Abstract
Purpose
Soil temperature is a fundamental parameter affecting not only microbial activity but also manganese (MnIII,IV) and iron (FeIII) oxide reduction rates. The relationship between MnIII,IV oxide removal from oxide-coated redox bars is missing at present. This study investigated the effect of variable soil temperatures on oxide removal by MnIII,IV and FeIII oxide-coated redox bars in water-saturated soil columns in the laboratory.
Materials and methods
The Mn coatings contained the mineral birnessite, whereas the Fe coatings contained a mixture of ferrihydrite and goethite. Additionally, platinum (Pt) electrodes designed to measure the redox potential (EH) were installed in the soil columns, which were filled with either a humic topsoil with an organic carbon (Corg) content of 85 g kg−1 (pH 5.8) or a subsoil containing 2 g Corg kg−1 (pH 7.5). Experiments were performed at 5, 15, and 25 °C.
Results and discussion
Although elevated soil temperatures accelerated the decrease in EH after water saturation in the topsoil, no EH decreases regardless of soil temperature occurred in the subsoil. Besides soil temperature, the importance of soil organic matter as an electron donor is highlighted in this case. Complete removal of the MnIII,IV oxide coating was observed after 28, 14, and 7 days in the soil columns filled with topsoil at 5, 15, and 25 °C, respectively. Along the Fe redox bars, FeIII reducing conditions first appeared at 15 °C and oxide removal was enhanced at 25 °C because of lower EH, with the preferential dissolution of ferrihydrite over goethite as revealed by visual differences in the FeIII oxide coating. Oxide removal along redox bars followed the thermodynamics of the applied minerals in the order birnessite > ferrihydrite > goethite.
Conclusions
In line with Van’t Hoff’s rule, turnover rates of MnIII,IV and FeIII oxide reduction increased as a result of increased soil temperatures. Taking into account the stability lines of the designated minerals, EH-pH conditions were in accordance with oxide removal. Soil temperature must therefore be considered a master variable when evaluating the oxide removal of redox bars employed for the monitoring of soil redox status.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soils are open and porous systems composed of solids, liquids, and gases, and are involved in a continuous exchange of matter and energy with the surrounding spheres. In wetland soils, flooding or other events cause water saturation that hampers the replenishment of oxygen (O2) from the atmosphere. Consequently, the availability of O2 is in a transient state and is not constant over time, because heterotrophic microorganisms consume O2 by reduction to water during the process of aerobic respiration (Glinski and Stepniewski 1985). Specialized microbes such as Geobacter or Shewanella are able to reduce tri- and tetravalent manganese (MnIII,IV) and trivalent iron (FeIII) oxides under the absence of more favorable terminal electron acceptors such as O2 or nitrate (NO3 −) (Martin 2005). Microbial activity is sensitive to changes in soil temperature and consequently has an impact on the reduction rates of MnIII,IV and FeIII oxides (Lovley 1991).
Various methods are available with which to document reducing conditions in soils, ranging from cost-intensive but fully automated systems using platinum (Pt) electrodes and data loggers (Patrick et al. 1996; Fiedler et al. 2007), laborious, time- and sampling-intensive techniques based on the terminal electron-accepting processes approach (Chapelle et al. 1995), through to inexpensive iron metal rods that provide little insight into the temporal extent of soil reducing conditions (Owens et al. 2008). Iron oxide-coated polyvinyl chloride (PVC) tubes were introduced by Jenkinson and Franzmeier (2006) to document and monitor soil redox status via the depletion of the synthesized FeIII oxide coating under soil reducing conditions. This method was later adapted using MnIII,IV oxides as the coating agent (Dorau and Mansfeldt 2015) to differentiate between weakly (EH 300 to 100 mV at pH 7; range of MnIII,IV oxide reduction) and moderately reducing conditions (EH 100 to − 100 mV at pH 7; range of ferrihydrite reduction) in the field. In waterlogged soils, whereas the critical value for the onset of MnIII,IV oxide reduction is 300 to 200 mV (Gotoh and Patrick 1972), the transformation of FeIII oxides starts at 100 mV under neutral soil reaction (Gotoh and Patrick 1974). Utilizing both types of bars (collectively referred to as “Mn and Fe redox bars”) enables the assessment of redox classes in soils, information that is of the utmost importance because various biogeochemical processes, e.g., greenhouse gas emissions or distinct trace metal mobility, can be linked to a specific redox window. Moreover, the possibility of short-term monitoring on a weekly basis using Mn redox bars may improve the temporal delineation of soil reducing conditions, as tri- and tetravalent Mn is the preferred electron acceptor and is more easily stripped from the white PVC surface than trivalent Fe (Dorau et al. 2016). Thus far, redox bars have been successfully applied to document sulfide in marsh pore water (Rabenhorst et al. 2010), to delineate hydric soils for land classification (Castenson and Rabenhorst 2006), to visually assess the in situ formation of FeIII oxides along the Mn oxide coating (Dorau et al. 2016), and further to study the sorption behavior of dissolved compounds to the remaining oxide coating (Dorau and Mansfeldt 2016). While the impact of soil temperature and EH has been related to oxide removal and depletion patterns along Fe redox bars (Castenson and Rabenhorst 2006; Rabenhorst and Castenson 2005), this relationship remains unconfirmed for Mn redox bars at present. Hence, the aim of this study was to assess the impact of soil temperature and EH on the reductive dissolution behavior of MnIII,IV and FeIII oxide coatings from redox bars in water-saturated soil columns under controlled conditions in the laboratory.
2 Materials and methods
2.1 Soil sampling and soil properties
Two samples were chosen for the column experiment. First, we sampled topsoil (Ah horizon) from a floodplain soil along the Wupper River at Leichlingen, North Rhine-Westphalia, Germany (51° 7′ 10.65″ N; 7° 0′ 40.79″ E). The soil is a Fluvisol (IUSS Working Group WRB, 2014), with sampling restricted to the humic topsoil (0 to 15 cm). Second, the loess parent material (C horizon) of a Haplic Cambisol (IUSS Working Group WRB, 2014) was sampled close to Mönchengladbach, North Rhine-Westphalia, Germany (51° 2′ 58.98″ N; 6° 27′ 39.16″ E). The two samples were selected based on their potential difference in soil organic carbon content (Corg) and pH. Besides the effect of temperature, sample selection accounted for the effect of variable soil properties on EH development and on oxide removal along the redox bars. For a more intuitive terminology, the former soil material is hereafter referred to as topsoil and the latter as loess. Soil samples were oven-dried at 40 °C and sieved <2 mm to determine the following soil properties: soil pH, which was measured potentiometrically using a glass electrode in a 0.01 M L−1 CaCl2 solution mixed 5:1 with soil (vol/vol); Corg, measured via dry combustion using a CNS analyzer (vario EL cube, Elementar, Hanau, Germany); grain size distribution, obtained by the sieve and settling method; and iron oxides (Fed), extracted using dithionite-citrate-bicarbonate (DCB). The solution was then analyzed for total iron (Fe) concentrations via flame atomic absorption spectroscopy.
2.2 Soil column experiments
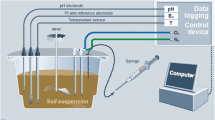
The soil columns were 20 cm in length and had an inner diameter of 11.5 cm. At temperature variants of 5, 15, and 25 °C, each of the four soil columns were incubated. Two soil columns were filled to the top with topsoil and the other two with loess at a unit bulk density of 1.2 g cm−3. One topsoil column was equipped with four Mn redox bars and the other topsoil column with four Fe redox bars, with the loess columns equipped with Mn and Fe redox bars in the same manner. Manganese redox bars were coated with the mineral birnessite (Dorau and Mansfeldt 2015), and the Fe redox bars with an FeIII oxide suspension containing the minerals ferrihydrite and goethite (Rabenhorst et al. 2008a). The length of the bars was 22 cm (Ø 21 mm), with the lower 20 cm coated. Removal and re-installation of the bars took place on a weekly basis for a total period of 28 days. Briefly, the oxide removal was quantified by (i) taking a picture of each 120° redox bar section, (ii) cropping of the distorted images to an overall image, and (iii) creation of a binary image by thresholding to quantify the oxide removal into depleted and non-depleted area by using an image analysis tool (e.g., GIMP v.2.8.4). The soil columns were placed in a water bath with the water table (WT) adjusted to the middle of the soil column to facilitate reducing conditions and to separate the redox bars into an aerobic (top 100 mm) and an anaerobic part (lower 100 mm). The water bath was placed in a climate chamber (KB400, Binder, Tuttlingen, Germany) and incubated for 28 days at a temperature of 5, 15, or 25 °C. After each temperature variant, new oven-dried and sieved topsoil or loess was used to fill the soil columns, which were equipped with newly manufactured redox bars. To determine EH during the course of the experiments, a silver/silver chloride (Ag/AgCl) electrode (3 M KCl; SE23, Sensortechnik Meinsberg, Meinsberg, Germany) was placed in the center of the soil column, with platinum (Pt) tipped electrodes in duplicate at 5-, 10-, and 15-cm depth in each of the topsoil and loess columns. Soil EH was recorded on an hourly basis for the total study period.
2.3 Assessment of mineral stability
As redox reactions cause changes in soil pH, the pH of the topsoil and loess samples used in the column experiment was measured potentiometrically via a glass electrode placed in a 0.01 M L−1 CaCl2 solution mixed 5:1 with soil (vol/vol). The pH was determined exclusively at the beginning and after the incubation period of 28 days to guarantee no soil disturbance during the experiment. A linear pH change was postulated from the beginning to the end of the corresponding temperature experiment at 5, 15, and 25 °C. In this context, redox equilibrium lines can be helpful with an emphasis on predicting the stability of MnIII,IV and FeIII oxides (Strawn et al. 2015). If the EH and pH of a soil are above the EH-pH equilibrium conditions, e.g., of goethite, then ferric Fe (Fe3+) is in this case predicted to be the stable species. If the conditions are below equilibrium, ferrous Fe (Fe2+) is predicted to be the predominant species and goethite might be reductively dissolved. We used the equations for birnessite (MnO2 + 4H+ + 2e− = Mn2+ + 2H2O; Eq. (1)), ferrihydrite (Fe(OH)3 + 3H+ + e− = Fe2+ + 3H2O; Eq. (2)), and goethite (α-FeOOH +3H+ + e− = Fe2+ + 2H2O; Eq. (3)) with the following Eqs. (4), (5), and (6)
by assuming a species concentration of either 10−6 mol Mn2+ or Fe2+ L−1, 25 °C, and 0.101 MPa (Bricker 1965; Fischer 1987).
3 Results and discussion
3.1 EH development
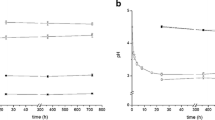
Figure 1 illustrates the development of EH during the soil column experiment. Analysis of this figure reveals that the value of EH decreased in the columns filled with topsoil from 493 to 258, 484 to -125, and from 428 to -202 mV at 5, 15, and 25 °C, respectively (Fig. 1a). In contrast, EH remained stable at between 543 and 482 mV under all temperatures for the loess samples (Fig. 1b). We attribute this behavior to the lack of a readily available Corg source (electron donor), as the loess sample contained very little Corg (1.98 g Corg kg−1, Table 1). Additionally, lack of adapted C-degrading microorganisms seems also reasonable as a possible explanation, since the non-fluviatile loess subsoil undergone no inundation history. On the other hand, the elevated topsoil content of 85.3 g Corg kg−1 soil favored a decrease in EH, even at the low temperature of 5 °C, and thus EH development in these samples was affected with respect to temperature. As indicated by the low standard deviation and proven by the two-tailed t test conducted at the 5% significance level, no significant differences between Pt electrode measurements in duplicates and with monitoring depth (5, 10, and 15 cm) were recorded. Therefore, EH development is displayed as the mean of multiple EH measurements per soil column (Fig. 1; n = 6).
3.2 Mn oxide removal
The EH-pH data for the topsoil plotted constantly below the stability line of birnessite at all temperatures (Fig. 2, Table 2). Sample pH values increased from 5.8 ± 0.01 at the beginning (Table 1) to pH 6.2 ± 0.01 (5 °C), 6.4 ± 0.02 (15 °C), and 6.6 ± 0.01 (25 °C) at the end of the incubation experiment. Equation 1 likely explains the slight increase in pH, since reduction is a proton-consuming process. In contrast, the EH-pH values for the loess were very close and slightly below the stability line of birnessite at all temperatures (Fig. 2, Table 2), with pH values before and after the incubation experiment remaining constant at 7.5 ± 0.01 (Table 1). Hence, it can be expected that MnIII,IV oxide removal from the redox bars was favorable for the topsoil but hampered for the loess. Indeed, whereas no MnIII,IV oxides were removed from Mn redox bars installed in the loess (Fig. 3d–f), MnIII,IV oxide removal was completed after 4 weeks of incubation at 5 °C (Fig. 3a), after 2 weeks of incubation at 15 °C (Fig. 3b), and after 1 week of incubation at 25 °C in the topsoil (Fig. 3c, Table 3). Even at a temperature of 5 °C and within a very short time frame of 7 days after water saturation, microorganisms effectively coupled the oxidation of organic matter with the reduction of MnIII,IV oxides in the topsoil, as evidenced by the partial depletion of the oxide coating (Fig. 3a, Table 3). As soil temperatures increased, Mn oxide loss was enhanced (Fig. 3b, c), with the temperature range between 5 and 15 °C and the first 7 days of activity considered to be the hot moment of MnIII,IV oxides reduction, as indicated by the differences in oxide removal (Fig. 3a, b, Table 3). Overall, it has to be considered that differences in Mn oxide removal between the topsoil and loess might be also due to the absence of heterotrophic microbes and not solely a function of thermodynamic predictions of stability.
EH development as a function of time and with respect to the stability line (dashed black line in figures a to f) of birnessite, ferrihydrite, and goethite. Soil pH was determined at the beginning and at the end of the topsoil and loess column experiment and a linear pH change was employed in the stability equation. Positive values indicate no reductive mineral dissolution and negative values indicate reductive dissolution of the designated mineral. Data are daily means with standard deviations of six Pt electrodes at temperatures of 5 (blue rectangles), 15 (green circles), and 25 °C (red triangles)
Images of Mn redox bars installed in soil columns filled with topsoil (a, b, c) and loess (d, e, f) at temperatures of 5 (a, d), 15 (b, e), and 25 °C (c, f). The removal/re-installation of redox bars took place on a weekly basis for a period of 28 days. Each image represents a composite image of three 120° sections from one bar
3.3 Fe oxide removal
Whereas EH-pH data were for half of the time slightly above the stability line of ferrihydrite at 5 °C, the reductive dissolution of ferrihydrite was constantly predicted at temperatures of 15 and 25 °C in the topsoil, respectively (Fig. 2, Table 2). In contrast, the reductive dissolution of goethite was not predicted at 5 °C, because EH-pH conditions were significantly above the stability line but predicted for 61% of the time at 15 °C and for 86% of the time at 25 °C, respectively (Fig. 2, Table 2). Although no change in color was evident for the Fe redox bars at 5 °C (Fig. 4a), a separation of the soil column into upper (above the WT) and lower parts (below the WT) became apparent after 2 weeks at 15 °C (Fig. 4b). As investigated by Rabenhorst et al. (2008b), the pale yellow areas present at 15 °C (Fig. 4b) and that occurred more intensively at 25 °C (Fig. 4c) reflect the preferential dissolution of ferrihydrite over goethite. This finding correlates with both the thermodynamic properties of ferrihydrite and the EH-pH data of the incubation experiment (Figs. 2 and 4), since ferrihydrite is predicted to be removed from the PVC surface. Considering goethite removal, white PVC was not visible after 28 days but it cannot be excluded that some goethite was still removed from the bars surface (Fig. 4b, c). The appearance of the Fe redox bars installed in the loess was not altered by the removal of the oxide coating at all temperature variants (Fig. 4d–f), a result again coherent with EH-pH data (Fig. 2).
Images of Fe redox bars installed in soil columns filled with topsoil (a, b, c) and loess (d, e, f) at temperatures of 5 (a, d), 15 (b, e), and 25 °C (c, f). The removal/re-installation of redox bars took place on a weekly basis for a period of 28 days. Each image represents a composite image of three 120° sections from one bar
3.4 Redox range of in situ FeIII oxide formation
Even though the processes (e.g., ligand-, proton-, or electron-promoted) that lead to the dissolution of birnessite (log K 0 35.38), ferrihydrite (log K 0 3.54), and goethite (log K 0 -0.02) are difficult to differentiate, organizational statements are possible, such as goethite dissolves more slowly than ferrihydrite, and FeIII oxides dissolve more slowly than MnIII,IV oxides (Lindsay 1979; Martin 2005). This information is also valid for the constructed EH plots (Fig. 2) and for the appearance of the oxide coating along redox bars. A number of studies have revealed that microsites in soils have an impact on the redox milieu under both laboratory (Yang et al. 2006) and field conditions (Dorau et al. 2016). A theory that accounts for the significantly more ferrihydrite (orange color) observed here remaining along the Fe redox bar coating in the upper 0 to 10 cm compared with the lower 10 to 20 cm (Fig. 4b). However, the low standard deviation of the EH measurements made at 5-, 10-, and 15-cm depth did not indicate any differences associated with the setting of the water table at 15 °C (Fig. 1). Hence, whereas visual differences caused by the depletion of the oxide coating enable the characterization of the soil milieu at very small scales, the equal EH values recorded in close proximity to the artificial water table do not account for the effect of the not fully water-saturated soil pores. This has to be taken into consideration when comparing depletion patterns along redox bars with EH measurements. Differences in oxide removal were not evident for the Mn redox bars, with no MnIII,IV oxide coating remaining after 28 days of installation, but less Mn oxide removal was observed from the upper part of the bars at 5 °C from day 14 to 21 (Fig. 3a–c).
Figure 5 shows a sketch of the incubation experiment, with one image of a Mn redox bar at 15 °C after 28 days of incubation. Along the artificial water table at between 9 and 10 cm (Fig. 5; see benchmark), a thin band of in situ formed FeIII oxides grew from days 21 to 28 and was present at all four replicates. The origin of this band is likely the bioavailable reducible FeIII oxides present in the topsoil sample at 21.3 g kg−1 (Table 1) that are reduced in the lower part of the soil column. This mobile ferrous Fe is translocated upwards by capillary rise and oxidizes above the artificial water table. Typically, such freshly precipitated Fe oxides are expected to be short-range ordered because of the low interfacial energies of nucleation (Stumm and Morgan, 1996). Indeed, protrusion of the Mn redox bar (Fig. 3b) into an oxalate-solution known to selectively extract short-range ordered FeIII oxides from soils (Schwertmann 1964) removed the thin Fe band within minutes (data not shown), verifying the assumption of a micro-crystalline nature. Interestingly, such thin bands of FeIII oxides were lacking along the Mn redox bars in the 5 and 25 °C incubation experiments because the EH was either too high to reductively dissolve and mobilize FeIII oxides (coherent with the appearance of the Fe redox bar in Fig. 4a), or too low for Fe2+ to be oxidized in the soil solution (Fig. 4c). Hence, there is a very narrow redox window responsible for the formation of FeIII oxides, as indicated by the differences in EH values observed from days 21 to 28 of the experiment, of -115 mV at 15 °C compared with -195 mV at 25 °C (Fig. 1a).
This has implications for the active formation of soil horizons having petrogleyic properties (e.g., bog iron; IUSS Working Group WRB 2014), which contains the following prerequisites: (i) a steady capillary rise of soil solution enriched in Fe2+, (ii) an O2 barrier above the water table where Fe2+ is oxidized and forms FeIII oxides, (iii) stable hydrologic conditions so that Fe precipitation takes place at a certain depth, and (iv) Fe enrichment then taking place over the course of centuries (Blume 1988). However, under acidic conditions, the abiotic oxidation of Fe2+ to Fe3+ by dissolved O2 has a half-life of years (Singer and Stumm 1970), although it may be accelerated by certain bacteria in low-pH environments (Nordstrom and Southam 1997). Determination of these boundary conditions is difficult to achieve and requires both aqueous and solid phase geochemical data (Mansfeldt et al. 2012; Stanton et al. 2007). The presence of thin circumferential Fe bands along Mn redox bars, if they are frequently observed throughout annual monitoring of the soil redox milieu, might thus be an additional indicator of FeIII oxide enrichment in distinct soil horizons. Manganese as an oxidant for Fe2+ seems to be an important precursor for the formation of durable FeIII oxide coatings because these circumferential bands were barely observed along Fe redox bars (Dorau and Mansfeldt 2016). This might also relate to the fact that newly formed Fe precipitates are difficult to differentiate from the orange background of FeIII oxide coatings from Fe redox bars. Apparently, FeIII oxide formation along redox bars takes place under a narrow range of EH-pH conditions. Analogous to the findings of Yu and Patrick (2004), there is also a less narrow EH range between 180 and − 150 mV where the emission of greenhouse gases is minimized (e.g., by carbon dioxide, nitrous oxide, and methane). Hence, the prevalence of a distinct redox window (spatially and temporally) has an impact on various biogeochemical processes taking place in soils. Further work is required to extend and verify the results from the laboratory experiments to field conditions.
4 Conclusions
This study demonstrated visually via the use of coated redox bars that the reductive dissolution behavior of MnIII,IV oxides is strongly affected by soil temperature. At low soil temperatures of 5 °C, microorganisms were able to reduce MnIII,IV oxides rapidly within a short period of days. Hence, monitoring of soil redox status on a daily basis and under moderately acidic conditions should be considered, especially in the summer when soil temperatures are high and water saturation is present. Additionally, the results obtained regarding temperature-dependent MnIII,IV oxide reduction have implications for soils affected by groundwater, perched water, flooding, and irrigation during low soil temperatures in winter, because two conditions are met in such settings: (i) water saturation prevails periodically and (ii) with generally low soil temperatures. Although the measured EH values do not necessarily match predicted and observed Mn2+ or Fe2+ concentrations in soil solution (e.g., due to kinetic constraints and chemical non-equilibrium under natural conditions), the depletion patterns observed along the Mn and Fe redox bars are still related to EH and pH conditions.
References
Blume HP (1988) The fate of iron during soil formation in humid-temperate environments. In: Stucki JW, Goodman BA, Schwertmann U (eds) Iron in soils and clay minerals. Springer Netherlands, Dordrecht, pp 749–777
Bricker O (1965) Some stability relations in the system Mn-O2-H2O at 25 °C and one atmosphere total pressure. Am Mineral 50:1296–1354
Castenson KL, Rabenhorst MC (2006) Indicator of reduction in soil (IRIS): evaluation of a new approach for assessing reduced conditions in soil. Soil Sci Soc Am J 70:1222–1226
Chapelle FH, McMahon PB, Dubrovsky NM, Fujii RF, Oaksford ET, Vroblesky DA (1995) Deducing the distribution of terminal electron-accepting processes in hydrologically diverse groundwater systems. Water Resour Res 31:359–371
Dorau K, Eickmeier M, Mansfeldt T (2016) Comparison of manganese and iron oxide-coated redox bars for characterization of the redox status in wetland soils. Wetlands 36:133–144
Dorau K, Mansfeldt T (2015) Manganese-oxide-coated redox bars as an indicator of reducing conditions in soils. J Environ Qual 44:696–703
Dorau K, Mansfeldt T (2016) Manganese and iron oxide-coated redox bars as a tool to in situ study the element sorption in wet soils. J Soils Sediments 16:976–986
Fiedler S, Vepraskas MJ, Richardson JL (2007) Soil redox potential: importance, field measurements, and observations. In: Sparks DL (ed) Advances in agronomy. Academic Press, San Diego, pp 1–54
Fischer WR (1987) Standard potentials (Eo) of iron(III) oxides under reducing conditions. Z Pflanz Bodenkunde 150:286–289
Glinski J, Stepniewski W (1985) Soil aeration and its role for plants. CRC Press, Boca Raton
Gotoh S, Patrick WH (1972) Transformation of manganese in a waterlogged soil as affected by redox potential and pH. Soil Sci Soc Am Pro 36:738–742
Gotoh S, Patrick WH (1974) Transformation of iron in a waterlogged soil as influenced by redox potential and pH. Soil Sci Soc Am Pro 38:66–71
IUSS Working Group WRB (2014) World reference base for soil resources 2014. World Soil Resources Reports No. 103. FAO, Rome, Italy
Jenkinson BJ, Franzmeier DP (2006) Development and evaluation of iron-coated tubes that indicate reduction in soils. Soil Sci Soc Am J 70:183–191
Lindsay WL (1979) Chemical equilibria in soils. John Wiley & Sons, Hoboken
Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55:259–287
Mansfeldt T, Schuth S, Häusler W, Wagner F, Kaufhold S, Overesch M (2012) Iron oxide mineralogy and stable iron isotope composition in a Gleysol with petrogleyic properties. J Soils Sediments 12:97–114
Martin ST (2005) Precipitation and dissolution of iron and manganese oxides. In: Grassian VH (ed) Environmental catalysis. CRC Press, Boca Raton, pp 61–81
Nordstrom DK, Southam G (1997) Geomicrobiology of sulfide mineral oxidation. Rev Mineral 35:381–390
Owens PR, Wilding LP, Miller WM, Griffin RW (2008) Using iron metal rods to infer oxygen status in seasonally saturated soils. Catena 73:197–203
Patrick WH, Gambrell RP, Faulkner SP (1996) Redox measurements of soils. In: Sparks DL (ed) Methods of soil analysis: chemical methods part 3. Soil Science Society of America and American Society of Agronomy, Madison, p 677
Rabenhorst MC, Bourgault RR, James BR (2008a) Iron oxyhydroxide reduction in simulated wetland soils: effects of mineralogical composition of IRIS paints. Soil Sci Soc Am J 72:1838–1842
Rabenhorst MC, Castenson KL (2005) Temperature effects on iron reduction in a hydric soil. Soil Sci 170:734–742
Rabenhorst MC, Megonigal JP, Keller J (2010) Synthetic iron oxides for documenting sulfide in marsh pore water. Soil Sci Soc Am J 74:1383–1388
Rabenhorst MC, Ming DW, Morris RV, Golden DC (2008b) Synthesized iron oxides used as a tool for documenting reducing conditions in soils. Soil Sci 173:417–423
Schwertmann U (1964) Differenzierung der Eisenoxide des Bodens durch Extraktion mit Ammoniumoxalat-Lösung. Z Pflanzenernähr Düng Bodenkd 105:194–202
Singer PC, Stumm W (1970) Acidic mine drainage: the rate-determining step. Science 167:1121–1123
Stanton MR, Yager DB, Fey DL, Wright WG (2007) Formation and geochemical significance of iron bog deposits. US Geological Survey, Washington
Strawn DG, Bohn HL, O'Connor GA (2015) Soil chemistry, fourth edn. John Wiley & Sons, Hoboken
Stumm W, Morgan JJ (1996) Aquatic chemistry, third edn. Wiley, New York
Yang J, Hu Y, Bu R (2006) Microscale spatial variability of redox potential in surface soil. Soil Sci 171:747–753
Yu KW, Patrick WH (2004) Redox window with minimum global warming potential contribution from rice soils. Soil Sci Soc Am J 68:2086–2091
Acknowledgements
The authors thank Mr. Roland Robel (University of Cologne, Germany) for his support and allowing the usage of facilities with which to manufacture redox bars. Dr. Gerhard Dumbeck and Mr. Manuel Endenich (both RWE POWER AG, Essen, Germany) are acknowledged for their help provided during soil sampling. We thank two anonymous reviewers for their recommendations to improve the quality of the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Claudio Zaccone
Rights and permissions
About this article
Cite this article
Dorau, K., Papenfuß, S. & Mansfeldt, T. Temperature-dependent oxide removal from manganese- and iron oxide-coated soil redox bars. J Soils Sediments 18, 680–687 (2018). https://doi.org/10.1007/s11368-017-1812-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1812-3