Gaonkar OD, Suresh Kumar G, Nambi IM (2016b) Numerical modelling on fate and transport of coupled adsorption and biodegradation of pesticides in an unsaturated porous medium. ISH J Hydraul Eng 22:236–246
García-Jaramillo M, Cox L, Cornejo J, Hermosín MC (2014) Effect of soil organic amendments on the behavior of bentazone and tricyclazole. Sci Total Environ 466–467:906–913
Gebremariam SY, Beutel MW, Flury M, Harsh JB, Yonge DR (2012a) Nonsingular adsorption/desorption of chlorpyrifos in soils and sediments: experimental results and modeling. Environ Sci Technol 46:869–875
Gebremariam SY, Beutel MW, Yonge DR et al (2012b) Adsorption and desorption of chlorpyrifos to soils and sediments. Reviews of environ Contam Toxicol. Springer, New York, pp 123–175
Golash N, Gogate PR (2012) Degradation of dichlorvos containing wastewaters using sonochemical reactors. Ultrason Sonochem 19:1051–1060
Graber ER, Dror I, Bercovich FC, Rosner M (2001) Enhanced transport of pesticides in a field trial with treated sewage sludge. Chemosphere 44:805–811
Guetzloff TF, Rice JA (1996) Micellar nature of humic colloids. Humic Fulvic Acids 651:18–25
Hassett JP, Anderson MA (1979) Association of hydrophobic organic compounds with dissolved organic matter in aquatic systems. Environ Sci Technol 13:1526–1529
Huang X, Lee LS (2001) Effects of dissolved organic matter from animal waste effluent on chlorpyrifos sorption by soils. J Environ Qual 30:1258–1265
Iglesias-Jiménez E, Poveda E, Sánchez-Martín MJ, Sánchez-Camazano M (1997) Effect of the nature of exogenous organic matter on pesticide sorption by the soil. Arch Environ Contam Toxicol 33:117–124
Johnson-Logan LR, Broshears RE, Klalne SJ (1992) Partitioning behavior and the mobility of chlordane in groundwater. Environ Sci Technol 26:2234–2239
Kalbitz K, Solinger S, Park J-H, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165:277–304
Katagi T (2006) Behavior of pesticides in water-sediment systems. Reviews of environ Contam Toxicol. Springer, New York, pp 133–251
Kihanda F, Warren G, Atwal SS (2004) The influence of goat manure application on crop yield and soil nitrate variations in semi-arid eastern Kenya. Managing nutrient cycles to sustain soil fertility in sub-Saharan Africa. Academy Science Publishers in association with the Tropical Soil Biology and Fertility Institute of CIAT, Nairobi, pp 173–186
Kovac N, Bajt O, Faganeli J, Sket B, Orel B (2002) Study of macroaggregate composition using FT-IR and 1H-NMR spectroscopy. Mar Chem 78:205–215
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004.
Martin-Neto L, Traghetta DG, Vaz CMP, Crestana S, Sposito G (2001) On the interaction mechanisms of atrazine and hydroxyatrazine with humic substances. J Environ Qual 30:520
Pan B, Ning P, Xing B (2008) Part IV - sorption of hydrophobic organic contaminants. Environ Sci Pollut Res 17:554–564
Pot V, Benoit P, Etievant V, Bernet N, Labat C, Coquet Y, Houot S (2011) Effects of tillage practice and repeated urban compost application on bromide and isoproturon transport in a loamy Albeluvisol. Eur J Soil Sci 62:797–810
Prosen H, Fingler S, Zupančič-Kralj L, Drevenkar V (2007) Partitioning of selected environmental pollutants into organic matter as determined by solid-phase microextraction. Chemosphere 66:1580–1589
Scaglia B, Baglieri A, Tambone F, Gennari M, Adani F (2016) Chlorpyrifos-methyl solubilisation by humic acids used as bio-surfactants extracted from lignocelluloses and kitchen wastes. Chemosphere 159:208–213
Schneider S, Coquet Y, Vachier P, Labat C, Roger-Estrade J, Benoit P, Pot V, Houot S (2009) Effect of urban waste compost application on soil near-saturated hydraulic conductivity. J Environ Qual 38:772
Smidt E, Meissl K (2007) The applicability of Fourier transform infrared (FT-IR) spectroscopy in waste management. Waste Manag 27:268–276
Soil Science Division Staff (2017) Soil survey manual. In: Ditzler C, Scheffe K, Monger HC (eds) USDA Handbook 18. Government Printing Office, Washington, DC, pp 120–131
Song NH, Chen L, Yang H (2008) Effect of dissolved organic matter on mobility and activation of chlorotoluron in soil and wheat. Geoderma 146:344–352
SP 36–1 (1987) Compendium of Indian standards on soil engineering: Part-1 LaboratoryTesting of soils for civil engineering purposes. Bureau of Indian Standards (BIS)
Spark KM, Swift RS (2002) Effect of soil composition and dissolved organic matter on pesticide sorption. Sci Total Environ 298:147–161
Thengodkar RRM, Sivakami S (2010) Degradation of Chlorpyrifos by an alkaline phosphatase from the cyanobacterium Spirulina platensis. Biodegradation 21:637–644
Thevenot M, Dousset S, Rousseaux S, Andreux F (2008) Influence of organic amendments on diuron leaching through an acidic and a calcareous vineyard soil using undisturbed lysimeters. Environ Pollut 153:148–156
Thevenot M, Dousset S, Hertkorn N, Schmitt-Kopplin P, Andreux F (2009) Interactions of diuron with dissolved organic matter from organic amendments. Sci Total Environ 407:4297–4302
Tiwari MK, Guha S (2012) Role of soil organic matter on the sorption and cosorption of endosulfan and chlorpyrifos on agricultural soils. J Environ Eng 138:426–435
Uhle ME, Chin YP, Aiken GR, McKnight DM (1999) Binding of polychlorinated biphenyls to aquatic humic substances: the role of substrate and sorbate properties on partitioning. Environ Sci Technol 33:2715–2718
Wang H, Lin K, Hou Z, Richardson B, Gan J (2010) Sorption of the herbicide terbuthylazine in two New Zealand forest soils amended with biosolids and biochars. J Soils Sediments 10:283–289
Wei Z, Xi B, Zhao Y, Wang S, Liu H, Jiang Y (2007) Effect of inoculating microbes in municipal solid waste composting on characteristics of humic acid. Chemosphere 68:368–374
Williams CF, Agassi M, Letey J, Farmer WJ, Nelson SD, Ben-Hur M (2000) Facilitated transport of napropamide by dissolved organic matter through soil columns. Soil Sci Soc Am J 64:590
Yu XY, Ying GG, Kookana RS (2009) Reduced plant uptake of pesticides with biochar additions to soil. Chemosphere 76:665–671
Zheng Y-Z, Lan W-S, Qiao C-L, Mulchandani A, Chen W (2007) Decontamination of vegetables sprayed with organophosphate pesticides by organophosphorus hydrolase and carboxylesterase (B1). Appl Biochem Biotechnol 136:233–241
Zsolnay Á (2003) Dissolved organic matter: artefacts, definitions, and functions. Geoderma 113:187–209
Zsolnay A, Baigar E, Jimenez M, et al (1999) Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 38:45–50. https://doi.org/10.1016/S0045-6535(98)00166-0
Zulin Z, Huasheng H, Xinhong W, Jianqing L, Weiqi C, Li X (2002) Determination and load of organophosphorus and organochlorine pesticides at water from Jiulong River estuary, China. Mar Pollut Bull 45:397–402
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Responsible editor: Xilong Wang
Electronic supplementary material
ESM 1
(DOCX 542 kb)
Rights and permissions
About this article
Cite this article
Gaonkar, O.D., Nambi, I.M. & Govindarajan, S.K. Soil organic amendments: impacts on sorption of organophosphate pesticides on an alluvial soil. J Soils Sediments 19, 566–578 (2019). https://doi.org/10.1007/s11368-018-2080-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-2080-6